Studies on the Attached Cultivation of Filamentous Oleaginous Microalga Tribonema minus
2020-09-28ZHANGYanJIChunliZHOUWenjunWANGHuiWANGJunfengandLIUTianzhong
ZHANG Yan, JI Chunli, ZHOU Wenjun, WANG Hui,4), *, WANG Junfeng,and LIU Tianzhong, 4)
Studies on the Attached Cultivation of Filamentous Oleaginous Microalga
ZHANG Yan1), 2), JI Chunli3), ZHOU Wenjun1), WANG Hui1),4), *, WANG Junfeng1),and LIU Tianzhong1), 4)
1),,,266101,2),100049,3),,030801,4),116023,
Attached cultivation is a promising method for microalgal biomass production. Filamentous oleaginous microalga(hereafter) has shown a remarkable potential for biofuel production in terms of its high lipid content. How- ever, the strain has only been cultivated in suspended cultivation systems including open pond and closed photobioreactors. Here, we attempted to study the attached cultivation of, which might be helpful for its scale-up cultivation and industrial applications. As the results, the optimal conditions forgrowth in the attached biofilm are 200μmolphotonsm−2s−1of light intensity and 5% of CO2, and the maximum biomass density of 223gm−2has been achieved under the light intensity. The non-woven fabric as substratum was found as the best substratum in thin layer attached bioreactor, on which the average biomass productivity ofis about (9.73±2.19)gm−2d−1. Furthermore, two attached bioreactor systems, rotary drum and rotation disc, were designed following the light dilution strategy and introduced intocultivationThe highest footprint areal biomass productivity of these two systems is 33 and 47.1gm−2d−1, respectively, much higher than that in suspended cultivation system. The results shows thatcan be cultured with attached cultivation method to improve its biomass productivity.
; attached culture; biomass productivity; light dilution
1 Introduction
In recent decades, the interests in photoautotrophically growing microalgae have rapidly increased because of the potential of microalgae as sustainable resources of renew- able energy, food, animal feed, high added value compo- nents, and environmental remediators (Brennan., 2010; Ooms., 2016). However, the lack of economic com- petitiveness of microalgal biomass production has hin- dered the commercialization of their cultivation, espe- cially for biofuel production (Barlow., 2016). Open ponds and closed photobioreactors usually are prevailing microalgal cultivation devices. These conventional culti- vation technologies limit the application of microalgae due to their low biomass density and high costs of harvesting and dewatering (Heimann, 2016; Mohd-Sahib., 2018). The major drawbacks of conventional suspended cultiva- tion system must be resolved in large-scale production.
Unlike the suspended cultivation system, attached cul- tivation systems produce biomass in the form of a biofilm-which is formed as the microalgal cells settle on the sur- face of artificial substratum materials (Schnurr., 2013;Shen., 2018). With this method, aqua-medium and the biomass are largely separated so that the disadvantages caused by huge water proportion can be largely avoided, resulting in the merits including water-saving, high bio- mass productivity and high efficient harvesting (Wang., 2017). As the attached cultivation system has the potential of obtaining an extremely high biomass footprint produc- tivity with a very low operation cost, the system has recent- ly been applied to many microalgal species with different environmental parameters for various purposes (Schnurr., 2015).
For photoautotrophically growing microalgae, especially in attached cultivation systems, light intensity and CO2con- centration are major environmental parameters that signi- ficantly affect the biomass growth since microalgae are di- rectly exposed to the ambient air where they use light energy to convert CO2into biomass (Ji., 2015; Kiper- stok.,2017). For this reason, fundamental studies on the effects of these parameters on biomass productivity of new microalgal strains should be preliminarily determin- ed and optimized in attached cultivation systems. More- over, from the engineering aspect, substratum material for algal cell attachment is a critical issue for scaling up the attached cultivation. The ideal supporting material for large scale cultivation should be cheap, durable, porous, non- toxic, water retentive among others (Wang., 2017). What else bioreactor structure is directly related to the light harvesting and utilization of biofilms, and the light dilution strategy should be considered during in the bio- reactors design. The maximum photosynthetic flux den- sity of full sunshine is generally over 2000μmolm−2s−1, far beyond the photosynthetic light saturation point (100– 150μmolm−2s−1) for commonly cultivated algal species (Liu., 2013). In attached cultivation systems, the full sun- shine intensity can be diluted, and one of the light dilution strategies is to array the algal biofilm in vertical multiple planes. The top light scatters penetrated into the glass chamber that can be diluted so as not to cause damage on the cells (Liu., 2013). Another strategy is to inter- mittently illuminate the biofilm with appropriate frequency to produce time-averaged irradiation so that more areas can receive proper illumination in a fixed total time (Gross., 2014; Toninelli., 2016). In general, cultivation condition, substratum material and bioreactor structure need to be studied in the attached cultivation of the photoauto- trophically growing microalgal cells.
