The tortuous path of lactate shuttle discovery:From cinders and boards to the lab and ICU
2020-10-09GeorgeBrooks
George A.Brooks
Exercise Physiology Laboratory,Department of Integrative Biology,University of California Berkeley,CA 94720-3140,USA
Abstract Once thought to be a waste product of oxygen limited(anaerobic)metabolism, lactate is now known to form continuously under fully oxygenated (aerobic) conditions. Lactate shuttling between producer (driver) and consumer cells fulfills at least 3 purposes; lactate is: (1) a major energy source, (2) the major gluconeogenic precursor, and (3) a signaling molecule. The Lactate Shuttle theory is applicable to diverse fields such as sports nutrition and hydration, resuscitation from acidosis and Dengue, treatment of traumatic brain injury, maintenance of glycemia,reduction of inflammation, cardiac support in heart failure and following a myocardial infarction, and to improve cognition. Yet, dysregulated lactate shuttling disrupts metabolic flexibility,and worse,supports oncogenesis.Lactate production in cancer(the Warburg effect)is involved in all main sequela for carcinogenesis:angiogenesis,immune escape,cell migration,metastasis,and self-sufficient metabolism.The history of the tortuous path of discovery in lactate metabolism and shuttling was discussed in the 2019 American College of Sports Medicine Joseph B.Wolffe Lecture in Orlando,FL.
Keywords: Anaerobic metabolism;Exercise;Glycolysis;Oxidative metabolism;Warburg Effect
1. Introduction
1.1. Author’s perspective
Fifty-five years ago, the author’s understanding of the biochemistry and physiology was simple:the accumulation of lactic acid indicated O2debt.Lactate was a dead-end metabolite,a metabolic waste that caused muscle fatigue, rigor, cramps,and soreness. Certainty of that understanding was assured because those ideas came from the founders of modern biochemistry(O.Meyerhof)1and muscle physiology(A.V.Hill).2However,from a teleological view,those ideas made no sense to the author,a 20-year-old at the time.Furthermore,the ideas seemed dated and inconsistent with emerging ideas in mitochondrial energetics,3,4muscle metabolism and contraction.5In contrfrom lactate shuttle theory(Fig.1),6-8 today we know that lactate is continuously produced under fully aerobic conditions (Fig. 2) and is a major fuel for muscle,9heart,10and brain;11the major gluconeogenic precursor;12,13and a signaling molecule.14Furthermore, lactate may serve to alleviate muscle fatigue due to extracellular K+accumulation15and,because it is the terminal step in glycolysis,catabolized by lactate dehydrogenase (LDH), produces lactate anion, not lactic acid.16-18
Given the early history,how paradoxical it is that we19and others20,21are evaluating the efficacy of using lactate-containing solutions to provide support in the setting of critical care medicine. Specifically, for treatment of traumatic brain injury (TBI), lactate formulations directly support neuronal metabolism when glucose uptake is limited following injury,achieve exquisite glycemic control by supplying the major gluconeogenic precursor for liver and kidneys,and limit postinjury cerebral swelling.19In the last century, a very insightful man said, “der Alte w€urfelt nicht!” (“the old one (i.e.,creator)does not play dice with the universe”).22So it is that lactate production and use is now to be viewed as a basic biological response that is accelerated to mitigate a variety of metabolic stresses.

Fig.1. The lactate shuttle concept,depicting lactate as the vehicle linking glycolytic and oxidative metabolism. Linkages between lactate “producer” and“consumer” exist within and among cells,tissues, and organs. As the product of one metabolic pathway (glycolysis) and the substrate for a downstream pathway of disposal(mitochondrial respiration),lactate is the link between the glycolytic and aerobic pathways. Importantly, according to the lactate shuttle hypothesis, this linkage occurs continuously under fully aerobic conditions,can transcend compartment barriers and occur within and among cells,tissues and organs.Modified from Refs 6,23,and 194 with permission.
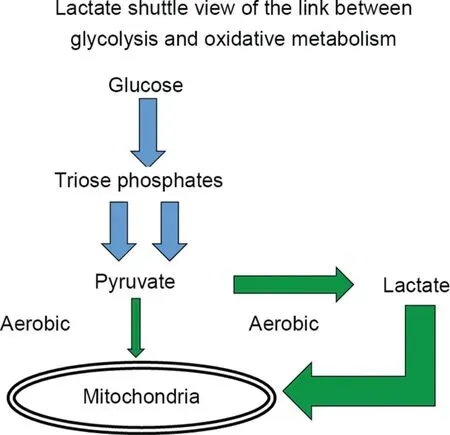
Fig. 2. Lactate production occurs continuously under fully aerobic conditions in intact animals,mammalian tissue preparations,intact animals,and humans in vivo.In muscles and arterial blood of resting healthy humans, lactate concentration approximates 1.0 mmol/L,while pyruvate concentration approximates 0.1 mmol/L.The lactate/pyruvate(L/P)approximates 10,with net lactate production and release from resting muscle of healthy individuals occurring when arterial partial pressure of oxygen(PO2)approximates 100 Torr and intramuscular PO2 approximates 40 Torr,well above the critical mitochondrial PO2 for maximal mitochondrial respiration(1-2 Torr).195-197 During exercise at about 65% of maximal oxygen consumption(VO2max),lactate production and net lactate release from working muscle beds rise and the L/P rises more than an order of magnitude(to approximately 500).57 However,the intramuscular PO2 remains at 3-4 Torr,well above the critical mitochondrial O2 level.Hence,it is appropriate to conclude that in healthy humans,glycolysis proceeds to lactate under fully aerobic conditions. Importantly, most (75%-80%)lactate is disposed of immediately within the tissue or subsequent to release and reuptake by working muscle,with significant uptake and oxidation by heart or oxidation by liver for gluconeogenesis.From diverse sources9,45,57,144,197,198 with permission.
