Response of Heterotrophic Bacteria Abundance and Community Structure to Asian Dust Addition in the Oligotrophic Northwest Pacific Ocean
2020-09-28CHENXiZHANGXiaohaoZHAOYangguoLIUGuangxingZHANGChaoandGAOHuiwang
CHEN Xi, ZHANG Xiaohao, 2), ZHAO Yangguo, 3), *, LIU Guangxing, 3), *, ZHANG Chao, 3), and GAO Huiwang, 3)
Response of Heterotrophic Bacteria Abundance and Community Structure to Asian Dust Addition in the Oligotrophic Northwest Pacific Ocean
CHEN Xi1), ZHANG Xiaohao1), 2), ZHAO Yangguo1), 3), *, LIU Guangxing1), 3), *, ZHANG Chao1), 3), and GAO Huiwang1), 3)
1)College of Environmental Science and Engineering, Ocean University of China, Qingdao 266100, China 2) Beijing Sound Environmental Engineering Co. Ltd., Beijing 101102, China 3) Key Lab of Marine Environmental Science and Ecology, Ministry of Education, Ocean University of China,Qingdao 266100, China
The dust storms from the continent usually affect the abundance and diversity of planktons by supplying trace elements. As such, the response of heterotrophic planktonic bacteria to dusts, nutrients (.., nitrogen and phosphorus) or ferrous dosages was investigated in the Kuroshio Extension region of the Northwest Pacific Ocean (NWPO) through on-board incubation experiments during an oceanographic survey in spring 2014. The flow cytometry and 16S rRNA high-throughput sequencing methods were applied to explore the abundance and community structure of bacteria, and the percentage of high nucleic acid bacteria (HNA%). The results showed that the heterotrophic bacteria abundance was low (average 2.55×105cellsmL−1) and subjected to both nitrogen (N) and ferrous (Fe) limitation. Sand-dust deposition observably promoted the activity of heterotrophic planktonic bacteria. The maximum abundance of heterotrophic bacteria was 6.98×105cellsmL−1in the dust-dosage group, which was 44% higher than the control (<0.05). The HNA% in the dust-dosage group was 1.37 times higher than the control (<0.05). The activation mechanism was mainly related to the dissolution of N and Fe in the dusts. The relative abundance of genuswas significantly increased by dust deposition while the relative abundance of the generaandwas decreased. These variations of bacterial community structure were ascribed to the dissolution of nutrients N and P. Comparing the results of different experimental groups, this study concluded that dust storm improved the abundance of heterotrophic bacteria by dissolution of N and Fe.
Northwest Pacific; Asian dust; heterotrophic bacteria; 16S rRNA high-throughput sequencing; bacteria abundance and community structure
1 Introduction
Marine heterotrophic planktonic bacteria are the main decomposers of organic matter in marine ecosystems. They play a key role in the cycle of biogenic elements. The bacterial abundance and community structure determine their ecological functions, and the changes of environmental conditions, sand-dust deposition for example, will significantly affect their biological activities, commu- nity composition, and ecological functions (Zubkov,2001).
Sand-dust deposition is the main form of atmospheric dry deposition, which releases inorganic nutrients into the sea after long-distance transport and deposition. During the process of long-distance transport of sand-dust, dif- ferent types of pollutants will be adsorbed to the surface of sand-dust. These pollutants will release nutrients and heavy metals after the sand-dust settles down into the sea (Gao, 2009). Atmospheric deposition affects every link of the microbial food loop in the ocean (Pulido-Villena, 2014) and thus impacts the activity and community structure of heterotrophic bacteria (Gasol and Moran, 1999; Zubkov, 2001; Jochem, 2004). In spring, Asian dust is transported to the Northwest Pacific Ocean and holds a relatively large deposition flux. The northwest Pacific Ocean is located in the subtropical zone and is a typical oligotrophic area (Gao, 2009). In this area, the chlorophyll and primary productivity are low and the number of heterotrophic planktonic bacteria is small, which is more susceptible to environmental changes. Therefore, it is of great significance to reveal the effects of Asian dust on the activity and community structure of heterotrophic bacteria in the poor nutrient area of the Northwest Pacific Ocean.
In most aquatic environments, heterotrophic planktonic bacteria were divided into high nucleic acid (HNA) and low nucleic acid (LNA) bacteria (Gasol and Moran, 1999; Zubkov, 2001; Andrade, 2003; Jochem, 2004) according to their nucleic acid content. Previous research (Gasol, 1999; Lebaron, 2001; Leba- ron, 2002) showed that the activity of heterotrophic planktonic bacteria was positively correlated with the content of nucleic acid, LNA revealed that the bacteria with slow metabolism or near death, while HNA indicated that the bacteria with strong metabolism. Thus, the percentage of high nucleic acid bacteria (HNA%) could approximately represent the activity of bacterial community.