Filamentous oleaginous microalgal species,sp., has shown a remarkable potential for biofuel produc- tion due to its rich lipid content and good industrial char- acteristics; however, it has been studied only in suspend- ed cultivation systems (Wang., 2013).sp. has unbranched filaments that are composed of a single row of elongated, cylindrical cells similar to those of. It has been proved that the filaments are helpful to adhere upon different substratum materials (Zhang., 2015). Therefore,sp. might be good candidate for the attached cultivation for biofuel production.
Here, in this study, the present work was carried out, aiming to implement attached cultivation technique for filamentous oleaginous microalga. The cultiva- tion conditions, substratum materials and bioreactor struc- ture were optimized, compared and designed, respectively. Our results should help to scale-up the cultivation of fila- mentous oleaginous.
2 Methods
2.1 Microalgal Strain and Culture Conditions
The filamentous microalgae(SAG 880-1) was provided by the Culture Collection of Algae of Gottingen University. The strain as inoculum was grown in Erlen- meyer flasks (50mL) containing 30mL of BG11 medium (Ernst, 1991) before the atteched cultivation experiments. The strains were cultured at 23℃±1℃ in an incubator with shaking at 120rmin−1and under a constant illumine- tion by white fluorescent lamps maintained at 60μmolpho- tonsm−2s−1.
2.2 Characteristics of Attached Cultivation Conditions of T. minus
The single-layer vertical attached photobioreactor de- signed by Liu. (2013) was adopted to cultivatecells. Briefly, a 0.2m×0.4m glass plate (3mm thick- ness) was placed in the center of a 0.5m×0.3m×0.05mglass chamber. One surface of the inserted plate, which would be illuminated in the following cultivation, was cov- ered by a layer of filter paper. A certain volume of the al- gal inoculum was evenly filtered onto a cellulose acetate/ nitrate membrane (with pore size 0.45μm) to form an algal‘disk’ with 10cm2±0.5cm2of the footprint. The algal ‘disk’ was then placed onto the filter paper, and the medium was dripped down to the space between the filter paper and the glass plate from a perforated nylon tubing which was placed onto the top brim of the glass plate so that the fil- ter paper, cellulose membranes as well as the algal ‘disk’ were kept wet as the culture medium was soaked in, as shown in Fig.1.

Fig.1 Thin-layer attached cultivation of T. minus.
The biomass growth ofwas measured every 3 days in order to determine if the growth behavior varies with respect to different light intensity and CO2concen- tration. The initial inoculation density ofwas 10gm−2and the temperature inside the glass chamber was kept at 24℃±1℃ during the whole experiment. To in- vestigate the influence of light intensity ongrowth,six light intensities (50, 100, 150, 200, 250, 300μmol pho- tonsm−2s−1) of artificial light were provided by cold fluo- rescence lamp. In addition, compressed air with different concentrations of CO2(0.03%, 2%, 5%, 10%, 20%) was supplied into the glass chambera nozzle installed at the bottom of the chamber with a superficial aeration rate of 0.0056ms−1.
2.3 Characteristics of Substratum Materials in Attached Cultivation of T. minus
The attached cultivation bioreactor used in this expe- riment was similar to that describe above. The difference was that this glass chamber (0.8m long, 0.3m wide) was vertically placed. A 0.2m×0.4m glass plate was inserted vertically at the center of a glass chamber, and one sur- face of the inserted plate was covered by a towel tightly and illuminated. Fourteen common substratum materials (Ta- ble 1) were tested in the attached cultivation ofin this study. These materials were cut into 0.03m×0.03m and immersed in suspended microalgal cultured to a high concentration and the algal cells will cover the full surfaces of substratum materials. Sometimes, the cells were artificially smeared onto the substratum surfaces. After that, the pre- pared ‘algal disks’ were evenly placed onto the towel.
Based on the optimal light intensity and CO2concentra- tion, compressed air enriched with 5% CO2(v/v) was aer- ated into the glass chamber at an aeration rate of 0.0056ms−1. The temperature inside the glass chamber was kept at 24℃±1℃. The light intensity of 200μmolphotonsm−2s−1of artificial light was provided by cold fluorescence lamp.
cells were sampled by gently scraping the sub- strate surface with a soft rubber blade every 3 days. The residual biomass was left to act as the seeds for the next 3 days’ growth cycle. The cells were cultured for 9 days and the average biomass productivity was calculated. In addi- tion, the attachments ofcells on different substra- tum materials and the harvesting performances were also evaluated.
