Isolation and Neuroprotective Activity of Phenolic Derivatives from the Marine-Derived Fungus Penicillium janthinellum
2020-09-28ZHENGYaoyaoCHENXuCHENLuminSHENLiFUXiumeiCHENQiuxiaCHENMinandWANGChangyun
ZHENG Yaoyao, CHEN Xu, CHEN Lumin, SHEN Li, FU Xiumei,CHEN Qiuxia, CHEN Min, and WANG Changyun, 5)
Isolation and Neuroprotective Activity of Phenolic Derivatives from the Marine-Derived Fungus
ZHENG Yaoyao1), 2), 3), CHEN Xu1), CHEN Lumin4), SHEN Li4), FU Xiumei2), 3),CHEN Qiuxia1), CHEN Min1), *, and WANG Changyun2), 3), 5), *
1),,,225127,2),,,266003,3),,266237,4),,,225001,5),,266003,
A new phenolic compound, 6-(2-acetyl-3,5-dihydroxybenzyl)-4-hydroxy-3-methyl-2-pyran-2-one (1), along with other six known phenolic derivatives (2−7), were isolated from the mangrove rhizosphere fungusHK1-6 cultured in potato dextrose broth medium containing 30gL−1of natural sea salt. The structure of the new compound (1) was elucidated by comprehensive analysis of spectroscopic data including 1D and 2D NMR spectra. The proposed biosynthetic pathway of compound 1 was also studied in this research. Interestingly, a brominated phenolic derivative, aryl bromide (compound 8), was obtained from this fungal strain cultured in medium containing 30gL−1of NaBr instead of natural sea salt. Compound 8 is proposed as a new natural product and formed through bromination of compound 7when the fungus was cultured with NaBr. The neuroprotective effect of compound 1 on oxygen-glucose deprivation (OGD)-induced injury was investigated in rat spinal cord astrocytes. MTT assay demonstrated that compound 1 can attenuate OGD-induced cell viability loss in rat spinal cord astrocytes.
marine fungus;; phenolic derivative;neuroprotective activity
1 Introduction
Neurodegenerative diseases have become increasingly common in last decades, and as were estimated they will surpass cancer as the second most common cause of death among elderly by 2040s (Prince., 2013). Proverbially, Alzheimer’s diseases, Parkinson’s disease, Huntington’s disease and stroke are the most common ones. Ischemic stroke, usually caused by a temporary or a permanent re- duction of local cerebral blood flow, is the leading cause of death and disability in humans due to its high prevalence, severity of insult, and lack of cell-targeted therapeutics (Chong., 2005; Kamel and Iadecola, 2012). Neu- roinflammation is one of the symbol features of ischemic scenarios. Several researches have shown that astrocytes are involved vitally in neuroinflammation, and they can re- lieve the blood-brain barrier disruption and delay neuro- nal damage (Volterra and Meldolesi, 2005; Blackburn., 2009). The neuroprotective function of astrocytes makes them excellent candidates as therapeutic targets to improve the recovery of stroke and other central nervous system injuries. With the aging society coming in both developed and developing countries, the health care costs for the pre- vention and treatment of neurodegenerative diseases will skyrocket (Pangestuti and Kim, 2011; Alghazwi., 2016). Hence, nowadays researchers have an increasing interest in studying natural bioactive compounds that can act as neuroprotective agents.
Marine microorganisms have been regarding as a pro- mising source of structurally diverse natural products with pronounced biological activities. It is well known that man- grove-associating microorganisms in tropical and subtro- pical intertidal estuarine zones have the biosynthetic potential of a wide range of novel chemical entities. In the past two decades, mangrove-associating microorganisms were proved to be rich sources of bioactive secondary metabolites, which play a highly significant role in drug discovery and development (Xu, 2015; Ancheeva., 2018; Blunt., 2018). Notably, several secondary metabolites isolated from mangrove-associating fungi have been reported to exhibit neuroprotective activity. For ex-ample, xyloketal B isolated from the mangrove fungussp. can significantly prevent OGD-induced decrease of MTT values in a concentration-dependent manner (Zhao., 2009), and an aurone glycoside from mangrove fun- gussp. exhibitslatent neuroprotective activity in 1-methyl-4-phenylpyridinium-induced oxidative damage in PC12 cells (Wu., 2015).
In our previous reports, a series of azaphilones including two chlorides penicilones C and D (Chen., 2017) and prenylated indole alkaloids (Zheng., 2020) were isolated from the mangrove rhizosphere fungusHK1-6 when it is cultured in potato dextrose broth and rice solid medium, respectively. In the present study,to further exploit the secondary metabolites of this fungal strain, a larger-scale fermentation was carried out using potato dextrose broth medium with 30gL−1of natural sea salt. In addition to thepredominant products penicilones A–D, the fungal extracts showed some red or orange spots in thin layer chromatography (TLC) analysis.Chemical investigation of the extract resulted in the isolation of a new phenolic compound (1) along with six known phenolic derivatives (2–7) (Fig.1). Furthermore, with the purpose of more halogenated derivatives, this fun- gal strain was cultured with 30gL−1of NaBr instead of natural sea salt. In addition to two brominated azaphilo- nes penicilones G and H (Chen., 2019), a brominated phenolic derivative (8)(Fig.1) was identified from the fun- gal cultures. Interestingly, compound 1 exhibited a neuroprotective effect against OGD-induced cell viability loss in rat spinal cord astrocytes. The isolation, structure elucidation, and biological activity of compounds 1–8 are pre- sented herein.