1.2. Physiological stress and lactate strain
In physiology, it is typical that lactate accumulates when there is a physiologic stress. The classical interpretation was that lactate accumulation was a stress (a fatigue agent, a dead-end metabolite, the result of O2insufficiency, etc.);1retrospectively, that was a biased and incorrect view. To the contrary, increased glycolysis in response to stress could have, and should have, been regarded as a compensatory strain response.23
That luminaries in biology and medicine were involved in O2Debt theory has historically been a problem for the field. For instance, in the unperfused and unoxygenated frog hemicorpus preparation of Meyerhof,1electrical stimulation led to glycogen depletion, lactate accumulation and fatigue. This was an ideal setup to quantitatively relate glycogen depletion to lactate accumulation,but the experimental setup(Fig.3)was nonphysiological and incapable of representing what happens in vivo.With the benefit of a century of research we now appreciate that in a closed, oxygen-limited environment, glycolysis was a major means to support adenosine triphosphate(ATP)flux.In concert,A.V.Hill,one of the founders of muscle and exercise physiology,measured postexercise O2consumption, but not lactate kinetics in humans recovering from exercise,24but he never measured either O2consumption or lactate production in the isolated frog muscle preparations he studied.2The evidence linking lactate production to muscle oxygen insufficiency was circumstantial, and,unfortunately, ill-considered. Regrettably, the Pavlovian and knee-jerk responses to lactate accumulation and attributing it to O2insufficiency have persisted.25

Fig.3. The Meyerhof calorimeter.m1 is the device for indirect electrical stimulation of frog hemicorpus thighs;the horizontal line(m2)is Ringers solution level.This device was used to demonstrate quantitative conversion of glycogen to lactate under nonperfused and nonoxygenated conditions.From Ref.1 with permission.
1.3. Controversies within the Nobel tradition
Despite widespread interpretation of data by Nobel Prize winners1,2that lactate production was the result of O2-limited metabolism, observations that lactate could be produced under fully aerobic conditions were made by other Nobelists.26,27Glucose consumption leading to lactate production under fully aerobic conditions was revealed to be a hallmark of cancer.27While originally ascribed to the presence of mitochondrial defects in cancer cells, subsequently the ability of mitochondria in cancer cells to respire with substrates, including lactate, was demonstrated.28Importantly,the efficacious use of lactate produced in fully oxygenated muscles to support gluconeogenesis and maintain euglycemia was demonstrated by the Coris.26However, while there was good reason to think that lactate could be efficacious in vivo,26the general belief of lactate as a metabolic waste and stress metabolite persisted through much of the 20th century.24,29
1.4. Origin of the Lactate Shuttle theory
The inspiration leading to articulation of the Lactate Shuttle hypothesis came from several lines of evidence using lab rats as models of mammalian metabolism;6evidence came from radiotracer studies of glucose and lactate kinetics and measurements of blood and muscle lactate concentrations in resting and exercising animals. At its essence, the hypothesis is that lactate shuttles among producer(driver)and consumer(recipient)tissues,cells,and cellular compartments.
1.5. Key results from isotope tracer studies
In contrast to the one-fifth/four-fifths oxidation/glyconeogenesis theory of lactate disposal during exercise recovery14C-lactate injected into lab rats resulted in label exhalation as14CO2,with little incorporation into muscle glycogen.30,31Second,technologies were developed to make metabolic measurements on resting, exercising, and recovering lab animals32and then determine concentrations and specific activities of CO2,glucose,lactate,and related metabolites33(Fig.4A and 4B),respectively.Indeed,it is likely that none of these advances would have been possible without isotope tracer technologies enabling measurements of metabolite production and disposal rates. With those methods available, it became possible to simultaneously compare glucose34and lactate35flux(turnover,production,and disposal)rates as well as rates of lactate disposal via oxidation and gluconeogenesis in trained and untrained rats both at rest and when exercising. Hence, the presence of lactate shuttling in a mammalian (rat) system was revealed (Fig. 5). In resting rats,lactate turnover and oxidation rates were surprisingly high, but were typically less than corresponding glucose flux rates.However,during exercise,lactate flux and oxidation easily exceeded corresponding glucose flux rates. This was because of the contribution of glycogen to glycolytic flux during exercise. Moreover, during exercise, most glucose production came from lactate via gluconeogenesis.34Training not only increased the capacity of gluconeogenesis, but also had dramatic effects on lactate the metabolic clearance rate (disposal rate/concentration). The classic effect of exercise training on lowering blood lactate concentration was observed in running rats. However,tracer data showed that while lactate production was high in running rats, lactate production was balanced by disposal.Hence, the lower circulating blood lactate concentration in trained rats during exercise was explained by greater clearance rates due to increased oxidation and gluconeogenesis.34,35Subsequently, with the advent of stable, nonradioactive isotope tracers, metabolite flux studies could be conducted on human subjects. In short, the same effects of exercise and exercise training on glucose and lactate production and disposal rates as observed in rats were replicated in cross-sectional36-39and longitudinal training studies on humans.9,13,40

Fig.4. Devices use to support the presence of lactate shuttling in resting and exercising mammals in vivo.These include(A)a motorized treadmill with sensors to determine oxygen consumption (VO2), rate of elimination of carbon dioxide (VCO2), electrocardiogram (ECG), RER (= VCO2/VO2)32 and (B) blood-specific activities of glucose,lactate glucose(Gluc),fructose di-phosphate(FDP),and other metabolic intermediates using 2-dimensional paper chromatography,autoradiography and enzymatic analyses.33 See original papers32,33 for details on how lactate flux(production and disposal,oxidation and gluconeogenic)rates were determined in a mammalian model organism during physical exercise.From Ref.23 with permission.