2.4 Evaluation of Attached Cultivation of T. minus with Different Structural Bioreactors
Two bioreactors including rotary drum and rotation disc were designed and used to culturecells in this study. As illustrated in Fig.2A, the rotary drum was main- ly composed of a screw and transparent objects with cir- cular surfaces at both ends. One side of the screw was connected with a motor so that the screw could rotate at a certain speed. In this study, 8 and 16 pieces of glasses are embedded between the rod and the circular objects, re- spectively. Each piece of glass was 0.6m long and 0.3m wide, and the non-woven fabric materials as substratum were fixed onto the two surfaces of the glasses. The whole device was fixed in a glass chamber at a certain height. The edge of the substratum was immersed into the BG11 me- dium, and was stored in the bottom of the chamber with the rotation of the screw. The medium could penetrate into the inside of the substratum to keep the cells wet.
The structure of the ration disc system was different from the rotary drum (Fig.2B). Although there was also a rotation screw fixed in a glass chamber, the embedded modes of substratum materials were totally different. In the rotation disc system, the substratum materials were made into circles and fixed on the rotating screw successively. The distances between the substratum materials could be ar- bitrarily adjusted, and the number of substratum materials was changed accordingly. Similarly, the bottom of the glass chamber was filled with BG11 medium, which could pe- netrate into the substratum materials with the rotation of the rod to keep the microalgal cells wet. Both of the two glass chambers were 0.8m length, 0.4m width and 0.4m height.
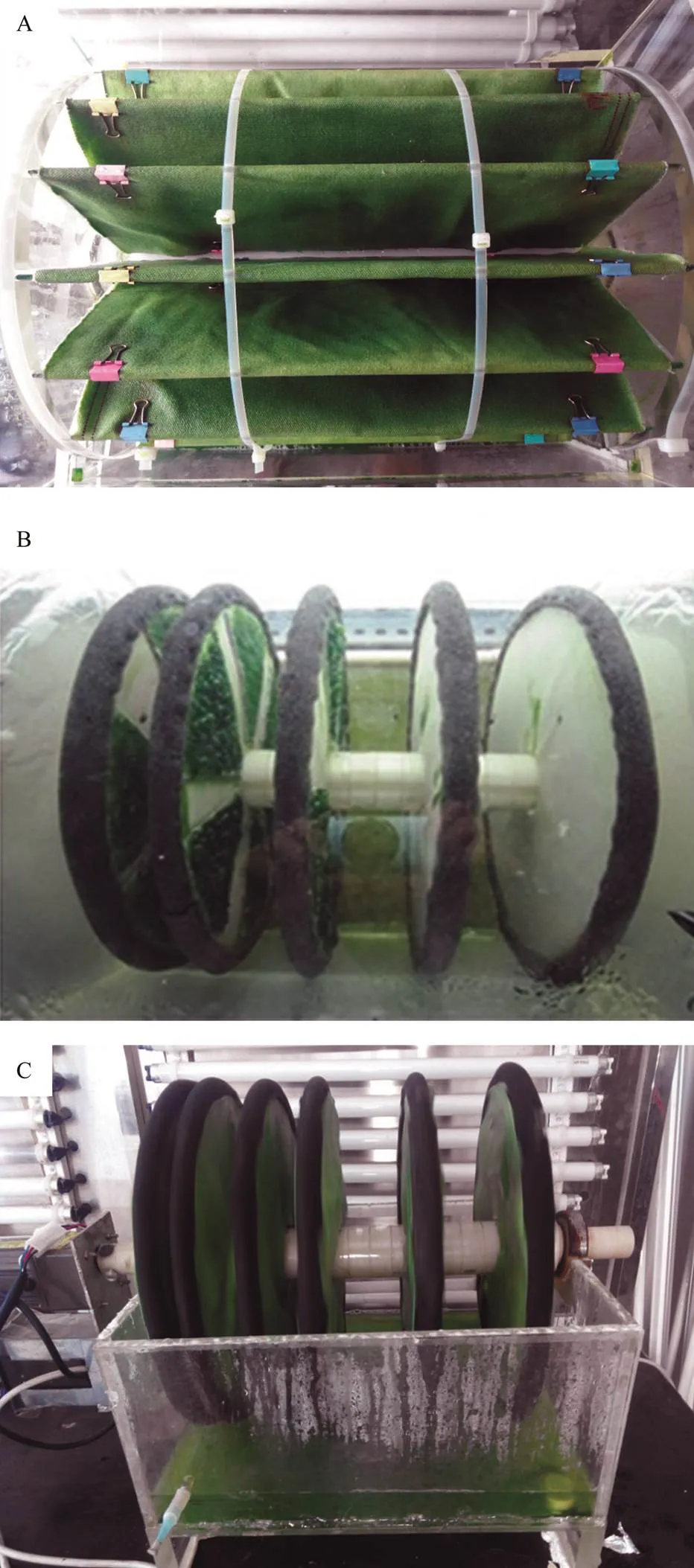
Fig.2 Attached cultivation of T. minus with two different structural bioreactors. (A) Rotary drum; (B–C) Rotation disc.