In this study, the effects of Asian dust on the abundance, HNA%, community composition and diversity of heterotrophic planktonic bacteria were investigated through ship-based field experiments at the oligotrophic area of the Northwest Pacific Ocean. The mechanism of sand- dust deposition impacting bacteria was analyzed by dosing N, P and Fe. This study could be helpful to further understand the mechanism and ecological effects of Asian dust deposition on ecosystems in the Northwest Pacific Ocean.
2 Materials and Methods
2.1 Establishment of Culture System
In March 2014, the nutrient supplying culture experiments were carried out at the Ar04 station (29.47˚N, 142.54˚E) of the Northwest Pacific Ocean on ‘.., control group, dust, N (NaNO3), P (NaH2PO4), NP (NaNO3+NaH2PO4) and NPFe (NaNO3+NaH2PO4+ FeSO4). All experiments were conducted in triplicates. The concentrations of dosing dust and nutrients are shown in Table 1.

Fig.1 Location of station Ar04 in the Northwest Pacific.
The seawater for ship-based culture was collected from sea surface layer (2–5m) and immediately filtered with 2 µm filter membrane to remove the zooplankton and microalgae. The filtered seawater was put into 2-liter sterile polycarbonate Nalgene polycarbonate bottle for culturing, then dust and nutrients were dosed according to the experimental design. The culture bottles were put into the culture box on the deck of the ship. The culture temperature was controlled by recycling the seawater into the culture box. The culture lasted for 7d.
The dust used in this study was the surface sand collected in May 2011 in Hunshandake Sandy Land, Inner Mongolia (42˚22´28´´N, 112˚58´34´´E). Samples were collected and frozen at −20℃. The sand samples were sieved to less than 20µm particle size to remove the large sand particle before artificial aging treatment (Guieu, 2010). The contents of nutrients and heavy metals were analyzed by ICS-1100 ion chromatography (Agilent, USA) according to previously described (Li, 2017). The results are shown in Table 2.

Table 1 Dust and nutrient addition concentrations in the experimental groups
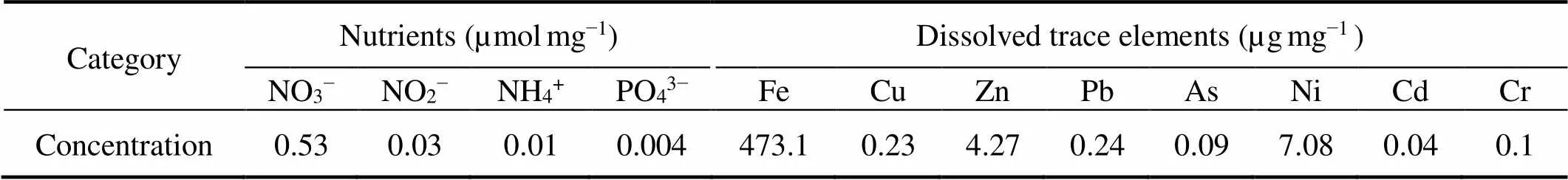
Table 2 Concentrations of nutrients and dissolved trace elements in dust particles
2.2 Determination of Nutrients in Seawater
Surface seawater samples from culture bottles were collected and filtered by 0.45µm acetic acid fiber membrane and put into acid-treated plastic bottles. NH4+-N was determined according to the indigo blue method, NO3−- N using a copper-cadmium reduction method, NO2−-N using naphthalene ethylenediamine hydrochloride method, PO43−using phosphomolybdenum blue method, with Qu- AAtro nutrient analyzer (Brown-Ruby, Germany).
2.3 Determination of Abundance and HNA% of Heterotrophic Bacteria
During the incubation, 10 mL of seawater in the culture bottle was taken and put into the sterile freezing tube every day. The polyformaldehyde with a final concentration of 0.5% (w/v) was added and mixture then stored at −80℃. The abundance of heterotrophic bacteria, HNA% and LNA% were determined by flow cytometry (BD, USA). For flow cytometric analyses, 1mL samples were incubated with 0.05mL of SYBR I (Molecular Probes Inc.), for 15min at room temperature in the dark. Counts were obtained with a flow cytometer equipped with an air- cooled argon laser (488nm, 15mW). Bacteria were discriminated and enumerated by using right angle light scatter (SSC) and green fluorescence measured at 530nm. A four-log decade was used for all cytograms. In a plot of green fluorescence (FL1) versus red fluorescence (FL3) we were able to distinguish photosynthetic prokaryotes from nonphotosynthetic prokaryotes. The group of HNA cells was discriminated from the group of LNA cells. Each subgroup was delimited on the SSC-versus-FL1 plot then cell abundance was determined for each subgroup.