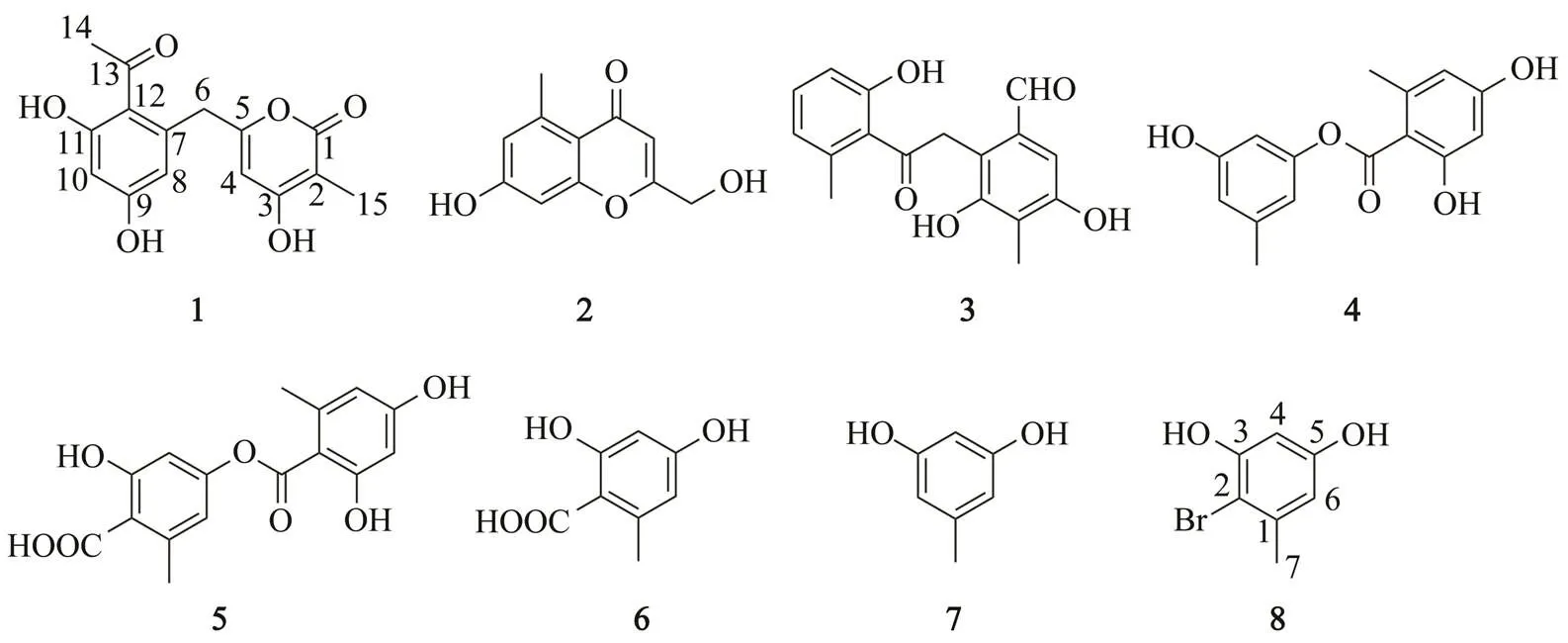
Fig.1 Structures of compounds 1−8.
2 Materials and Methods
2.1 General Experimental Procedures
UV spectra were obtained on a Beckman DU 640 spectrophotometer. IR spectra were recorded on a Cary 610/ 670 spectrometer using KBr pellets. 1D and 2D NMR spectra were acquired using an AVANCE 600 NMR spectrometer (600MHz for1H and 150MHz for13C), using TMS as internal standard. HRESIMS spectra were obtain- ed from a maXis spectrometer. GC-MS spectra were acquired from a Trace ISQ spectrometer (Thermofisher). Semi- preparative HPLC was performed on a HITACHI system using a semi-preparative C18(Kromasil, 5μm, 10mm×250mm) column coupled with a 2400 UV detector. Silica gel (Qing Dao Hai Yang Chemical Group Co.; 200–300 mesh), Sephadex LH-20 (Amersham Biosciences) and octadecyl- silyl silica gel (Unicorn; 45–60μm) were used for column chromatography (CC). Precoated silica gel plates (Yan Tai Zi Fu Chemical Group Co.; G60, F-254) were used for thin layer chromatography (TLC).
2.2 Fungal Material
The isolation and identification of the mangrove rhizo- sphere fungusHK1-6 has been described previously (GenBank accession no. KY412802) (Chen., 2017).
2.3 Fermentation and Isolation
2.3.1 Culturing in medium with natural sea salt
The fungus was cultured in potato dextrose broth medium (20g of dextrose and 30g of natural sea salt in 1L of potato infusion; 1L Erlenmeyer flasks each containing 400mL of culture broth) at 25℃ without shaking for 4 weeks. The culture (30L) was filtered to separate the brothfrom the mycelia. Then the broth was extracted three times with an equal volume of EtOAc, and the mycelia were extracted three times with MeOH. The organic extracts were combined and concentrated under vacuum to afford a to- tal extract (broth extract and mycelia extract, 13.0g). The organic extracts were separated through a silica gel column chromatography using a step gradient elution with EtOAc-petroleum ether (0−100%) and then with MeOH- CH2Cl2(0−100%) to provide 10 fractions (Fr.1−Fr.10). Fr.3 was isolated by silica gel CC using a step-wise gradient of EtOAc-petroleum ether. Then it was transferred to an ODS column and eluted with 80% MeOH-H2O to produce compound 4 (7.0mg). The remaining fraction was purified by HPLC eluting with 75% MeOH-H2O to produce compounds 6 (12.0mg) and 7 (9.2mg). Fr.6 was subjected to silica gel CC using gradient elution with EtOAc- petroleum ether to afford three subfractions (Fr.6-1−Fr.6-3). Fr.6-2 was purified by semipreparative HPLC eluted with 65% MeOH-H2O to produce compounds 1 (16.0mg) and 2 (9.0mg). Fr.6-3 was further separated by ODS column eluted with 70% MeOH-H2O and finally was purified by semipreparative HPLC eluted with 60% MeOH-H2O to produce compounds 3 (6.0mg) and 5 (14.0mg).