According to the results, non-woven fabric (#2) was adopted in this study as the substratum, and the cells were artificially smeared onto the substratum surfaces to make the initial inoculation density of(5gm−2). Com- pressed air with 5% CO2was pumped into the glass cham- ber. Enough artificial light was provided by cold fluores- cence lamp from the top of the glass chamber to make the surface intensity of the substratum materials 100–200μmolphotonsm−2s−1. And the ration rate was 2 revolutions per minute.
The algal film was sampled by gently scraping the sub- stratum surface with a soft rubber blade every 3 days as was described above. The residual biomass was left to act as seeds for the next 3 days’ growth cycle and the whole culture period was 9 days. The harvested algal slurry was washed twice with distilled water and then was over-dried for biomass productivity measurement.
2.4 Growth and Lipid Analysis
The biomass productivity (gm−2d−1) was measured by weighing. The algal cells were totally washed off or scrapp- ed from the substratum materials. The collected algal cellswere then filtered on a pre-weighed (0, g) NC mem- brane (pore size 0.45μm). The algal paste was washed twice with distilled water to remove all soluble nutrients. The membrane was then oven-dried at 105℃ for 12h and then weighed (W, g) after cool down.
The biomass productivity based on unit area of the substrate afterdays culture (g, n, gm−2d−1) is calculated as Eq. (1):

where thegwas the substratum materials area.
The biomass productivity base on the footprint area of the bioreactor afterdays culture (f, n, gm−2d−1) is calculated as shown in Eq. (2).

where ∑ indicated the summary weight of algal cells from all substratum materials,fwas the footprint area occu- pied by the incidence light area.
Total lipid was extracted with methanol and chloroform according to modified Bligh and Dyer’s method (Chen., 2012). Approximately 50mg (A) of dried algal pellet was ground with quartz firstly and then mixed with 7.5mL methanol/chloroform (2:1, v/v) at 37℃ overnight. The mixture was then centrifuged at 8000for 10min. Then the supernatant was collected and the residual biomass was extracted one more time. The supernatants were combin- ed, and chloroform and 1% sodium chloride solution were added into to a final volume ratio of 1:1:0.9 (chloroform/ methanol/water). The organic phase was carefully trans- ferred to a vial and dried to constant weight with nitro- gen flow. The lipid weight (L) was the difference of the weights of a vial with and without lipid. The total lipid con- tent was calculated as described previously (Huang., 2014).

whereLwas the difference of the weights of vial with and without lipid.
Lipid yield was calculated as follows:
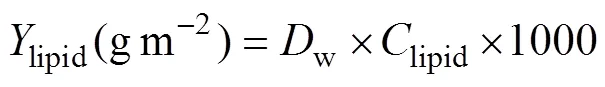
wherewis the biomass, andlipidis the lipid content of dry weight.
3 Results and Discussion
3.1 Preliminary Evaluation of Attached Cultivation of T. minus
Prior to evaluating the effect of light intensity and CO2concentration on attached cultivation of, com- pressed air enriched with 1.5% CO2(v/v) and 100μmol photonsm−2s−1of artificial light were firstly provided to culture the. Microalgal cells were harvested every 3 days, and the biomass and lipid content were measured and calculated as described above. As illustrated in Fig.3A, biomass ofincreased continuously and reached over 160gm−2after 18-day-cultivation when about10gm−2cells were selected as the initial inoculum density. It can also be found that the biomass productivity, both at the third day and ninth day, increased to the maximum of 12.2 and 11.4gm−2d−1, respectively, then the biomass pro- ductivity declined. As shown in Fig.3B, the total lipid content ofincreased from 22.3% of dry weight to 35.7% within 18 days, and the lipid productivity based on the biomass was growing well during the cultivation. Based on the findings, it can be found that,cells could grow well with the attached cultivation and used for the remaining experiments.