2.4 Total DNA Extraction
The cultured seawater was filtered by 0.22µm polycarbonate membrane. The total DNA was extracted from the membranes by using DNA extraction kit (Mobio, USA) and frozen at −80℃ until use.
2.5 16S rRNA High-Throughput Sequencing
The V3-V4 variable regions of 16S rRNA were amplified by using universal primers. The polymerase chain reaction products were sequenced on Illumina MiSeq PE300 platform. The paired reads obtained by sequencing are spliced into a long sequence by using overlapping through PANDAseq software (Masella, 2012), and finally the long reads with high variable region are obtained. After quality control for the original data, the sequences with 97% similarity were clustered into one operational taxonomic unit (OTU) by UPARSE software (http://drive5.com/uparse/) (Edgar, 2013). Using Qlime software, the dilution curve of Alpha diversity index (Kemp and Aller, 2004) was obtained. According to the curve, reasonable flattening parameters were determined and random flattening was carried out. After flattening, the most abundant reads were selected from OTUs as the representative sequence. Ribosomal database project search tool (http://rdp.cme.msu.edu/) was used to compare the sequences with the 16S rRNA gene database. Finally, each OTU was classified into a known species (Wang, 2007; Cole, 2013).
2.6 Data Statistics
DPS software was used to analyze the experimental data by one-way ANOVA and the significant differences among the groups were tested.
3 Results and Discussion
3.1 Environmental Conditions of Station Ar04
Station Ar04 is located in the middle of Izu Ogasawara Trench in the Northwest Pacific Ocean, it belongs to the Kuroshio Extension Area in the Northwest Pacific Ocean. Due to the Kuroshio Current originating from the northern equator, the surface water temperature and salinity are relatively high. The environmental factors of station Ar04 are shown in Table 3. The surface water temperature (T), salinity (S), pH, dissolved oxygen (DO) and dissolved organic carbon (DOC) of station Ar04 are high. The concentration of dissolved inorganic nitrogen (DIN) is 0.26 μmolL−1, PO43−is 0.05μmolL−1, N/P is 5 (<16), chlorophyll is 0.24μgL−1. Both Nutrient and chlorophyllare at a low level, indicating that this station is in a state of poor nutrition and the growth of phytoplankton and bacteria is prone to be limited by nitrogen. The heterotrophic bacteria abundance (HBA) at station Ar04 is 4.2×105cellsmL−1.

Table 3 Environmental factors and heterotrophic bacterial abundance at Ar04 station
3.2 Effect of Dust Dosage on Abundance of Heterotrophic Bacteria
The variation of heterotrophic bacterial abundance in dust and different nutrients supplementation culture experiments is shown in Fig.2.
As shown in this figure, the abundance of heterotrophic bacteria increased rapidly in the dust-adding group and reached a maximum of 6.98×105cellsmL−1on the 5th day. It was 44% higher than the control group in the same period (<0.05). Similarly, Lekunberri (2014) added different amounts of dusts and nutrients in different culture systems and they found that a small amount of dust dosage increased the bacterial abundance and productivity by 1.8 times and 5.1 times, respectively. Research indicated that the nutrients such as N and P released from dusts promoted the growth of phytoplankton and they continuously provided the dissolved organic matter (DOM) for bacterial growth, which finally improved the activity and amount of bacterial populations (Falkowski, 1992). In the later period of culture, the decrease of bacterial quantity may be related to the consumption of DOM and nutrients in the culture system and the accumulation of harmful substances such as heavy metals from dust (Gao, 2009).
The variation of heterotrophic bacterial abundance in N group presented the same trend as that of dust addition group. The maximum value appeared on the 6th day with 6.42×105cellsmL−1, higher than that of C group (5.67 × 105cellsmL−1) (<0.05). This indicated that the growth of heterotrophic bacteria in this station was limited by N concentration. However, compared with the dust group, the heterotrophic bacterial abundance in N group was significantly lower at the later stage of culture (<0.05). It is speculated that the release of nutrients in dust is a continuous process, which can continuously supplement nutrients for planktons (Herut, 2005).