2.3.2 Culturing in medium with NaBr
The fungus was cultured with bromine-modified potato dextrose broth medium, which included 20g of dextrose and 30g of sodium bromide in 1L of potato infusion. The fungus was cultured in 1L Erlenmeyer flasks containing 400mL of culture broth at 25℃ without shaking for 4 weeks. Then the culture (30L) was filtered to separate the broth from the mycelia. With the similar extraction process, the organic extracts were combined and concentrated under vacuum to produce a total extract of 9.0g, which included broth extract and mycelia extract. The extract was subjected to silica gel CC using gradient elution with petroleum ether-EtOAc to produce 9 subfractions (Fr.1− Fr.9). Fr.7 was subjected to ODS column and eluted with 70% MeOH-H2O to obtain Fr.7-3. Then it was purified by semipreparative HPLC eluted with 60% MeOH-H2O to produce compound 8 (5.5mg). The phenolic derivatives 5 (5.0mg), 6 (15.0mg) and 7 (12.5mg) were also isolated from the fungal cultures in NaBr containing medium.
2.4 Physical-Chemical Property
6-(2-Acetyl-3,5-dihydroxybenzyl)-4-hydroxy-3-methyl-2-pyran-2-one (1).white amorphous powder; UV (MeOH)max(log) 206 (1.9), 290 (0.9)nm; IR (KBr)max3158, 1570, 1411, 1268, 1169, 1120, 1025, 850, 758, 649cm−1;1H NMR (600MHz, acetone-6) and13C NMR (150MHz, acetone-6) see Table 1; HRESIMS (positive)/313.0694 [M+Na]+(Calcd. for C15H14NaO6, 313.0683).
Aryl bromide (8).1H NMR (600MHz, acetone-6) and13C NMR (150MHz, acetone-6) see Table 1; GC-MS 10.91min,/202, 204 [M]+.
2.5 GC-MS Analysis of Compound 8
Compound8was analyzed by GC-MS under the following conditions: capillary column, DB-5 (30m×0.25mm×0.25μm); detector temperature, 200℃; injection temperature, 280℃; initial temperature was maintained at 50℃ for 3 min and then raised to 330℃ at the rate of 20℃min−1, and final temperature was maintained for 3min; carrier, He. The retention time of compound 8 was 10.91min.
2.6 Biological Assays
2.6.1 Neuroprotective effect
Cell culturing The cerebral cortex was taken from Sprague-Dawley (SD) rats within 24h after birth. The cells were dispersed by treating with 0.25% trypsin at 37℃. After being centrifuged, the cells were cultured in high glucose DMEM medium with 10% FBS, penicillin G (100unitsmL−1) and streptomycin (100μgmL−1) at 5% of CO2and 37℃. A single layer of astrocytes were obtained in 12−14 days.
Cell viability assay The rat spinal cord astrocytes were seeded into 96-well plates at a density of 1×104cells perwell and cultured to 70%−80% confluence at 37℃ in an incubator with 5% CO2. Different concentrations of compound 1 (0, 0.625, 1.25, 2.5, 5.0, 10.0 and 20.0μgmL−1) was added to the astrocyte culture for 24h. Then 20μL modified tetrazolium salt MTT (5mgmL−1) was added to each well for 4h at 37℃. Afterwards, 100μL DMSO was added to each well to dissolve the insoluble purple formazan. The final concentration of DMSO was 0.2%. The absorbance of the culture medium at 490nm was measured using a Benchmark microplate reader.
Neuroprotective activity assay The rat spinal cord astrocytes were seeded into 96-well plates with at a density of 1×104cells per well and cultured at 37℃ in a 5% CO2incubator to 70%−80% confluence. Compound 1 at different concentrations (0.3125, 0.625, 1.25, 2.5, 5.0, 10.0 and 20.0μgmL−1) was added into the astrocytes culture 24h prior to oxygen-glucose deprivation (OGD). The blank control group was maintained in normal DMEM (Dulbecco’s modified Eagle’s medium) under normoxic conditions at 37℃in the absence of OGD exposure and com- pound 1 pretreatment. The negative control group was sub- jected to OGD for 6h (5% CO2, 1% O2and 94% N2) without compound 1 pretreatment. The cell viability was also detected with MTT method as above.
2.6.2 Antibacterial activity
The antibacterial activity assay was carried out with con- ventional broth dilution method using 96-well microtiter plates (Pierce., 2008). Seven bacterial species (Gram- positive(ATCC 43300),(ATCC 33591),(ATCC 29213),(ATCC 25923),(ATCC 51299),(ATCC 35667), and Gram-negative(ATCC 25922)) were cultured at 37℃ in LB broth overnight. The compounds were dissolved in DMSO. LB broth was used as a blank control and DMSO was used as a negative con- trol while oxacillin sodium and vancomycin hydrochloride were used as positive controls.
2.6.3 Lethality to brine shrimp
The test of brine shrimp toxicity onwas performed according to the standard protocols (Meyer., 1982; Solis., 1993). DMSO was used as a positive control.
3 Results and Discussion
3.1 Structure Determination
To discover the trace components in the metabolites of fungusHK1-6, a large-scale fermentation (30L) was carried out using potato dextrose broth medium containing 30gL−1ofnatural sea salt at 25℃ without shaking for 4 weeks. The fungal culture was extracted by organic solvents and the residues wereanalyzed with chromatographic techniques, including silica gel column chromatography, Sephadex LH-20, and semi-preparative HPLC, to obtain the phenolic derivatives 1–7. Compounds1–7 were showedby the consistent characteristic red or orange spots from TLC analysis.