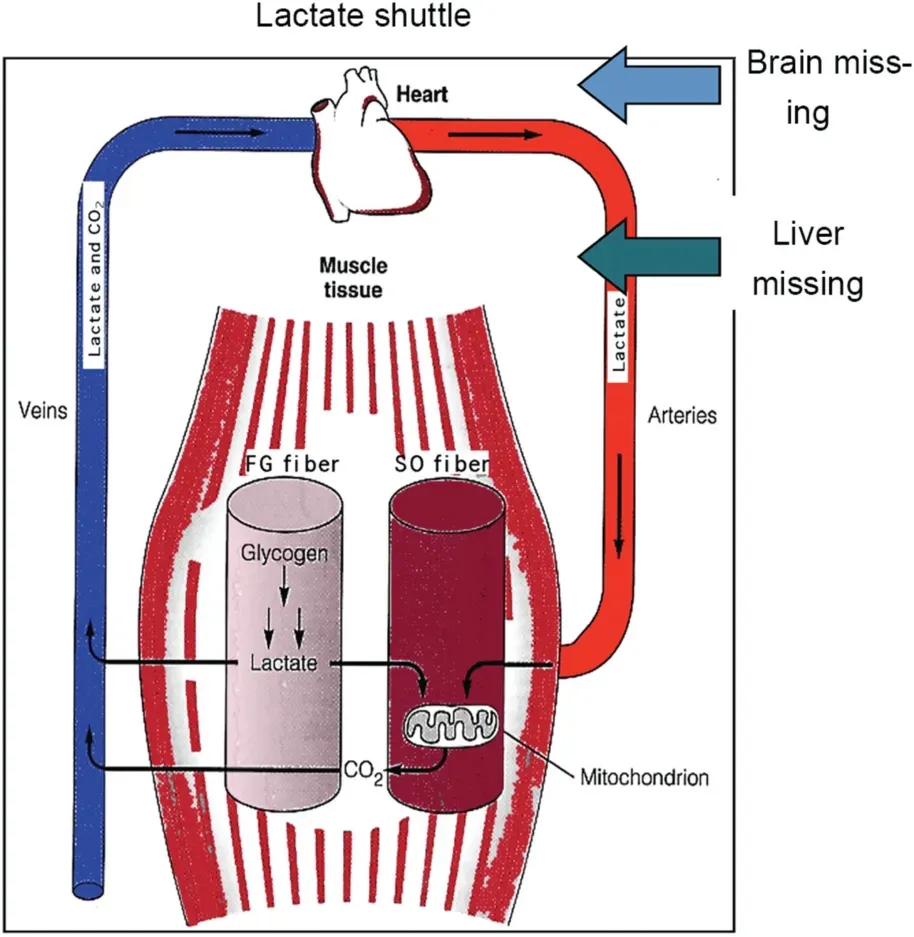
Fig.5. Depiction of the lactate shuttle as it fulfills 3 physiologic functions:(1)lactate as a major energy source, (2) lactate as the major gluconeogenic precursor,and(3)lactate as a signaling molecule with autocrine-,paracrine-,and endocrine-like effects(called a“lactormone”).“Cell-Cell”and“Intracellular Lactate Shuttle”concepts describe the roles of lactate in the delivery of oxidative and gluconeogenic substrates as well as in cell signaling.Examples of the Cell-Cell Lactate Shuttles include lactate exchanges between white glycolytic and red oxidative fibers within a working muscle bed and between working skeletal muscle and heart,brain,liver,and kidneys. Examples of Intracellular Lactate Shuttles include cytosol-mitochondrial and cytosol-peroxisome exchanges. Indeed, most,if not all,lactate shuttles are driven by a concentration or pH gradient or by redox state. FG =fast glycolytic; SO = slow oxidative. The figure is annotated because the original model did not anticipate cerebral lactate oxidation and hepatic and renal gluconeogenesis. Compiled from diverse sources6-8 and194 with permission.
1.6. Key results from tissue lactate concentration studies
The results of isotope tracer studies34,35showing high rates of lactate disposal via tissue exchange and oxidation were complemented by results of carefully done rat muscle studies by Paul Mol-e,Kenneth Baldwin,and their associates.41,42The Lactate Shuttle hypothesis was necessary to explain why in working red skeletal muscles, lactate concentrations were not only lower than those in white sections of the same muscle but also were lower than those in the blood perfusing them.Thus, the concept of a lactate shuttle6was supported by independent lines of confirmatory data,6which were subsequently described more fully(Figs.1,2,and 5).43,44
1.7. Key results from tissue net and isotope tracer balance studies
Results of whole body tracer and tissue lactate concentration studies were informative but provided minimal information on the tissue sites of lactate production and disposal in vivo. However, in the early 1980s, studies on tissue specificity of lactate metabolism were under way,9,45,46and those efforts served as templates for further research.9,10,40For example,in 1988,fueling of the heart during exercise with lactate released from working muscle beds was observed and was key to envisioning lactate shuttling in humans.10In terms of Cell-Cell, or Tissue-Tissue lactate shuttling,it was subsequently recognized that lactate shuttled and carbon recycled between and among tissue beds,such as between skeletal muscle, heart and liver (Fig. 5). In terms of driver cells, fast white skeletal cells and tissues42,47-49and the integument50were identified. In terms of recipient cells and tissues,the beating heart takes up and oxidizes lactate,10,46,51as do working skeletal muscle beds.9,45However, what is the path between lactate uptake and the formation and release of CO2within an organ,tissue or cell?Was there something else,perhaps an Intracellular Lactate Shuttle,to be discovered and elucidated?
1.8. The intracellular,cytosol-to-mitochondrial lactate shuttle
Observations of lactate exchange between cells,tissue,and organs in humans and other mammals52,53provided impetus for discovery of intracellular lactate shuttling. Studies on humans38,39and other mammals35,54,55revealed that most lactate was disposed of via intramuscular oxidation, a result inverse of the one-fifth/four-fifths theory of O2Debt (vide supra). Lactate disposal via oxidation was particularly prominent when muscles and heart were engaged and blood flow,oxygen consumption, and metabolite flux rates were high.9,10,45,46With that realization, issues arose about where within a working muscle fiber or beating heart cell lactate was oxidized.At the time,much thinking about this issue was that the first step in lactate oxidation to pyruvate occurred in the cytosol,but for several reasons that idea made no sense.Beating heart46and working red muscle45,56simultaneously consume glucose and take up and oxidize glucose and lactate.Additionally, measurements of the lactate (L) to pyruvate (P)ratio (L/P) were inconsistent with the one-fifth/four-fifths theory of lactate disposal.In a resting person,the L/P in leg muscle venous blood ranged from 10 to 20;29,57however, when muscles contracted to achieve a moderate exercise power output, the L/P in venous effluent of working muscle rose more than an order of magnitude (i.e., L/P >500).57Because of the presence LDH in erythrocytes and lung parenchyma,58,59relative rise of the L/P in arterial blood of exercising individuals is significant but blunted.9,29,57Given these data, the notion that lactate oxidation occurs in the cytosol was implausible. If not in the cytosol,where,then,does lactate oxidation commence?Where else,but in the mitochondrial reticulum!