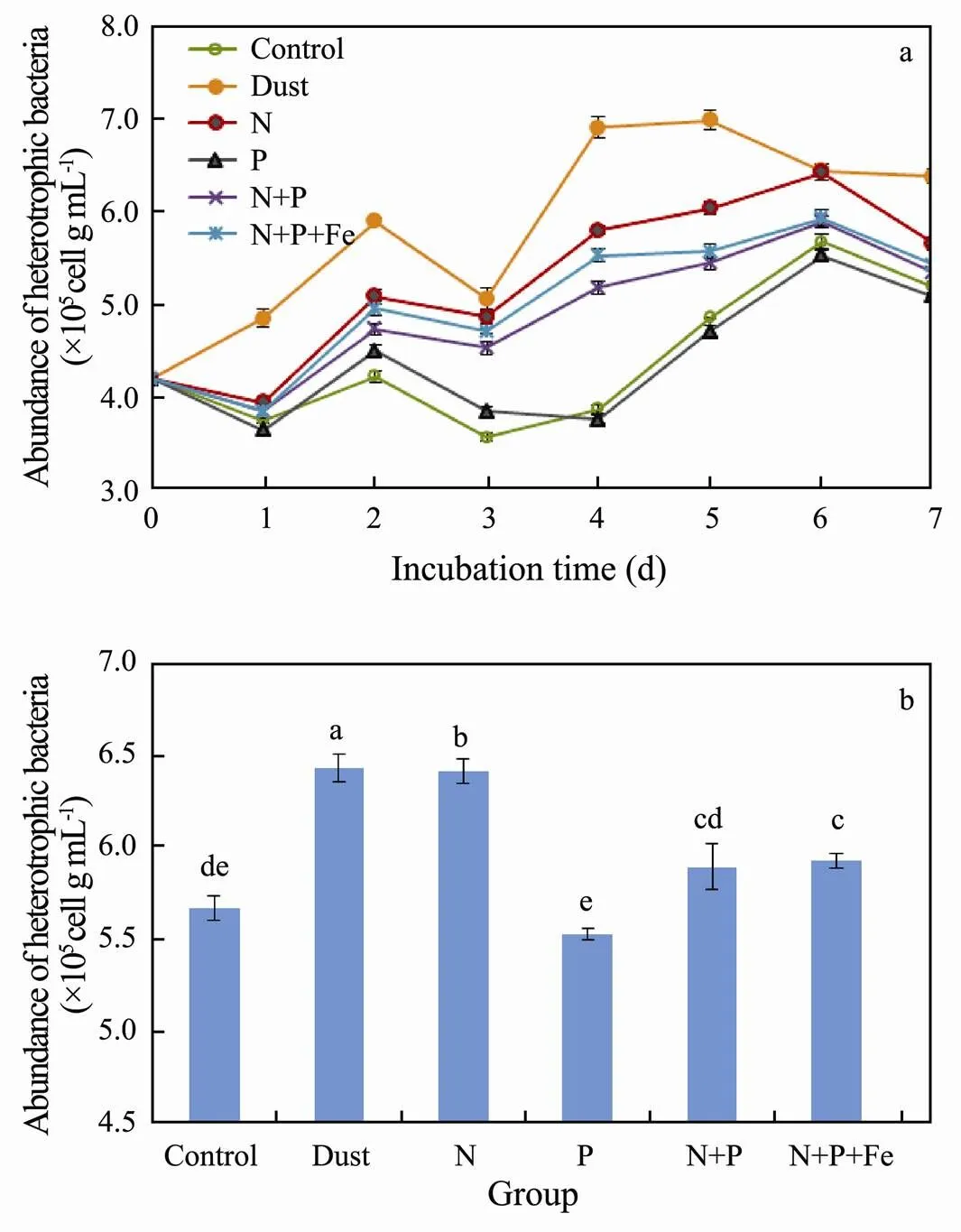
Fig.2 Variation of bacterial abundance (a) and one-way ANOVA among dust and different nutritional treatments on 6th day (b).
The abundance of heterotrophic bacteria in group P was lower than that in control group at the later stage of culture, but there was no significant difference (>0.05). This indicated that phosphorus dosage had no obvious promoting effect on the growth of bacteria in this station. The addition of phosphorus might even aggravate the nitrogen limitation in the later stage of culture and thus inhibit the growth of heterotrophic bacteria (Li, 2017; Zhang, 2018). This result also indicated that this sea area was not restricted by phosphorus in March.
The variation of heterotrophic bacterial abundance in NP group presented the same trend as that in N group. The abundance was significantly higher than that in control group, but significantly lower than that in N group. This further indicated that the addition of phosphorus dosage would enhance the nitrogen limitation.