Compound 1 was obtained as a white powder. Its molecular formula was determined as C15H14O6basing on the HRESIMS ion peak at/313.0694 [M+Na]+(calcd. for C15H14NaO6, 313.0683), indicating nine degrees of unsaturation. The1H NMR spectrum (Table 1) reveals two aromatic protons (H6.38 and 6.36), one olefinic proton (H5.84), one methylene group (H3.89),and two methyl groups (H2.53 and 1.83). The13C NMR and DEPT spec- trum (Table 1) exhibited the presence of 15 carbon resonances including two methyls (C32.4 and 8.5), one methylene group (C38.3), three methine groups (C111.7, 102.8, and 100.8), and nine quaternary carbons (C203.6, 165.7, 165.0, 162.5, 161.7, 161.6, 138.5, 119.6, and 98.6). Careful comparisons of the1H and13C NMR data indicated that the structure of compound 1 greatly resembles that of TW93a obtained from the type II minimal PKS of(Yu., 1998). The most obvious difference between them is that compound 1 contains an additional methyl signal atH1.83 (H3-15) in1H NMR spectrum and a corresponding carbon signal atC8.5 (C-15) in13C NMR spectrum. In HMBC spectra (Fig.2). The correlations between H3-15 and C-1, C-2 and C-3 showed that the methyl group (Me-15) locates at C-2. Considering the HMBC correlations between H-4 and C-2, C-3 and C-5, the fragment structure of 4-hydroxy-3-me- thyl-2-pyran-2-one can be revealed in compound 1. Furthermore, the HMBC correlations (Fig.2) between H3- 14 and C-12 and C-13;between H-8 and C-9, C-10 and C-12; and between H-10 and C-8 indicated a partial struc- ture of 1-(2,4-dihydroxyphenyl)ethan-1-one in compound 1. Additionally, the HMBC correlations between H2-6 and C-4, C-5, C-7, C-8, and C-12 confirmed that the two partial structures, 4-hydroxy-3-methyl-2-pyran-2-one and 1-(2,4-dihydroxyphenyl)ethan-1-one, are connected through the methylene bridge at C-6. Then the whole structure forcompound 1 is 6-(2-acetyl-3,5-dihydroxybenzyl)-4-hydro- xy-3-methyl-2-pyran-2-one.
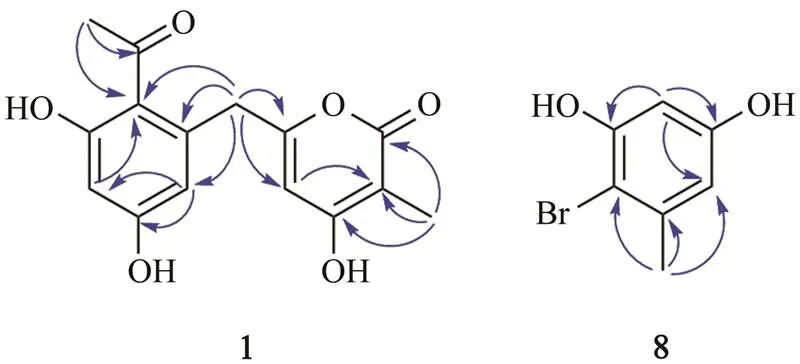
Fig.2 Key HMBC correlations for compounds 1 and 8.
Structurally, compound 1 belongs to the polyketide fa- mily. Considering the results of other researchers (Mizuuchi., 2009), a plausible biosynthetic route of com- pound 1the polyketide pathway was proposed (Scheme 1). The biosynthesis is initiated by the formation of the polyketide chain through Claisen condensation using one acetyl-CoA starter unit and six malonyl-CoA extender units. Thereafter, the first cyclization event involves an al- dol condensation between C-7 and C-12. After aromati- zation of the first ring and formation of the α-pyrone, a methyl is attached to C-2 in the presence of-adenosyl- methionine (SAM) to give compound 1.
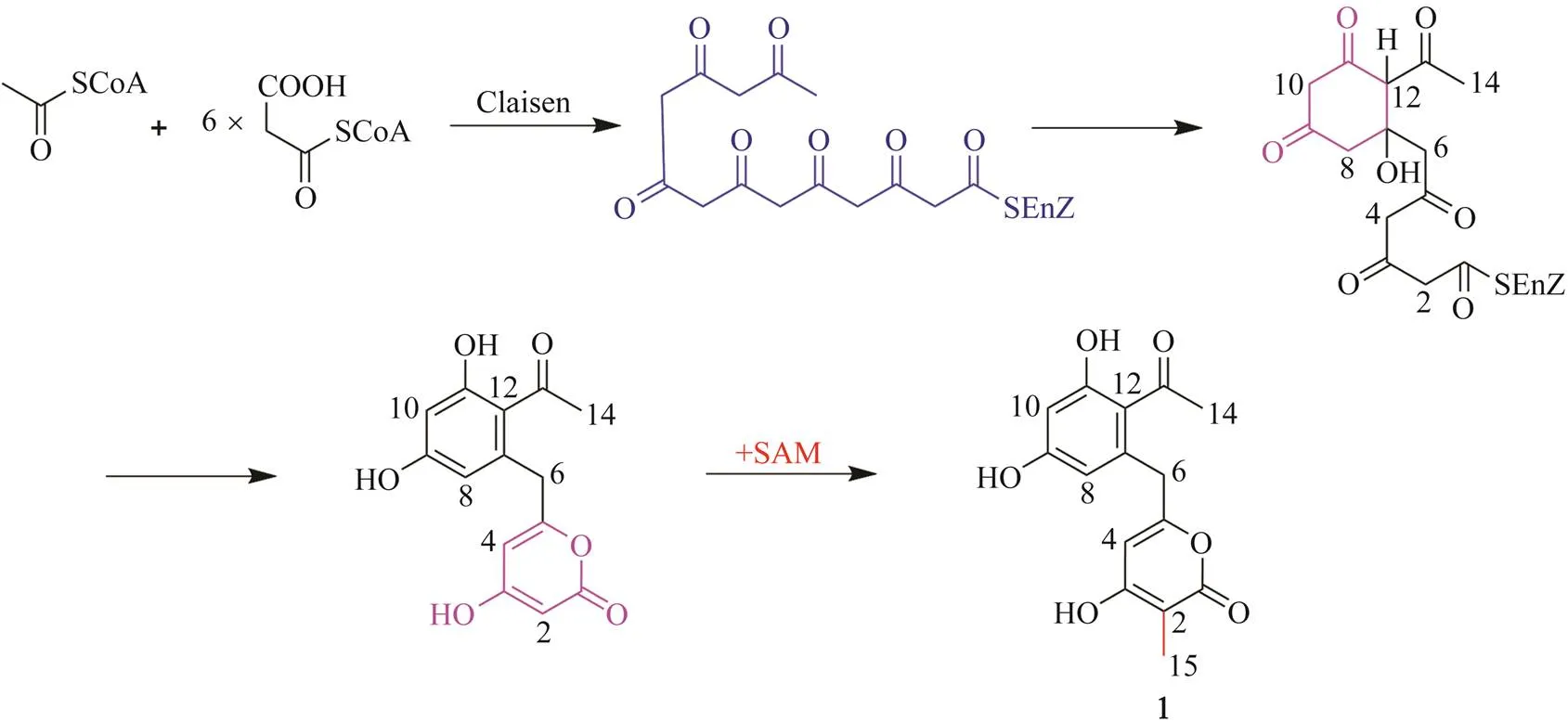
Scheme 1 Proposed biosynthetic pathway ofcompound 1.