3.2 The Influence of Light Intensity and CO2 Con-centration on Attached Cultivation of T. minus
The biomass and lipid content at different light intensi- ties and CO2concentrations were shown in Fig.3. Light intensity is reported to significantly affect the growth of microalgal cells (Bezerra., 2012). According to Fig.3C, the biomass increased as the light intensity was increased from 50μmolphotonsm−2s−1to higher intensities. The maximum biomass density of 223gm−2was achieved at the light intensity of 200μmol photonsm−2s−1, and then decreased when light intensity increases. It was also wor- thy to mention that the light intensity of attachedwas higher than that of other microalgal species in- cluding(150μmolphotonsm−2s−1) (Ji., 2015),sp(100μmolphotonsm−2s−1) (Ji., 2014), indicating thatmight be more resistant to strong irradiation. However, different from biomass density, lipid content in cells did not show a significant change with respect to different light intensi- ties. The lipid contents ofat tested light intensity maintained at 28.4%–33.7% of dry weight. According to the biomass density and lipid content, lipid yield ofwas calculated. From 50μmolphotonsm−2s−1to the higher, the lipid yield was increased continuously till 72.3gm−2at 200μmol photonsm−2s−1and then decreased.
Ji. (2017) found that the CO2concentration, other than aeration rate or medium flow rate, dominates the bio- mass accumulation of microalgal biofilm. Hence, the op- timization of CO2concentration was carried out in this ex- periment. With ambient air (0.03% of CO2), attached cul- tivation ofshowed the least biomass production, as the final biomass density within 12 days increased from 10 to 64gm−2while the lipid productivity kept almost constant (4.5gm−2d−1). With the supplement of 5% and 10% CO2, biomass growth increased dramatically to 153 and 162gm−2, respectively at day 12. However, when the CO2concentration increased to 20%, the biomass density reduced dramatically. For attached cultivation, the aqua layer between the microalgal cells and ambient gas phase is greatly reduced to a thin liquid film. So the transporta- tion of CO2from gas bulk to the algal cells and the O2release from cells to gas bulk are much easier (Wang.,2017). However, an inappropriate aeration condition with too high content of CO2may faintly acidify the contact region between the wet biofilm and the gaseous phase, which results in inhibiting the growth of algal cells (Lang- ley., 2012). Fig.3E also demonstrated the CO2con- centration did not affect the lipid content of attachedbiofilm significantly. However, because of the bio- mass continuously increase, the CO2concentration caused a dramatical change on the lipid yield of. Thus, according to the results, 200μmolphotonsm−2s−1and 5% CO2were optimal conditions forgrowth in attach- ed biofilm.

Fig.3 Thin-layer attached cultivation of T. minus. (A) Biomass and biomass productivity of T. minus under with 1.5% CO2 (v/v) and 100μmolphotonsm−2s−1 of artificial light. (B) Lipid content and productivity of T. minus with 1.5% CO2 (v/v) and 100μmolphotonsm−2s−1 of artificial light. (C) Effect of light intensity on biomass accumulation and lipid content of T. minus at day 6. (D) Effect of light intensity on lipid yield of T. minus at day 6. (E) Effect of CO2 concentration on biomass accumulation and lipid content of T. minus at day 6. (F) Effect of light intensity on lipid yield of T. minus at day 6.
3.3 Characteristics of Substratum Materials in Attached Cultivation of T. minus
The filamentous microalgae were much easier to well attach onto the substratum surface than the unicellular strains (Wang., 2017). Fourteen different substratum materials were tested in the attached bioreactor to culturecells in this study (Fig.4 and Table 1), and the sur- faces of these materials were covered with micro-level pores or fluffy. Well attachment of microalgal cells was es- sential for substratum materials evaluation. Therefore, the tested substratum materials were placed on the glass plate vertically and the medium could drop down from pipes on the top of the towel and flow across the surfaces (Fig.5). During the cultivation, we observed if the microalgal cells were easily washed off from the substratum materials with the flow of the culture medium and we also calculated the biomass productivities of cells on different substratum ma- terials.

Fig.4 The different substratum materials used in attached culture of T. minus.
After the substratum materials covered with cells were placed onto the surface of towel, we found that microal- gal cells are easily washed off from substratum materials #3, #4 and #9 (attachment as ‘–’) when the medium dripped down even with very slow flow rate. Hence, the three kinds of substratum materials could not be used in attached cultivation ofThe attachmene of cells on other materials, especially #1, #2, #6, #7 #11 and #13, showed very good status. From Table 1, it suggested that, flocking cloth, cotton and polyethylene film with shorter fibers are the best materials for attachment culture ofDifferent from the results of Gross. (2016), the nylon and polypropylene mesh with mm openings are the best for initial attachment. The attachment of micro- algae on substratum is influenced by many factors include- ing the algal properties (, cell size, surface charge, geo-metric structure, secretion,.), substratum properties (,surface charge, hydrophile, surface structure,.), medium composition and the hydrodynamic condition (Naumann., 2013;Zheng., 2016). The difference between this study and Gross. (2016) might be caused by the size and structure of cells between filamentous microalgae and unicellular ones.