The heterotrophic bacterial abundance in NPFe group indicated the same trend as that in NP group. However, it was significantly higher than that in NP group during the whole culture process (<0.05), indicating that the addition of Fe contributed to the growth of heterotrophic bacteria. Fe acts as a catalyst in the reduction of NO3−to NH4+or N2and thus Fe limitation is often observed in the open ocean (DiTullio, 1993). In the long-distance atmosphere transportation of dusts, Fe in the dusts usually appears in soluble Fe(II) form due to the photochemical reaction (Paerl, 1997). After depositing into the sea, Fe(II) can be rapidly utilized by phytoplankton and bacteria (Martin, 1994). By this process, it promotes bacteria to absorb nutrients such as DOM and nitrogen source and finally enhances the bacterial productivity in the ocean.
Calculation showed that the complete release of total nitrogen in dust addition group approached to 1.14μmolL−1, less than that of 2μmolL−1in other experimental groups. The maximum release of Fe in dust addition group in culture system reached 8.45nmolL−1, far higher than that of 2nmolL−1in NPFe group and 0.07±0.04 nmolL−1in oceanic surface seawater (Johnson, 1997). The bacterial abundance of dust group was higher than that of other experimental groups over the culturing time. The most possible reason might be that the large amount of Fe release from dusts promoted the absorption efficiency for N and P nutrients of planktons and they thus provide more DOM for heterotrophic bacteria growth. Therefore, dust deposition changed the abundance of heterotrophic planktonic bacteria and enhanced the bacterial productivity through the combined action of N and Fe release in the Northwest Pacific poor nutrient ocean in spring.
3.3 Effect of Dust on Activity of Heterotrophic Bacteria
The change and statistical analysis of bacterial HNA% in dust and nutrient supplementation culture experiments are shown in Fig.3. Different treatments present the same variable trend as the control except a little higher in percentage. The background value of HNA% of bacteria in station Ar04 was 53.33%. After one day of cultivation, HNA% of bacteria increased, and then decreases to the lowest markedly from the second day to the third day. Afterwards, it gradually recovered and increased. The decrease of HNA% in the early stage of culture was related to the gradual consumption of nutrients in the culture system, which led to the decrease of bacterial metabolic ability and activity. However, the increase in the later stage of culture might be ascribed to the continuously release of large quantities of DOM and nutrients by the deceased picoplankton (<2µm). The DOM and nutrients further enhanced the bacterial activity (Li, 2017). As shown in the figure, the significant difference of HNA% in each group was also observed. Except phosphorus dosing group (>0.05), the HNA% of dust, N, NP and NPFe groups was significantly higher than that of the control (<0.05). Especially, HNA% increased significantly in dust and NPFe group, which were 1.37 times and 1.33 times of the control, respectively. The N and NP dosage groups were followed with 1.27 times and 1.18 times of the control.
Interestingly, the HNA% of the control began to decrease on the 5th day of culture, and the same trend was also observed in the N and P supplementation groups. Compared with that, the HNA% of the dust and the NPFe groups kept rising to the end of culture. The HNA% of the Fe supplementation group was always higher than that of the dust group from the 5th day. The above results suggested that the dust deposition could significantly promote the activity of heterotrophic bacteria. N and the combined dosage of Fe held the same effect as dust, in which Fe might play a stronger role, followed by N alone, but P had no significant effect on bacterial activity. It concluded that dust deposition enhanced the activity of heterotrophic planktonic bacteria in the study area by dissolving out N and Fe, especially Fe but not P.

Fig.3 Variation of HNA% (a) and one-way ANOVA among dust and different nutritional treatments of 6th day (b).
3.4 Effects of Dust on Diversity of Heterotrophic Bacterial Community
The difference of heterotrophic planktonic bacterial community diversity (Shannon index) between each experimental group was analyzed by using 16S rRNA high-throughput sequencing method and the result is shown in Fig.4. At the end of culture, the diversity of bacterial communities in the N, P and NP supplementation groups was significantly lower than that in the control group (3.47), indicating that the addition of nutrients promoted the growth of some functional bacteria and increased their dominance thus decreased community diversity. This effect was more obvious for NP combined addition. The Shannon index of bacterial community diversity in NPFe group was 3.74, which was significantly higher than that of other groups, indicating that Fe could promote the growth of various heterotrophic planktonic bacteria, especially for non-dominant species. The Shannon index of the bacterial community in the dust addition group was 3.04, which was lower than the other groups except the NP combined addition group. This indicated that the dust promoted the growth of some bacteria and inhibited other bacteria growth at the same time, it might related to the dissolution of nutrients, Fe, and, Fe was able to improve the diversity of heterotrophic planktonic bacteria community, while nutrients and toxic heavy metals (Cu, Zu, Pb, Cd, Cr) decreased the diversity.