The structure of the known compounds were determin- ed as 7-hydroxy-2-(hydroxymethyl)-5-methyl-4-chromen- 4-one (compound 2)(Kimura., 1992), 3,5-dihydro- xy-2-(2-(2-hydroxy-6-methylphenyl)-2-oxoethyl)-4-me- thylbenzaldehyde (compound 3) (Matsunaga., 2015), 3-hydroxy-5-methylphenyl 2,4-dihydroxy-6-methylben- zoate (compound 4)(Eamvijarn., 2012), lecanoric acid (compound 5)(Narui., 1998), orsellinic acid (com- pound 6) (Lopes., 2008) and orcinol (compound 7)(Lopes., 2008) considering their NMR and MS ana- lysis data and those previously reported results. Com- pound 3 has only been reported once, which was isolated from a fungussp. obtained from sand col- lected in Kasai, Tokyo, Japan (Matsunaga., 2015).
To explore the metabolic potential ofHK1-6, we tried to optimize fermentation conditions. When this fungal strain was cultured using potato dextrose broth medium with NaBr (30gL−1) instead of natural sea salt, the TLC analysis of the extract surprisingly exhibited a special color spot with deep pink in the center and light pink around the periphery. A brominated phenolic derivative, aryl bromide (compound 8), was separated from the treated fungal cultures. In addition, quite unexpectedly, com- pared with the yields from the fungal cultures with natural sea salt, compounds 6 and 7 significantly increased while compound 5 reduced from the NaBr-treated fungal cultures.
Compound 8 was obtained as a light red powder. Its molecular formula was established as C7H7BrO2based on GC-MS and NMR spectra. The GC-MS analysis showed bromine-containing peaks in a ratio of 1:1 (/202, 204 [M+H]+) at 10.91min (Fig.S7), indicating the presence of one bromine atom in compound 8. Comparing the1H and13C NMR spectra of compound 8 with those ofcompound 7, it was found that a close relationship exists between them. In the1H NMR spectrum ofcompound 8 (Table 1), only two aromatic-hydrogens (H6.39 and 6.37) were observed, suggesting that the bromine atom replaced one of aromatic protons of compound 7. Considering the key HMBC correlations as well (Fig.2), the structure of compound 8 was determined as shown. It is noteworthy that compound 8 is proposed as a new natural product, although a synthetic sample has been reported (Liang., 2013). Its13C NMR and 2D NMR data are reported for the first time in the present work. The halogenated analogues might be caused by halogen sources in culture me- dia used for the fermentations (Yamazaki., 2015; Ali., 2017). Considering the two different fermentations ofHK1-6, it can be concluded that compound 8 is formed through bromination of 7 in the NaBr- containing medium.