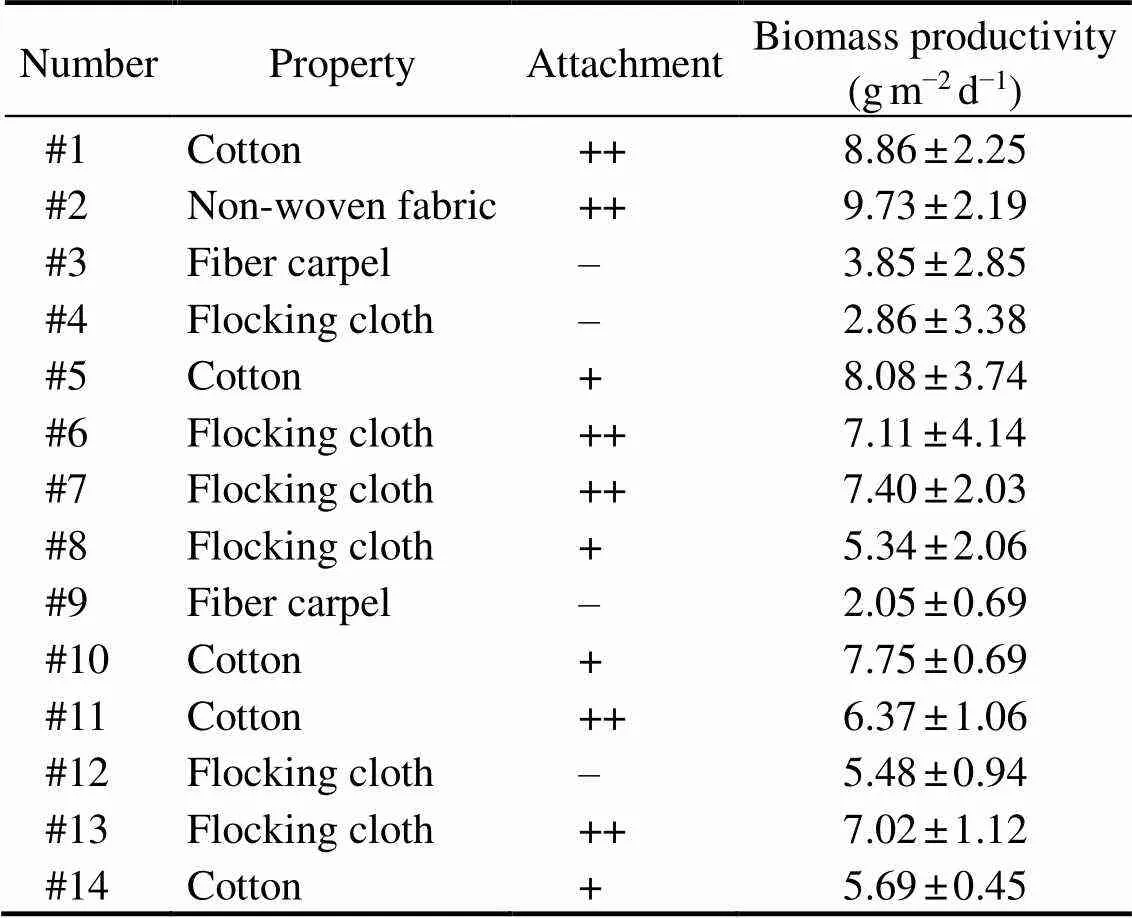
Table 1 Evaluation of substratum materials in attached culture of T. minus
The average biomass productivity ofwas also calculated and listed in Table 1. It can be found that there were significant changes in the biomass productivity ofon different substratum materials. The properties of substrate, in particular the hydrophilicity/hydrophobicity ratio and the surface structure of substrate, play important roles on the growth of algae attached biofilm (Singh, 2011;Cui., 2013). The least lipid productivity (2.05gm−2d−1) was got when the cells were attached on substratum #9. However, the three maximum lipid productivities ofwere got on #1, #2 and #5 substratum materials, which were 8.86, 9.73 and 9.08gm−2d−1, respectively. Overall, from the attachment performance and growth rates of cells, material #2 with non-woven fabric was the most ideal substratum for the attached cultivation of.