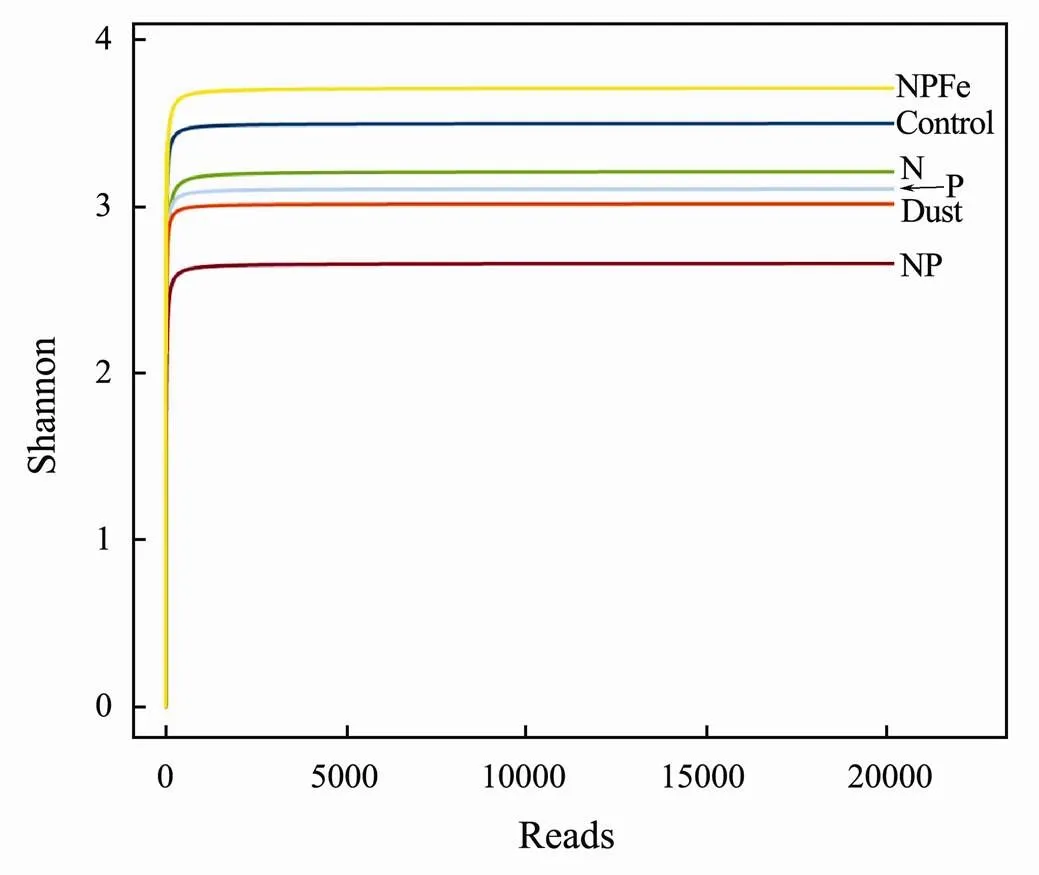
Fig.4 Shannon indexes of bacteria communities of dust and different nutritional treatments.
3.5 Effects of Dust on Composition and Structure of Heterotrophic Bacteria Community
The generic distribution of bacterial communities in different treatment groups is shown in Table 4.
The dominant bacterial genera in the control group are,,andwith relative abundance of 26.34%, 21.45% and 19.47%. Com- pared with that, the relative abundance ofincreased significantly to 31.45% in the dust- adding group, whileanddeceased to 11.63% and 4.95%.
The station Ar04 in this study is located in the Northwest Pacific Ocean with a low nutritional level. The relative abundance ofandis relatively high. They both belong toand contained proteorhodopsin gene, a series excellent genes of light-sensing and light-responding, which can provide considerable energy for their growth. Thus, this make them competitive in nutrient-poor surface seawater (González, 2008). In addition, mostcontain MED152 genome, which regulates the behavior of gliding, floating and attachment of bacteria (González, 2008). The relative abundance ofincreased significantly in the dust-adding group. It is spe- culated that this gene may make them more easily attaching to the dust surface, facilitating nutrient absorption and accelerating growth.
The genusincan tolerate extreme oligotrophic condition by regulating growth rate, degrading complex aromatic organic compounds or using a variety of simple molecules (White, 1996), the genusinholds proteorhodopsin gene which help them more competitive in oligotrophic surface seawater. However, compared with the control, the relative abundance ofdecreased significantly whileincreased, indicating thatwas less competitive thanin dust addition group.