2. The mitochondrial lactate oxidation complex (mLOC)
Controversy surrounds the idea of mitochondrial lactate oxidation; some investigators have produced mitochondrial preparations that oxidize lactate,60-65whereas some others66-68have failed in the attempt. Conceptually the issue is resolved if one realizes that the cellular respiratory apparatus is not located in discrete, capsular-shaped organelles (i.e., mitochondria), but rather in an extensive network,the mitochondrial reticulum.69,70Hence,attempts to isolate mitochondria for ex vivo respiratory studies inevitably results in disruption of the mitochondrial reticulum69,71and lability and fragility of mitochondrial constituents such as cytochrome c and LDH.Still,it is possible to show lactate oxidation in mitochondrial preparations from mammalian muscle,including human skeletal muscle.72Additionally,the presence of muscle mitochondrial lactate oxidation can be demonstrated by magnetic resonance spectroscopy.73,74Moreover,studies of muscle cells and tissues using confocal laser scanning microscopy,immunoprecipitation and immunohistochemistry show the presence of LDH in the mLOC.75,76Seemingly, then, the issue of mitochondrial lactate oxidation has been resolved. In retrospect,the inability of some investigators to produce mitochondrial preparations that do respire lactate is not unique. A similar problem with the ability of muscle mitochondrial preparations to oxidize long chain fatty acids has been ascribed to use of the proteolytic enzyme nagarse, which increases muscle mitochondrial protein yield, but the resulting preparations lose the ability to oxidize fatty acids or their carnitine derivatives.77In this instance, the inability of mitochondria preparations to recapitulate what is known to happen in vivo can be ascribed to isolation artifact.It is the same with mitochondrial preparations which lose LDH during isolation and are unable to oxidize lactate.Losing or knocking out mLDH,an essential part of the mLOC,only proves that mLDH is essential for mitochondrial lactate oxidation.
In vivo tissue lactate production, uptake, and oxidation occur because glycolysis and mitochondrial respiration occur simultaneously. For the mitochondrial reticulum to oxidize lactate,the reticulum contain a transporter or transporters64,78and LDH,62,75both of which were found as soon as probed for using immunohistochemistry and immunocoprecipitation.75,76,79LDH is now listed in mitochondrial constituent databases such as the MitoCarta80,81(https://www.broadinstitute.org/scientific-commu nity/science/programs/metabolic-disease-program/publications/mitocarta/mitocarta-in-0) and MitoMiner (http://mitominer.mrcmbu.cam.ac.uk/release-4.0/begin.do).
The first clues to the structure of mLOC (Fig.6) and mitochondrial ability to oxidize lactate as well as pyruvate was deduced based on the functionality of mitochondrial preparations and a single report in the literature on how to mitigate the effects of LDH-contaminated mitochondrial preparations.82To oxidize lactate mitochondrial preparations requires all the usually understood components (pyruvate dehydrogenase,Krebs cycle enzymes, components of the electron transport chain), a lactate transporter, and LDH. Upon investigation,mitochondrial preparations from rat and human muscle were found to contain monocarboxylate transporter isoform 1(MCT1), its membrane chaperone basigin (BSG or CD147),LDH and cytochrome oxidase.75Subsequently, essential elements of the mLOC were found in mitochondrial preparations from liver,63,65brain,76and various model systems, such as brain slices,83primary neuronal cultures,76,84normal breast,and transformed breast cancer cells28and tumors.85Immunohistologic evidence of the presence of mLOC is presented in Fig.7.At the time of discovery,it was known that,while lactate was preferred over pyruvate as an oxidizable substrate,monocarboxylate transporters (MCTs) could also transport pyruvate and b-hydroxybutyrate,86,87but a unique mitochondrial pyruvate transporter had not been identified.

Fig. 6. A schematic showing the putative mitochondrial lactate oxidation complex (mLOC). The lactate-pyruvate transporter (MCT1) is inserted into the mitochondrial inner membrane,strongly interacting with its chaperone protein CD147,and is also associated with cytochrome oxidase(COx)as well as mitochondrial lactate dehydrogenase(mLDH),which could be located at the outer side of the inner membrane.Lactate,which is always produced in the cytosol of muscle and other tissues because of the abundance,activity,and characteristics of cytosolic LDH,is oxidized to pyruvate via the lactate oxidation complex in mitochondria of the same cell.This endergonic lactate oxidation reaction is coupled to the exergonic redox change in COx during mitochondrial electron transport.ETC=electron transport chain; GP=glycerol phosphate; Mal-Asp=malate-aspartate; MCT=monocarboxylate (lactate) transporter; mPC=mitochondrial pyruvate carrier;TCA=tricarboxylic acid.Redrawn from Ref.75 with permission.
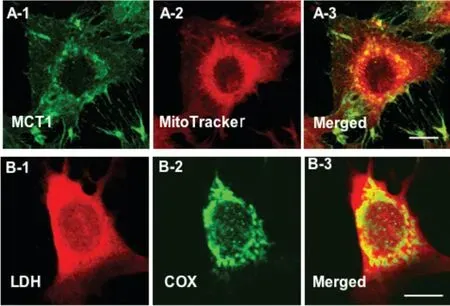
Fig.7. Immunohistochemical images demonstrating some components of the mitochondrial lactate oxidation complex(mLOC)in L6 cells.The mLOC contains the inner mitochondrial membrane cytochrome oxidase (COX), the lactate-pyruvate transporter(MCT1),lactate dehydrogenase(LDH)and the MCT anchoring protein,CD147(Basigin).MCT1 was detected at both sarcolemmal and intracellular domains (A-1). Mitochondrial reticulum (MR), identified by MitoTracker, was extensively elaborated in L6 cells (A-2). The merged images of MCT1(green,A-1)and MR(red,A-2)showed intense yellow,indicating co-localization of MCT1 and components of the MR, particularly at perinuclear cell domains (A-3). (B) LDH (B-1) and COX (B-2) are imaged.Superposition of signals for LDH(red,B-1)and COX(green,B-2)shows colocalization of LDH in the MR (yellow) of muscle cells (B-3). Depth of field approximately 1 mm, scale bar=10 mm. Similar data have been obtained on rat plantaris leg muscles in vivo.From Ref.75 with permission.
2.1. Mitochondrial lactate and pyruvate carriers
Following reports of discovery of the mitochondrial pyruvate carrier (mPC),88,89and with access to our own custom antibodies to MCT1 as well as commercially available antibodies to the putative mPC, we obtained images assessing co-localization of MCT1 and mPC in L6 cells.In those preliminary studies,co-localization analysis of mMCT1 and mPC1 in Imaris software showed an r2of 0.8. It appears that both MCT1 and mPC are co-localized to the mitochondria(r2=0.8)(Fig.8).However,at the light microscopic level it is impossible to know if mMCT and mPC interact physically and functionally. Immunocoprecipitation, x-ray crystallography, mass spectrometry, and protein deletion (knockout) studies are needed to definitively answer questions about mMCT and mPC co-localization and functionality and the role of the mPC in mitochondrial lactate oxidation.