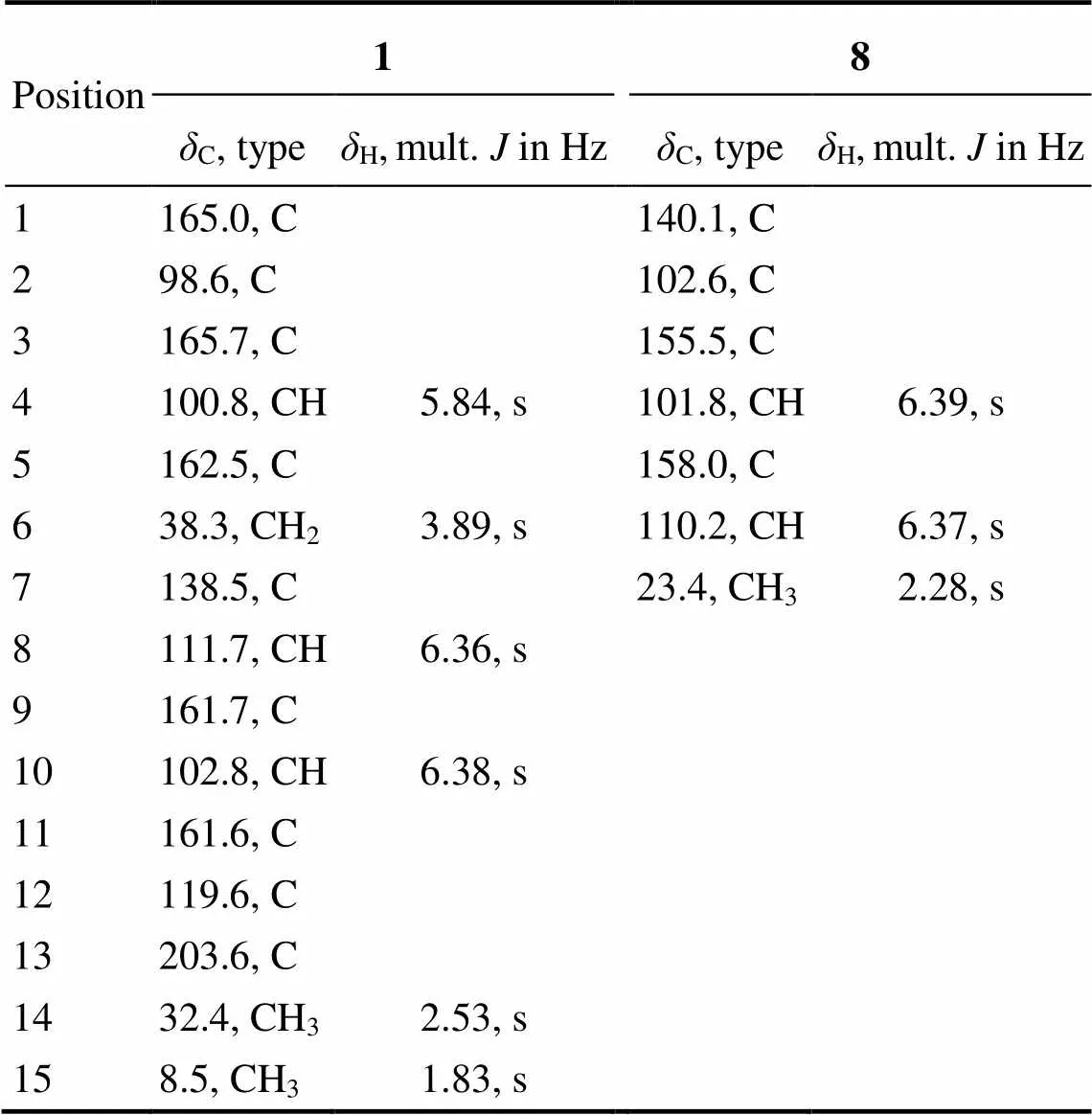
Table 1 1H (600MHz) and 13C (150 MHz) NMR data for compounds 1 and 8 inacetone-d6
3.2 Bioactivity
A literature search revealed that many phenolic compounds have been reported to show a large variety of biological activities such as antibiotic, antiviral, and antitumour (Huneck, 1999; Muller, 2001). In our study, the new phenolic compound 1 was evaluated for neuroprotec- tive effect to astrocytes.neuroprotective effect on oxygen-glucose deprivation (OGD) damaged rat spinal cord astrocytes was investigated by using MTT method (Bao., 2016). The results indicated that compound 1 has no toxicity to normal spinal cord astrocytes (Fig.3A) but increases the cell viability of model rat spinal cord astro- cytes (OGD for 6h) pretreated with different concentrations of compound 1 (0.3125–5.0μgmL−1) (Fig.3B). Thuscompound 1 may have neuroprotective effect by attenu- ating OGD-induced cell viability loss in rat spinal cord astrocytes.
Compounds 1–8 were also tested for their antibacterial activity against seven bacterial strains including Gram- positive(ATCC 43300),(ATCC 33591),(ATCC 29213),(ATCC 25923),(ATCC 51299),(ATCC 35667), and Gram-negative(ATCC 25922). However, none of these compounds were proved to be active. Furthermore, compound 1 also showed no le- thality towards brine shrimp.
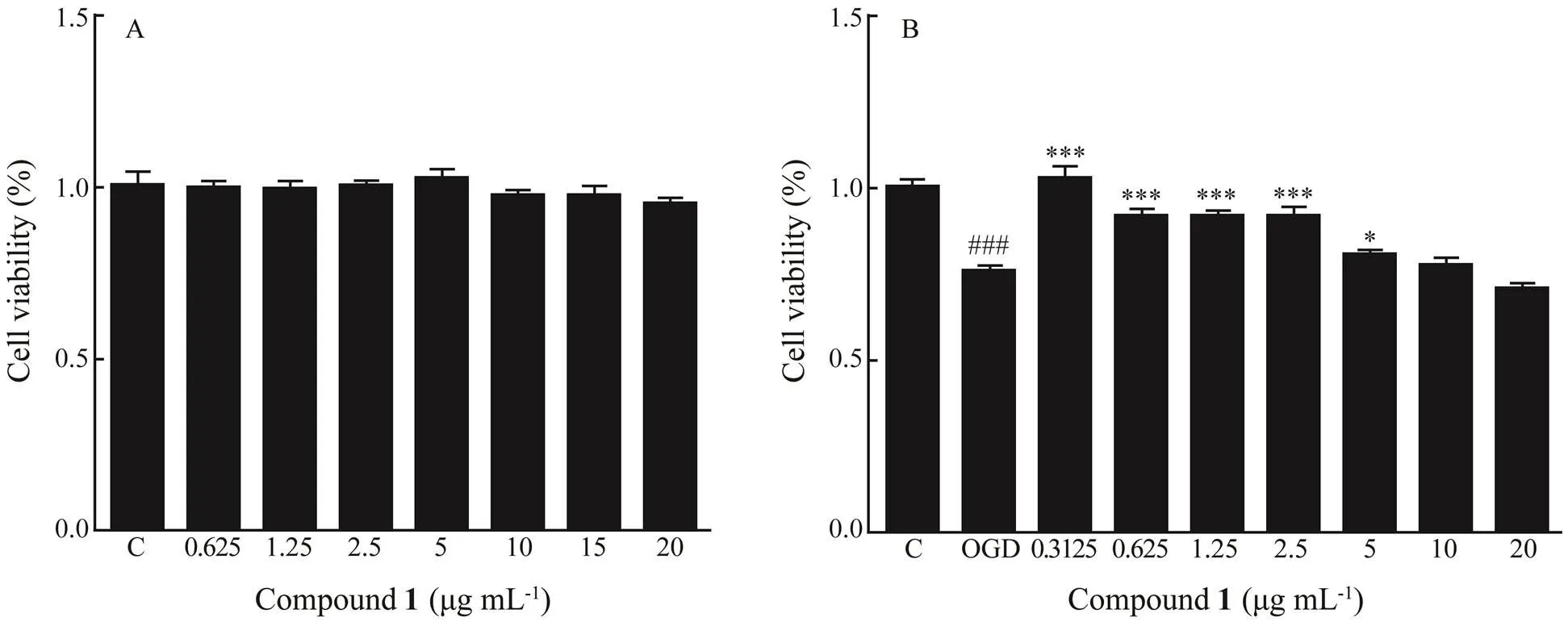
Fig.3 Effect of compound 1 on cell viability of normal rat spinal cord astrocytes (A) and model rat spinal cord astrocytes (OGD for 6h) (B). The cell viability was assayed with MTT mothed. ###P<0.001 vs. control, *P<0.05 vs. OGD, ***P<0.001 vs. OGD.