3.4 Growth of T. minus on Rotary Drum and Rotation Discs Systems
Based on the optimal conditions, the cultivation ofin two different structures of attached bioreactors, the rotary drum and the rotation discs, were carried out, and the biomass and the productivities were measured. In order to fully understand the light dilution strategy on the biomass accumulation of the microalgal cells, 8 and 16 pieces of glasses were installed in the rotary drums, re- spectively, forming different irradiation frequencies on the cells. The biomass productivity ofon the rotary drum is shown in Fig.6. Considering the differences of the amount of the harvested algal pastes and the amount of retained algal cells after manual harvesting, which may influence the next 3 days’ growth, there were little varia- tions of the biomass productivity among different har- vesting times. After calculation, the biomass productivity per unit area of the substrate on the 8 plates and 16 plates were 4.7–6.1 and 3.2–3.7gm−2d−1, respectively. Intermit-tently illumination the algal biofilm with different frequen- cies produce different irradiation and then with the same ration rate and different amounts of plates, the light absorption time of cells on the surfaces of the substratum based on 8 plates was higher than that on 16 plates. There- fore, the biomass productivity based on unit area of the substrate recorded on 8 plates was higher than that on 16 plates.
Fig.5 The growth of T. minus on different substratum materials. (A) The growth of T. minus after 1 day. (B) The growth of T. minus after 3 days. The number in the figure corresponds to the number of the material in Fig.4.
However, the culture area recorded on 16 plates was much larger than that recorded on 8 plates. Hence, accord- ing to the effective calculation, the final biomass produc- tivities based on the footprint area of rotary drum ranged from 21.3 to 27.1gm−2d−1and from 28.3 to 33gm−2d−1, respectively, recorded on the 8 plates and 16 plates. With the multiple increases of the culture area, the total bio- mass productivity based on the footprint area also raised; however, the increment was less than twice. Hence, we speculated that the biomass productivity can reach the highest and then the value will start to fall, rather than increase with culture area. The value obtained from pres- ently attached cultivation was much higher than the high- est biomass (6.87gL−1)that is from suspended cultivation of(Wang., 2016).

Fig.6 Biomass productivity of T. minus attached on rotary drum bioreactor. (A) Biomass productivity on substratum surface. (B) Areal productivity of footprint.
For rotation disc systems, as illustrated in Fig.7A, the biomass productivity per unit area of the substrate on the 5 discs and 10 discs ranged from 17.6 to 19.8gm−2d−1and from 10.7 to 12.4gm−2d−1, respectively. Furthermore, due to the different culture areas, the biomass productivities based on the footprint area ranged from 38.5 to 43.4gm−2d−1and from 44.3 to 50.3gm−2d−1, respectively. These bio- mass productivities were similar to some other microalgal studies, including the culture ofsp., where the biomass productivity reached about 45gm−2d−1 in a 2 days’ culture(Shen., 2018).

Fig.7 Biomass productivity of T. minus attached on rota- tion disc system. (A) Biomass productivity on substratum surface. (B) Areal productivity of footprint.
In the attached culture ofin the rotation disc system, a larger culture area made higher biomass pro- ductivity of footprint area, which is consistent with the condition of the rotary drum. However, the design of ro- tation disc systems is different from rotary drum. The ro- tary drum is designed by intermittently illuminating the biofilm to produce time-averaged irradiation, whereas the design principle of the rotation disc system is to scatter the strong light by putting the algal biofilm in vertical mul- tiple planar arrays. The biomass productivity ofin rotation disc system was higher than that in the rotary drum. It indicated that, in the same light intensity condi- tion, the light scattering strategy was much more effective than intermittently illuminating for the growth of microal- gal cells. With the increment of the light intensity or/and the amounts of plates, the tendency of the biomass pro- ductivity in two attached bioreactors might change, and the further study will be carried out outdoor. Overall, the biomass productivities ofcultured in two attach- ed bioreactors designed with different light dilution strate- gies were both higher than 6.87gL−1 in suspended culti- vation bioreactors in previous studies(Wang., 2016). It can be concluded that the attached cultivation was suit- able for the cultivation of filamentous microalgae.