Finally,with regard to the site of intramuscular lactate oxidation, Gladden and associates,18in an attempt to integrate ideas about the intracellular lactate oxidation, modified Fig. 6 to include the hypothesis that mitochondrial lactate oxidation to pyruvate occurred in the cytosol or mitochondrial intermembrane space. However, that conclusion is inconsistent with what we28,76and others63,65,84have found concerning the location of mitochondrial LDH.

Fig.8. First images assessing co-localization of the monocarboxylate(lactate,pyruvate,b-hydroxybutyrate)transporter(MCT1)and mitochondrial the pyruvate carrier(mPC)in L6 cells,which shows the localization of DAPI-positive nuclei (A), MCT1 (B), mPC1 (C), and Mito Tracker-positive MR (D) in L6 cells. The merged images are shown in E. Co-localization analysis of mPC1(C) and mitochondria (D) showed a Pearson correlation coefficient (r2) value of 0.8. Co-localization analysis of MCT1 (B) and mPC1 (C) showed an r2 of 0.3,largely because MCT1 occupies sarcolemmal,mitochondrial,and peroxisomal compartments.A channel to represent the co-localization of MCT1 and mitochondria was created to image mMCT1; subsequent co-localization of mMCT1 with mPC1 resulted in an r2 of 0.8 (F). White dots indicate the colocalization of mMCT1 and mPC1 as observed in Image J software. Whole images were contrast enhanced in A, B, C, D, and E. Similar results were observed for mPC2. Scale bar=20 mm. It appears that both MCT1 and the putative mPC are co-localized to the mitochondria (r2=0.8). However, at the light microscopic level,it is impossible to know if the 2 proteins interact physically and functionally.Also,with benefit of the Orbitrap liquid chromatography/mass spectrometry device, we would be able to determine fractional synthesis rates of mitochondrial lactate oxidation complex and mPC proteins.
Future research efforts to better define mitochondrial lactate and pyruvate oxidation complexes, perhaps using Betzig-type super-resolution microscopy,electron microscopy,or hyperpolarized C studies,13are eagerly anticipated. Moreover, all extant models may prove to be too simplistic. For instance,others have proposed90that intracellular lactate shuttling is associated with the well-known malate-aspartate shuttle.Because redundancy in regulation is a physiologic principle,we shall not be surprised if intracellular lactate shuttling will eventually be shown to be accomplished by multiple mechanisms in vivo.
2.2. Other Lactate Shuttle and other roles of lactate
Because of the recognition of the presence of lactate shuttling within and among various cells,tissues and organs such as muscle,heart,and liver,6,7,43,44we and others have extended the concept to include other cells, tissues, and organs such as the brain,90,91lungs,58,59sperm,92,93adipose,94-96 and peroxisomes.97Descriptions of these lactate shuttles are described elsewhere.23
3. Lactate signaling,lipolysis,fatty acid oxidation,glucose tolerance,and inflammation

Fig.9. Illustration of how lactatemia affects blood(glucose)and peripheral glucose uptake as well as the production,uptake and oxidation of FFA,giving rise to metabolic inflexibility in muscle.Lactate is the inevitable consequence of glycolysis,18 the minimal muscle lactate (L) to pyruvate (P) ratio (L/P) being 10 and rising to an L/P of >100 when glycolytic flux is high.57 Lactate is the favored oxidizable substrate and provides product inhibition of glucose and FFA oxidation.As the products of glycolysis,lactate and pyruvate provide negative feedback inhibition of glucose disposal(blue dashed lines).Also,as the predominant mitochondrial substrate, lactate gives rise to acetyl-coenzyme A(CoA), and in turn malonyl-CoA. Acetyl-CoA inhibits b-ketothiolase and,hence, b-oxidation, while malonyl-CoA inhibits mitochondrial FFA-derivative uptake via CPT1(T).199 Moreover,lactate is the main gluconeogenic precursor raising glucose production and blood(glucose)(red lines).Via GPR81 binding,lactate inhibits lipolysis in WAT (T), depressing circulating FFA.96,104 This model explains the paradoxical presence of lactatemia in high-intensity exercise and insulin-resistant states with limited ability to oxidize fat (green lines).Modified from Ref. 76. CPT1=carnitine palmitoyl transporter-1; FAT=fatty acid translocator comprised of CD36 and FABPc; FFA=free fatty acid;GLUT=glucose transporter; m=mitochondrial; Malonyl=CoA formed from exported TCA citrate controlled by the interactions of malonyl-CoA decarboxylase (MCD) and acetyl-CoA carboxylase (ACC); MCT=monocarboxylate transporter; mPC=mitochondrial pyruvate transporter; PDH=pyruvate dehydrogenase; s=sarcolemmal; T=inhibition; WAT = white adipose tissue. Not shown is fatty acyl-Co(FA-CoA)that will accumulate if FFAs are taken up by myocytes,but blocked from mitochondrial entry by the effect of malonyl-CoA on CPT1.Accumulated intracellular FA-CoA will give rise to intramyocellular triglyceride (IMTG) and the formulation of LC-FA, DAG, and ceramides via inhibition of PI3 Kinase (PI3-K) and reducing GLUT4 translocation; from Ref.23 with permission.
An inverse relationship between blood and plasma free fatty acid (FFA) concentration and oxidation has long been recognized,98but the associations are under-appreciated (Fig.9).In the 1960s, Issekutz and colleagues99,100noted the effect of lactacidemia on diminishing circulating FFA in dogs and humans during hard exercise,and lactate infusion into running dogs caused circulating FFA to decline.99,101,102In their work,these investigators could clearly observe an effect of the lactate on circulating FFA, but whether the mechanism was an inhibition of lipolysis or a stimulation or re-esterification was not addressed.
The mechanism by which lactatemia suppresses circulating FFA is now known to be due to suppression of adipose lipolysis. Recently, several groups of investigators94-96,103 have shown that,independent of pH or sodium ions,lactate inhibits lipolysis in fat cells through activation of a previously identified orphan G-protein coupled receptor, now termed hydroxycarboxylic acid receptor 1 (HCAR-1). In mouse, rat, and human adipocytes, HCAR-1 appears to act as a lactate sensor with the inhibitory effect on lipolysis operating through cyclic adenosine monophosphate and cyclic adenosine monophosphate response element binding.104-106
Most recently, a large international group of investigators has expanded knowledge of the role of lactate signaling via transforming growth factor b (TGF-b) secreted from adipose.107TGF-b is a multifunctional cytokine belonging to the TGF superfamily that includes 3 different mammalian isoforms (TGF-b1 to TGF-b3). Because of its role in immune and stem cell regulation and differentiation, TGF-b2 is a highly researched cytokine in the fields of cancer, autoimmune and infectious diseases,disruption of the blood-brain barrier in epilepsy, aging, and TBI.108While all TGF-b isoforms are known to be secreted by white blood cells, Takahashi et al.107showed that after endurance training, adipose of mice secreted TGF-b2 in response to lactate signaling.In turn,TGF-b2 improved glucose tolerance in mice, leading the authors to conclude that exercise training improves systemic metabolism through an inter-organ (adipose to liver) communication via a“lactate-TGF-b2 signaling cycle”.