4 Conclusions
In this study, a new phenolic compound 6-(2-acetyl- 3,5-dihydroxybenzyl)-4-hydroxy-3-methyl-2-pyran-2-one (1) and seven phenolic compounds (2−8) were obtained from the mangrove rhizosphere fungusHK1- 6. The structure of compound 1 was elucidated by spectroscopic data. A possible biosynthesis of compound 1 through the polyketide pathway was also discussed. Com- pound 8 is proposed as a new natural product and produced through bromination of compound 7 in the NaBr- supplemented culture medium. The bromine-modified ap- proach is a simple and useful method to dig various metabolites from a single fungal strain. Compound 1 was in- dicated to possess neuroprotective activity by MTT assay.
Supplementary Materials
The MS and NMR spectra of compounds 1 and 8 are available.
Acknowledgements
The Testing Center of Yangzhou University is appreciated for the testing assistance. This work was supported by the National Natural Science Foundation of China (Nos.81703411, 41830535, U1606403), the Marine S & T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao) (No. 2018SD KJ0406-5), the National Science and Technology Major Project for Significant New Drugs Development (No. 2018 ZX09735-004), the Program of Open Studio for Druggability Research of Marine Natural Product, the Pilot National Laboratory for Marine Science and Technology (Qingdao, China), and Taishan Scholars Program, China.
Alghazwi, M., Kan, Y. Q., Zhang, W., Gai, W. P., and Yan, X. X., 2016. Neuroprotective activities of marine natural products from marine sponges., 23 (4): 360-382, DOI: 10.2174/0929867323666151127201249.
Ali, T., Inagaki, M., Chai, H., Wieboldt, T., Rapplye, C., and Ra- kotondraibe, L. H., 2017. Halogenated compounds from directed fermentation of, an endophytic fungus of the liverwort., 80 (5): 1397-1403, DOI: 10.1021/acs. jnatprod.6b01069.
Ancheeva, E., Daletos, G., and Proksch, P., 2018. Lead compounds from mangrove-associated microorganisms., 16 (9): 319, DOI: 10.3390/md16090319.
Bao, G., Li, C., Qi, L., Wang, N., and He, B., 2016. Tetrandrine protects against oxygen-glucose-serum deprivation/reoxyge- nation-induced injuryPI3K/AKT/NF-κB signaling pathway in rat spinal cord astrocytes.,84: 925-930, DOI: 10.1016/j.biopha.2016.10.007.
Blackburn, D., Sargsyan, S., Monk, P. N., and Shaw, P. J., 2009. Astrocyte function and role in motor neuron disease: A future therapeutic target?,57 (12): 1251-1264, DOI: 10.1002/ glia.20848.
Blunt, J. W., Carroll, A. R., Copp, B. R., Davis, R. A., Keyzers, R. A., and Prinsep, M. R., 2018. Marine natural products., 35 (1): 8-53, DOI: 10.1039/C7NP0005 2A.
Chen, M., Shen, N. X., Chen, Z. Q., Zhang, F. M., and Chen, Y., 2017. Penicilones A–D, anti-MRSA azaphilones from the ma- rine-derived fungusHK1-6., 80 (4): 1081-1086, DOI: 10.1021/acs.jnat prod.6b01179.
Chen, M., Zheng, Y. Y., Chen, Z. Q., Shen, N. X., Shen, L., Zhang, F. M., Zhou, X. J., and Wang, C. Y., 2019. NaBr-induced production of brominated azaphilones and related tricyclic polyketides by the marine-derived fungusHK1-6., 82 (2): 368-374, DOI: 10.1021/acs.jnatprod.8b00930.
Chong, Z. Z., Li, F., and Maiese, K., 2005. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease., 75 (3): 207-246, DOI: 10.1016/j.pneurobio.2005.02.004.
Eamvijarn, A., Kijjoa, A., Bruyere, C., Mathieu, V., Manoch, L., Lefranc, F., Silva, A., Kiss, R., and Herz, W., 2012. Secondary metabolites from a culture of the fungusand theircytostatic activity in human cancer cells., 78 (16): 1767-1776, DOI: 10.1055/ s-0032-1315301.
Huneck, S., 1999. The significance of lichens and their metabolites., 86 (12): 559-570, DOI: 10. 1007/s001140050676.
Kamel, H., and Iadecola, C., 2012. Brain-immune interactions and ischemic stroke: Clinical implications., 69 (5): 576-581, DOI: 10.1001/archneurol.2011.3590.
Kimura, Y., Shiojima, K., Nakajima, H., and Hamasaki, T., 1992. Structure and biological activity of plant growth regulators produced bysp. No. 31f.,56 (7): 1138-1139, DOI: 10.1271/bbb. 56.1138.