Table 4 Composition and relative abundance of bacterial community at genus level in dust and different nutrient addition groups
The relative abundance of genusincreased from 26.34% to 31.45%, 48.81%, 49.01%, 61.41% and 40.72% in dust, N, P, NP and NPFe groups. This genus was the absolutely predominant bacteria among all experiment groups. The relative abundance ofin NPFe group was lower than that of other nutrient supplementation groups, while it was closer to that of dust group. In addition, the relative abundances ofandin NPFe group were 10.13% and 5.37% respectively, which was also closer to that of dust group (11.63% and 4.95%). It can be concluded that the effect of dust deposition on the composition and structure of bacterial community originates from the dissolutions of N and P nutrients, especially of Fe in the dusts.
4 Conclusions
1) Dust deposition significantly promoted the abundance of high nucleic acid bacteria and heterotrophic planktonic bacteria through the dissolution of N and Fe.
2) Dust deposition and nutrient addition decreased the diversity while increased the dominance of heterotrophic planktonic bacteria community. The genusinsignificantly increased in dominance, while the generainandinreduced in dominance.
3) The effect of dust deposition on the composition and structure of heterotrophic planktonic bacteria community originated from the dissolution of N, P nutrients, especially Fe in the dusts.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (No. 41210008), and the Major State Basic Research Development Program of China (973 Program No. 2014CB953701).
Andrade, L., Gonzalez, A. M., Araujo, F. V., and Paranhos, R., 2003. Flow cytometry assessment of bacterioplankton in tro- pical marine environments., 55 (3): 841-850.
Cole, J. R., Wang, Q., Fish, J., Chai, B., Mcgarrell, D. M., Sun, Y., Brown, C. T., Porras-Alfaro, A., Kuske, C. R., and Tiedje, J. M., 2013. Ribosomal database project: Data and tools for high-throughput rRNA analysis., 42: 633-642.
DiTullio, G. R., Hutchins, D. A., and Bruland, K. W., 1993. Interaction of iron and major nutrients controls phytoplankton growth and species composition in the tropical North Pacific Ocean., 38 (3): 495-508.
Duan, J., Chen, Z., and Wu, L., 2014. Study on intraseasonal variation of current at the source region of Kuroshio by analyzing the buoy observation data., 29 (4): 523-530 (in Chinese with English abstract).
Edgar, R. C., 2013. UPARSE: Highly accurate OTU sequences from microbial amplicon reads., 10 (10): 996- 998.
Falkowski, P. G., Woodhead, A. D., and Vivirito, K., 1992.. Springer Science & Business Media, New York, 256-287.
Gao, H., Qi, J., Shi, J., Shi, G., and Feng, S., 2009. Long range transport of Asian dust and its effects on ocean ecosystem., 24 (1): 1-10.
Gasol, J. M., and Moran, A. G., 1999. Effects of filtration on bacterial activity and picoplankton community structure as assessed by flow cytometry., 16 (3): 251-264.
Gasol, J. M., Zweifel, U. L., Peters, F.,Fuhrman, J., and Hagström, Å., 1999. Significance of size and nucleic acid content heterogeneity as assessed by flow cytometry in natural planktonic bacteria., 65 (10): 4475-4483.
González, J. M., Fernández-Gómez, B., Fernandez-Guerra, A., Gomez-Consarnau, L., Sánchez, O., Coll-Lladó, M., Campo, J. D., Escudero, L., Rodríguez-Martínez, R., Alonso-Sáez, L., Latasa, M., Paulsen, I., Nedashkovskaya, O., Lekunberri, I., Pinhassi, J., and Pedrós-Alió, C., 2008. Genome analysis of the proteorhodopsin-containing marine bacteriumsp. MED152 (Flavobacteria)., 105 (25): 8724-8729.
Guieu, C., Dulac, F., Desboeufs, K., Wagener, T., Pulido-Villena, E., Grisoni, J., Louis, F., Ridame, C., Blain, S., Brunet, C., Nguyen, E., Tran, S., Labiadh, M., and Dominici, J., 2010. Large clean mesocosms and simulated dust deposition: A new methodology to investigate responses of marine oligotrophic ecosystems to atmospheric inputs., 7: 2765- 2784.
Herut, B., Zohary, T., Krom, M. D., and Mantoura, R. F. C., 2005. Response of East Mediterranean surface water to Saharan dust: On-board microcosm experiment and field observations., 52 (22): 3024-3040.
Jochem, F., Lavrentyev, P., and First, M., 2004. Growth and grazing rates of bacteria groups with different apparent DNA content in the Gulf of Mexico., 145 (6): 1213-1225.
Johnson, K. S., Gordon, R. M., and Coale, K. H., 1997. What controls dissolved iron concentrations in the world ocean., 57 (3): 137-161.
Kemp, P. F., and Aller, J. Y., 2004. Bacterial diversity in aquatic and other environments: What 16S rDNA libraries can tell us., 47 (2): 161-177.