On first impression,one might think that the effects of lactate on HCAR-1 inhibiting lipolysis and mitochondrial lactate oxidation(Fig.6)96are contradictory with increasing glucose tolerance via TGF-b2.107However,a reasonable alternative explanation is that the effects of lactate on HCAR-1 in adipose are acute (as occurs during hard exercise) and that the effects of TGF-b2 are long term (as occurs during recovery from exercise) when glucose tolerance and lipid oxidation are improved in men and women.109While the purported effects of lactate signaling HCAR-1 and TGF-b2 observed in rodent models await validation in humans,for the present it is certain that lactate both inhibits lipolysis and mitochondrial FFA oxidation and stimulates mitochondrial biogenesis and glucose tolerance and lipid oxidation in humans in vivo.
Beyond a role in inhibiting lipolysis, lactate also plays an important role in limiting inflammation following injury.Consequently,lactate-containing solutions are being evaluated as antiinflammatory resuscitation fluids for use in a variety of other therapies, including acute pancreatitis,104,110hepatitis,104and dengue fever.21Compared to normal saline, lactate-containing resuscitation solutions offer the advantage of providing calories in addition to fluid and electrolytes. However, it was not until Hoque and colleagues104found that lactate binding to HCAR-1 negatively regulates toll-like receptor induction of the pyrindomain-containing protein 3 inflammasome and production of Interleukin (IL)-1b, via Arrestin b 2. It was then appreciated that lactate and HCAR-1 were involved in suppression of inflammation in patients with acute organ injury. For the treatment of brain swelling and elevated intracranial pressure following TBI,hypertonic sodium-L-lactate,a hypertonic solution,can help manage intracranial pressure by its osmotic effects.111-113
4. The dark side of lactate metabolism—when lactate production and accumulation may or may not be efficacious
Some table salt improves the taste of many foods, but too much salt can ruin a soup or entr-ee.Similarly,insulin and insulin action are keys to metabolic flexibility and health. However, too much exogenous insulin can be lethal. So it is with lactate accumulation,metabolism,and supplementation.
4.1. Glucose-lactate interactions and cancer metabolism
Warburg and Minami114first described the metabolic phenotype characteristic of cancer cells. They noted high glucose uptake and excessive lactate formation in cancer cells even under fully oxygenated conditions. This discovery was subsequently named the Warburg Effect.115While the high glucose uptake/lactate release phenotype remains a hallmark of cancer,116,117today there is no consensus on the meaning of the Warburg Effect.The excessive lactate formation of cancer cells and tumors led Warburg to propose that cancer was an injury to the cellular respiratory apparatus. More recently,however, we28and others118observed that cancer cell mitochondria are capable of respiring with lactate. In a recent review,San-Mill-an and Brooks119described many similarities between cancer and healthy exercise phenotypes. Consequently,we proposed that augmented lactate production(lactagenesis)initiated by gene mutations is the reason and purpose of the Warburg Effect and that dysregulated lactate metabolism and signaling are key elements in carcinogenesis.119Specifically, we identified the following steps by which lactagenesis may support carcinogenesis: angiogenesis, immune escape, cell migration, metastasis, and self-sufficient metabolism. Justification for our hypothesis that dysregulated lactate metabolism and signaling are key elements in carcinogenesis are presented separately.23,119In this regard, it is to be noted that we are not the only exercise physiologists and biochemists to have commented on the meaning of exaggerated lactate shuttling in cancer.120
4.2. Blocking lactate shuttles in cancer
Since Sonveaux et al.85in the Feron lab identified lactate shuttling in tumors,and as commented on by Semenza,121there have been serious attempts to repress tumorigenesis by blocking the release of lactate from glucose-consuming and highly glycolytic cells and cells respiring lactate. That cancer cells respire with lactate drawn from the tumor microenvironment is an important realization in itself.28,118However,oxidative lactate disposal within tumors also sets up the concentration gradient necessary for lactate shuttling.Following the lead of Sonveaux et al.,85the search is on to develop MCT1 and MCT4 inhibitors.85,122-125However, the lack of MCT specificity has been a problem,even for investigators in big pharma.126Because a quest to find cancer-specific MCT blockers has as yet been unsuccessful,others are looking for alternative approaches to blocking lactate shuttling in tumors and cancer,such as by limiting the expression of CD147,the scaffold for MCT insertion into cell membranes(vide supra).127-130
4.3. Exercise,lactatemia,and carcinogenesis
Endurance training and cancer phenotypes have a lot in common, including the presence of high glycolytic rates and lactate production and accumulation.119Accordingly, it is of concern that elevated circulating lactate levels resulting from high-intensity interval exercise training can provoke cancer-prone cells to transform. Fortunately, epidemiologic studies support the idea that regular physical activity reduces the risk of many common cancers, including cancer of the breast, colon, bladder, uterus, esophagus, kidney,lung, and stomach.131It is noteworthy that the organs of cancer have apparently little to do with regard to exercise itself, suggesting the systemic circulation of a protective cytokine, myokine, adipokine, or metabolite during exercise.Given this observation, a suggestion is that intermittent lactate release and circulation of lactate during physical activity improves lactate clearance and preconditions cells,tissues, and organs for reducing the chance that lactagenesis promotes carcinogenesis.119
4.4. Lactate insulin and hypoglycemia
Lactate-glucose interactions can be complex,and interference with lactate shuttling by glucose-insulin signaling can be disruptive. As recognized in the Lactate Shuttle6,43and Cori Cycle,26glucose and glycogen are the precursors to lactate formation,7,132and lactate is the major gluconeogenic precursor.13,26,36,133,134However, whereas the blood glucose level provides important feedback in the regulation of insulin and counter-regulatory hormones, lactate normally plays only a small role in the regulation of insulin secretion,and is, by nature’s design, excluded from the regulatory processes.