Liang, D., Luo, H., Liu, Y. F., Hao, Z. Y., Wang, Y., Zhang, C. L., Zhang, Q. J., Chen, R. Y., and Yu, D. Q., 2013. Lysilactones A-C, three 6-dibenzo[b,d]pyran-6-one glycosides from, total synthesis of Lysilactone A., 69 (9): 2093-2097, DOI: 10.1016/j.tet.2013.01.029.
Lopes, T. I. B., Coelho, R. G., Yoshida, N. C., and Honda, N. K., 2008. Radical-scavenging activity of orsellinates., 56 (11): 1551-1554, DOI: 10.1248/ cpb.56.1551.
Matsunaga, H., Kamisuki, S., Kaneko, M., Yamaguchi, Y., Takeuchi, T., Watashi, K., and Sugawara, F., 2015. Isolation and structure of vanitaracin A, a novel anti-hepatitis B virus com- pound fromsp.,25 (19): 4325-4328, DOI: 10.1016/j.bmcl.2015. 07.067.
Meyer, B. N., Ferrigni, N. R., Putnam, J. E., Jacobsen, L. B., Ni- chols, D. E., and McLaughlin, J. L., 1982. Brine shrimp: A con- venient general bioassay for active plant constituents.,45 (1): 31-34, DOI: 10.1055/s-2007-971236.
Mizuuchi, Y., Shi, S. P., Wanibuchi, K., Kojima, A., Morita, H., Noguchi, H., and Abe, I., 2009. Novel type III polyketide syn- thases from.,276 (8): 2391- 2401, DOI: 10.1111/j.1742-4658.2009.06971.x.
Muller, K., 2001. Pharmaceutically relevant metabolites from li- chens.,56 (1-2): 9- 16, DOI:10.1007/s002530100684.
Narui, T., Sawada, K., Takatsuki, S., Okuyama, T., Culberson, C. F., Culberson, W. L., and Shibata, S., 1998. NMR assignments of depsides and tridepsides of the lichen family-., 48 (5): 815-822, DOI:10.1016/S0031- 9422(97)00958-8.
Pangestuti, R., and Kim, S. K., 2011. Neuroprotective effects of marine algae., 9: 803-818, DOI: 10.3390/md90 50803.
Pierce, C. G., Uppuluri, P., Tristan, A. R., Wormley, F. L. J., Mo- wat, E., Ramage, G., and Lopez-Ribot, J., 2008. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing., 3 (9): 1494-1500, DOI: 10.1038/nprot. 2008.141.
Prince, M., Bryce, R., Albanese, E., Wimo, A., Ribeiro, W., and Ferri, C. P., 2013. The global prevalence of dementia: A systematic review and metaanalysis., 9 (1): 63-75, DOI: 10.1016/j.jalz.2012.11.007.
Solis, P. N., Wright, C. W., Anderson, M. M., Gupta, M. P., and Phillipson, J. D., 1993. A microwell cytotoxicity assay using(brine shrimp).,59 (3): 250-252, DOI: 10.1055/s-2006-959661.
Volterra, A., and Meldolesi, J., 2005. Astrocytes, from brain glue to communication elements: The revolution continues., 6 (8): 626-640, DOI: 10.1038/nrn1722.
Wu, Y. Z., Qiao, F., Xu, G. W., Zhao, J., Teng, J. F., Li, C., andDeng, W. J., 2015. Neuroprotective metabolites from the endo- phytic fungusof the mangrove.,12: 148-152, DOI: 10. 1016/j.phytol.2015.03.007.
Xu, J., 2015. Bioactive natural products derived from mangrove-associated microbes.,5 (2): 841-892, DOI: 10. 1039/C4RA11756E.
Yamazaki, H., Rotinsulu, H., Narita, R., Takahashi, R., and Namikoshi, M., 2015. Induced production of halogenated epi- dithiodiketopiperazines by a marine-derivedcf.with sodium halides., 78 (10): 2319-2321, DOI: 10.1021/acs.jnatprod.5b00669.
Yu, T. W., Shen, Y. M., McDaniel, R., Floss, H. G., Khosla, C., Hopwood, D. A., and Moore, B. S., 1998. Engineered biosyn- thesis of novel polyketides from streptomyces spore pigment polyketide synthases.,120 (31): 7749-7759, DOI: 10.1021/JA9803658.
Zhao, J., Li, L., Ling, C., Li, J., Pang, J. Y., Lin, Y. C., Liu, J., Huang, R., Wang, G. L., Pei, Z., and Zeng, J. S., 2009. Marine compound Xyloketal B protects PC12 cells against OGD-induced cell damage.,1302: 240-247, DOI: 10. 1016/j.brainres.2009.09.034.
Zheng, Y. Y., Shen, N. X., Liang, Z. Y., Shen, L., Chen, M., andWang, C. Y., 2020. Paraherquamide J, a new prenylated indole alkaloid from the marine-derived fungusHK1-6., 34 (3): 378-384, DOI: 10.1080/14786419.2018.1534105.
. E-mail: dieying0719@163.com
E-mail: changyun@ouc.edu.cn
October 15, 2019;
December 17, 2019;
March 20, 2020
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Diffusion Characteristics of Swells in the North Indian Ocean
- Analysis of the Dynamic System of Wave Glider with a Towed Body
- Provenance and Tectonic Implications of Paleozoic Strata in the South Yellow Sea Basin, China–Revealed from the Borehole CSDP-2
- Ecological Risk of Heavy Metals in Sediment Around Techeng Island Special Marine Reserves in Zhanjiang Bay
- Geochemical and Grain-Sized Implications for Provenance Variations of the Central Yellow Sea Muddy Area Since the Middle Holocene
- Grain-Size Distribution of Surface Sediments in the Bohai Sea and the Northern Yellow Sea: Sediment Supply and Hydrodynamics