Lebaron, P., Servais, P., Agongué, H., Courties, C., and Joux, F., 2001. Dose the nucleic acid content of individual bacterial cells allow us to discriminate between active cells and inactive cells in aquatic systems., 67 (4): 1775-1782.
Lebaron, P., Servais, P., Baudoux, A., Bourrain, M., Courties, C., and Parthuisot, N., 2002. Variations of bacterial-specific activity with cell size and nucleic acid content assessed by flow cytometry., 28 (2): 131-140.
Lekunberri, I., Lefort, T., Romero, E., Vázquez-Domínguez, E., Romera-Castillo, C., Marrasé, C., Peters, F., Weinbauer, M., and Gasol, J. M., 2014. Effects of a dust deposition event on coastal marine microbial abundance and activity, bacterial community structure and ecosystem function., 32 (4): 381-396.
Li, J. H., Zhang, C., Liu, Y., Gao, H., Shi, J., and Yao, X., 2017. Impacts of dust and haze particles deposition on phytoplankton growth in Yellow Sea during springtime., 37 (1): 112-120 (in Chinese with English abstract).
Masella, A., Bartram, A., Truszkowski, J., Brown, D., and Neufeld, J., 2012. PANDAseq: Paired-end assembler for illumina sequences., 13: 1-7.
Martin, J. H., Coale, K. H., Johnson, K. S., Fitzwater, S. E., Gordon, R. M., Tanner, S., Hunter, C. N., Elrod, V. A., No- wicki, J. L., Coley, T. L., Barber, R. T., Lindley, S. T., Watson, A. J., Vanscoy, K., Law, C. S., Liddicoat, M. I., Ling, R., Stanton, T. P., Stockel, J., Collins, C. A., Anderson, A., Bidigare, R. R., Ondrusek, M., Latasa, M., Millero, F. J., Lee, K., Yao, W., Zhang, J., Friederich, G., Sakamoto, C., Chavez, F., Buck, K., Kolber, Z., Greene, R. M., Falkowski, P., Chisholm, S. W., Hoge, F., Swift, R., Yungel, J., Turner, S., Nightingale, P. D., Hatton, A., Liss, P. S., and Tindale, N. W., 1994. Testing the iron hypothesis in ecosystems of the equatorial Pacific Ocean., 371: 123-129.
Paerl, H. W., 1997. Coastal eutrophication and harmful algal blooms: Importance of atmospheric deposition and groundwater as new nitrogen and other nutrient sources., 42: 1154-1165.
Pulido-Villena, E., Baudoux, A., Obernosterer, I., Landa, M., Capparros, J., Catala, P., Georges, C., Harmand, J., and Guieu, C., 2014. Microbial food web dynamics in response to a Saharan dust event: Results from a mesocosm study in the oligo- trophic Mediterranean Sea., 11: 337- 371.
Wang, Q., Garrity, G. M., Tiedje, J. M., and Cole, J. R., 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy., 73 (16): 5261-5267.
White, D. C., Sutton, S. D., and Ringelberg, D. B., 1996. The genus: Physiology and ecology., 7 (3): 301-306.
Zhang C., Gao, H. W., Yao, X. H., Shi, Z. B., Shi, J. H., Yu, Y., Meng L., and Guo, X. Y., 2018. Phytoplankton growth response to Asian dust addition in the northwest Pacific Oceanthe Yellow Sea.15: 749-765.
Zubkov, M. V., Fuchsa, B. M., Burkill, P. H., and Amann, R., 2001. Comparison of cellular and biomass specific activities of dominant bacterioplankton groups in stratified waters of the Celtic Sea., 67 (11): 5210-5218.
. E-mail: ygzhao@ouc.edu.cn E-mail: gxliu@ouc.edu.cn
February 11, 2019;
August 28, 2019;
September 13, 2019
(Edited by Ji Dechun)
杂志排行
Journal of Ocean University of China的其它文章
- Diffusion Characteristics of Swells in the North Indian Ocean
- Analysis of the Dynamic System of Wave Glider with a Towed Body
- Provenance and Tectonic Implications of Paleozoic Strata in the South Yellow Sea Basin, China–Revealed from the Borehole CSDP-2
- Ecological Risk of Heavy Metals in Sediment Around Techeng Island Special Marine Reserves in Zhanjiang Bay
- Geochemical and Grain-Sized Implications for Provenance Variations of the Central Yellow Sea Muddy Area Since the Middle Holocene
- Grain-Size Distribution of Surface Sediments in the Bohai Sea and the Northern Yellow Sea: Sediment Supply and Hydrodynamics