MCTs are bidirectional symporters facilitating movement of protons and lactate anions down concentration gradients.While MCTs are ubiquitous and scaffolded in plasma membranes of most cells, including erythrocytes cells in the heart,muscle, and brain,8,135,136via small interfering RNAs MCTs are excluded from insertion into pancreatic b-cell plasma membranes.137,138In pancreatic b-cells, MCT expression is silenced to keep extracellular lactate from affecting intracellular redox and thereby interfering with glucose sensing and insulin secretion.139The silencing of MCT1 in pancreatic b-cells is evolutionary proof of how lactate overrides glucose in regulating energy substrate partitioning in general and insulin secretion in particular when the dominant role of lactate must be suppressed.In this regard,it is noteworthy that,in persons with failed suppression of pancreatic b-cells, MCT expression becomes hypoglycemic during hard exercise. If MCT1 is not silenced, lactate will gain entry into pancreatic b-cells and affect cell redox,just as if blood glucose were elevated. Consequently, individuals in whom pancreatic b-cell MCT expression is not silenced during exercise experience lactatemia and become hypoglycemic due to hyperinsulinemia and increased glucose disposal through metabolism.140
4.5. The“Anaerobic Threshold”
The exercise intensity at which a rise in arterial blood lactate concentration commences is the“Anaerobic Threshold”(AT).At one time, the AT was reasonably associated with a limitation in working muscle oxygen delivery, which resulted in a Pasteur effect involving augmented glycolysis due to oxygen limitation.25,141The idea was straightforward and at the time was based on the widely accepted O2Debt theory.24However, because the AT concept assumed that lactatemia during exercise was due to increased lactate production rather than an imbalance between lactate production(appearance in the blood,Ra)and lactate disposal(disappearance from the blood,Rd),9,142the AT concept has been severely challenged.43,143Importantly, and regrettably, the AT concept failed because of the recognition that muscle produced and released lactate9,38,45,142under fully aerobic conditions.144Still, in the hands of knowledgeable and skilled clinicians, AT testing can be part of an armamentarium in dissecting changes in blood lactate Ra due to chronic obstructive pulmonary disease,pulmonary hypertension, cardiovascular disease, mitochondrial defects, catecholamine or other endocrine effects,145pharmacologic toxicity,146altered carbohydrate nutrition,147environmental effects,148or other effects.
4.6. Lactate-brain fuel after TBI
In comatose TBI patients,the role of lactate in brain fueling was found to be impressive because most (70%-80%) of circulating blood glucose was produced via gluconeogenesis from lactate.11,12Also, net lactate uptake provided 12% of brain fuel. Hence, most (57%) brain fuel was from lactate,either directly(12%)or indirectly via gluconeogenesis(45%).
In healthy humans, plasma lactate levels during exercise can increase by an order of magnitude or more. For example, with a 10-fold rise in concentration, net lactate uptake provides 25% of total brain energy needed.149,150Disregarding for a moment that cerebral disposal of glucose is accomplished by conversion to lactate,preference of cerebral lactate over glucose has been found in studies on rats151and humans,150,152and the provision of lactate to a healthy brain decreases glucose net uptake.This observation indicates an important cerebral lactate shuttling phenomenon.Alternatively, some might consider that lactate can serve as an alternative brain fuel.However,as emphasized by Schurr,lactate is the brain fuel because,regardless of whether extracellular glucose or lactate is taken up by brain,the path of glucose disposal is through conversion to lactate and a Cell-Cell Lactate Shuttle.153,154
4.7. Lactate and brain-derived neurotrophic factor
In addition to serving as a cerebral energy substrate, circulating lactate can signal secretion of cerebral brain-derived neurotrophic factor(BDNF).As discussed in part elsewhere in this work, circulating blood lactate can be raised during exercise by production in muscle, the integument and other epinephrine sensitive tissues. Also, circulating lactate level can be raised during rest or exercise by vascular L-lactate infusion into the systemic circulation. Indeed, infused sodium lactate raises circulating BDNF levels.155BDNF is a member of the neurotrophic family of proteins and facilitates neurogenesis,neuroprotection, neuroregeneration and synaptic plasticity, as well as formation, retention, and memory recall.156BDNF is produced both in the central nervous system and in other tissues, including the vascular endothelium. High levels of BDNF messenger RNA are found in the hippocampus and in the cerebral cortex, and in rodent models physical exercise is the strongest known stimulus to BDNF expression in the hippocampus.157In contrast,attenuated expression of BDNF messenger RNA in the hippocampus may constitute a pathogenic factor common to Alzheimer’s disease and major depression.158Circulating BDNF is typically elevated in exercise,but levels are reduced in patients with depression and type 2 diabetes.159Even though acute exercise increases BDNF production in the hippocampus and cerebral cortex, studying the effects of exercise on BDNF expression in the human brain is difficult.In what may become noted as a classic study in neurochemistry,Seifert et al.156compared BDNF levels in arterial and internal jugular vein blood and demonstrated the effects of exercise and exercise training on cerebral BDNF net release.
While many posit a positive role for BDNF in terms of cognition, it is important to know also that lactate therapy has been shown to raise BDNF levels and improve cognitive function in rodent models following brain injury.160-162 Most recently,in an extraordinary set of experiments on healthy men who volunteered to exercise at high intensities with arterial and jugular bulb catheters in place,Hashimoto et al.163showed that executive function was directly correlated to blood (lactate) and cerebral lactate uptake. Wang et al.164also recently provided data showing that optogenetic activation of astrocytes in the anterior cingulate cortex triggers lactate release and improves decision making in rats with chronic visceral pain.As predicted in papers resulting from studies on rodents164,165and healthy and injured humans,11Hashimoto et al.163found that brain fueling with lactate improved cerebral functioning.Additionally,with respect to cognition,it has been shown that long-term memory consolidation in rat hippocampus relies on lactate and the Astrocyte-Neuron Lactate Shuttle(ANLS).166,167
5. A role for lactate supplementation in sepsis
Although we have discussed numerous instances in which it may be clinically beneficial to achieve elevated blood lactate concentrations,it is noteworthy to mention that a blood lactate value of 4 mmol/L is a biomarker of severe sepsis.168,169Interestingly, the lactate threshold in exercise frequently corresponds with 4 mmol/L blood lactate.170What then is the significance of a slightly elevated blood lactate concentration(e.g., >2.0 mmol/L) in a febrile patient?171Concern over the prospect of a blooming bacterial,viral,or fungal attack leading to sepsis needs to be appreciated from the context of the huge lactate clearance capacity in a healthy person.172Also,lactatemia is unlikely to be due to oxygen insufficiency (hypoxia)in either exercise23,143or sepsis.20,173,174Alternatively, is the body readying for some challenge by raising the level of a key myocardial51and whole body energy substrate,7a gluconeogenic precursor13,36,44or an anti-inflammatory moiety?104,110In realizing the importance of lactate in supporting metabolism, some have proposed that lactated Ringers solution be used as a resuscitation fluid in sepsis,174and, by extension of the same thinking, that the use of isotonic or hypertonic sodium-L-lactate and SanguisalTM(a physiologically balanced mixture of Na+-, K+-, Mg2+-, and Ca2+-lactate plus phosphate buffer)also needs to be evaluated for resuscitation in sepsis.
6. Conclusion
Studies on humans and mammals such as rats and dogs during rest and exercise led to discovery of the Lactate Shuttle,a mechanism by which lactate production in rapidly glycolyzing driver cells provides energy substrate for recipient cells where lactate is a fuel energy source,gluconeogenic precursor,and signaling molecule.In heart and red skeletal muscle,lactate disposal is by mitochondrial respiration. In the liver and kidneys, mitochondria play roles in oxidizing lactate to pyruvate and then in the conversion to oxaloacetate and phosphoenolpyruvate via pyruvate carboxylase and phosphoenolpyruvate carboxykinase.In the liver and kidneys,the oxidation of lactate and other fuel sources also provides energy for completion of gluconeogenesis and glycogen synthesis. By changes in cell redox owing to the production and oxidation of lactate,as well as allosteric binding to HCAR-1 and TGF-b signaling,lactate affects numerous processes. Discoveries of intracellular,cell-cell,and organ-organ lactate exchanges has led to articulation of numerous lactate shuttles,including peroxisomal and astrocyte-neuron lactate shuttles, the basis of which are interactive effects between driver producer and recipient consumer cells.
In terms of the organization of energy substrate partitioning in humans and other mammals,lactate is at the fulcrum of intermediary metabolism. Lactate is the inevitable product of glycolysis.With rapid glycolysis, as occurs in hard muscle exercise, lactate limits lipid metabolism in 2 ways. First, lactate released into the blood reaches white adipose tissue, and by binding to HCAR-1,lactate acts to inhibit lipolysis via a cyclic adenosine monophosphate-dependent pathway(CREB),which limits subsequent release of fatty acids into blood.Second,lactate produces an abundance of mitochondrial acetyl-CoA that gives rise to malonyl-CoA, which in turn inhibits carnitine palmitoyl transporter-1 (CPT-1) and blocks mitochondrial FFA uptake.
Recognizing that lactate is produced under full aerobic conditions, as well as understanding the roles of lactate shuttling in biology,is providing new opportunities to treat the ill and injured.For example,exogenous L-lactate vascular infusion is being evaluated in the treatment of heart failure,175-177 TBI,19,113,178,179pancreatitis,104,110hepatitis,104dengue fever,21and sepsis.174
Recognizing that lactate,particularly rising blood lactate concentration,is a biomarker for an imbalance between lactate production and removal provides practitioners in diverse fields with important information on physiologic status of athletes and the ill and injured.The applications for this information include providing fluid,energy and electrolyte support to athletes and others,180and apply to fields as diverse as pulmonary medicine,141sports medicine,181,182critical care medicine,173and oncology.85Indeed,the recognition that lactate shuttles among driver producer and recipient consumer cells in tumors offers the exciting possibility of reducing carcinogenesis and tumor size by blocking either or both producer and recipient arms of lactate shuttles within and among tumor cells.With growing appreciation of the importance and extent of lactate shuttling in vivo,it is to be anticipated that by controlling blood(lactate)via a closed loop,a continuous monitoring system can improve care of the ill,injured,and malnourished.183-185
In closing,profound thanks are offered to those students,postdoctoral fellows,visiting scientists and University of California at Berkeley colleagues who participated in the discovery of lactate shuttling; those contributors are identified in the references, typically as first authors. Also, it is important to fully recognize the many contributions of the American College of Sports Medicine(ACSM) and to recognize that our contributions, as described in this paper,occurred within the context of a greater whole.It is risky to limit the examples of how ACSM colleagues have advanced science and medicine, since other important contributions are easily and unintentionally omitted.Nevertheless,contributions of the following individuals to physiology, and sports medicine should not be overlooked: Jere Mitchell, in cardiovascular physiology and medicine;186John Holloszy, in mitochondrial biogenesis;187L.Bruce Gladden,in the regulation of intermediary metabolism;18,188Jim Barnard, V. Reggie Edgerton, and Ken Baldwin, in muscle fiber heterogeneity;47,48Scott Powers,in redox signaling and preconditioning of the heart to cardiovascular insults;189Karl Wasserman and Jerry Dempsey, in pulmonary physiology and medicine;25,190Tom Fahey,in translating the lessons of physiology and sports medicine to the literature for health care professionals and the lay public;191-193 and Tim White,in the administration of higher education (https://www2.calstate.edu/csu-system/chancel lor/Pages/meet-the-chancellor.aspx). In the aggregate, our scientists, clinicians, and staff at the ACSM know well how the body works under stress, in illness, and after injury. Thus, collectively we can think of and accomplish things that few others could imagine. One such example is an idea from exercise physiology that has wide applicability to science and medicine—the Lactate Shuttle—in which the product of one process, glycolysis, is the substrate for another process, oxidative metabolism. The linkage between the two is lactate,the metabolite that can transcend cellular, tissue and organ compartment barriers to be a preferred fuel energy source,the major gluconeogenic precursor and a powerful signaling molecule.
Acknowledgments
This review is due to the combined efforts of all students,fellows, visiting scientists, colleagues, and human subjects associated with GAB. Appreciation is also expressed to the officers and staff of the American College of Sports Medicine(ACSM) for the privilege of delivering the 2019 J.B. Wolffe Memorial Lecture. Supported by NIH R01 AG059715-01 and Pac-12 Conference CAL-Brooks-17-02 and the University of California at Berkeley’s Center for Research and Education on Aging(CREA).
Competing interests
The author declares that he has no competing interests.
杂志排行
Journal of Sport and Health Science的其它文章
- The role of mitochondria in redox signaling of muscle homeostasis
- Physical exercise in the prevention and treatment of Alzheimer’s disease
- The roles of microRNA in redox metabolism and exercise-mediated adaptation
- Exercise-induced oxidative stress:Friend or foe?
- Epidemic-specific social capital and its impact on physical activity and health status
- Team sport,power,and combat athletes are at high genetic risk for coronavirus disease-2019 severity