4 Conclusions
The attached cultivation technique of the filamentous oleaginous microalgawas investigated in this study. The fundamental conditions including light inten- sity and CO2concentration were first optimized with a thin layer attached bioreactor. And after 12 days of culti- vation, the biomass density of 223gm−2and 153gm−2were achieved at the light intensity of 200μmolphotonsm−2s−1and 5% CO2, respectively. Moreover, this filamen- tous species showed well attachment on many substratum surfaces and finally, the optimum substratum material was screened. Material #2 with non-woven fabric was regard- ed as the ideal substratum in the attached cultivation ofbased on the attachment performance and growth rates of cells, and finally the maximum lipid productivity, 9.73gm−2d−1, was achieved with material #2. Two attach- ed bioreactor systems (rotary drum and rotation disc) were designed following the light dilution strategy and intro- duced into the cultivation ofThe final biomass productivity based on the footprint area of rotary drum ranged from 21.3 to 27.1gm−2d−1and from 28.3 to 33gm−2d−1, which was recorded on the 8 plates and 16 plates. For rotation disc systems, the biomass productivities based on the footprint area ranged from 38.5 to 3.4gm−2d−1and from 44.3 to 50.3gm−2d−1, respectively, which was re- corded on the 5 discs and 10 discs. Both average biomass productivities were much higher than those reported in suspended cultivation. The results demonstrated thatis a good candidate for the attached cultivation of fil- amentous microalgaeis feasible and promising.
Acknowledgements
This work was supported by the Strategic Priority Re- search Program of Chinese Academy of Sciences (Trans- formational Technologies for Clean Energy and Demon- stration (No. XDA21010211), the Shandong Provincial Natural Science Foundation (No. ZR2017QC007), and the Youth Innovation Promotion Association, CAS.
Barlow, J., Sims, R. C., and Quinn, J. C., 2016. Techno-economic and life-cycle assessment of an attached growth algal biore- finery., 220: 360-368.
Bezerra, R. P., Matsudo, M. C., Sato, S., Perego, P., Converti, A., and Carvalho, J. C. M. D., 2012. Effects of photobioreactor configuration, nitrogen source and light intensity on the fed-batch cultivation of(). Bio- energetic aspects., 37: 309-317.
Brennan, L., and Owende, P., 2010. Biofuels from microalgae– A review of technologies for production, processing, and ex- tractions of biofuels and co-products., 14 (2): 557-577.
Chen, L., Liu, T., Zhang, W., Chen, X., and Wang, J., 2012. Bio- diesel production from algae oil high in free fatty acids by two-step catalytic conversion., 111: 208-214.
Cui, Y., and Yuan, W., 2013. Thermodynamic modeling of algal cell-solid substrate interactions., 112: 485-492.
Ernst, A., 1991. Cyanobacterial picoplankton from Lake Cons- tance. I. Isolation by fluorescence characteristics., 13(6): 1307-1312.
Gross, M., and Wen, Z., 2014. Yearlong evaluation of perfor- mance and durability of a pilot-scale Revolving Algal Biofilm (RAB) cultivation system., 171: 50-58.
Gross, M., Zhao, X., Mascarenhas, V., and Wen, Z., 2016. Effectsof the surface physico-chemical properties and the surface textures on the initial colonization and the attached growth in algal biofilm., 9: 38-38.
Heimann, K., 2016. Novel approaches to microalgal and cyano- bacterial cultivation for bioenergy and biofuel production., 38: 183-189.
Huang, L., Xu, J., Li, T., Wang, L., Deng, T., and Yu, X., 2014. Effects of additional Mg2+on the growth, lipid production, and fatty acid composition ofsp. FXY-10 under different culture conditions., 64(3): 1247-1256.
Ji, B., Zhang, W., Zhang, N., Wang, J., Lutzu, G. A., and Liu, T., 2014. Biofilm cultivation of the oleaginous microalgaesp., 37(7): 1369-1375.
Ji, C., Wang, J., and Liu, T., 2015. Aeration strategy for biofilm cultivation of the microalga., 37(10): 1953-1958.
Ji, C., Wang, J., Li, R., and Liu, T., 2017. Modeling of carbon dioxide mass transfer behavior in attached cultivation pho- tobioreactor using the analysis of the pH profiles., 40(7): 1079-1090.
Kiperstok, A. C., Sebestyén, P., Podola, B., and Melkonian, M., 2017. Biofilm cultivation ofenables a highly productive one-phase process for astaxanthin pro- duction using high light intensities., 21: 213-222.
Langley, N. M., Harrison, S. T. L., and van Hille, R. P., 2012. A critical evaluation of CO2supplementation to algal systems by direct injection., 68: 70-75.
Liu, T., Wang, J., Hu, Q., Cheng, P., Ji, B., Liu, J., Chen, Y., Zhang, W., Chen, X., Chen, L., Gao, L., Ji, C., and Wang, H., 2013. Attached cultivation technology of microalgae for ef- ficient biomass feedstock production., 127: 216-222.
Mohd-Sahib, A. A., Lim, J. W., Lam, M. K., Uemura, Y., Ho, C.D., Oh, W. D., and Tan, W. N., 2018. Mechanistic kinetic mo- dels describing impact of early attachment betweenand polyurethane foam material in fluidized bed bio- reactor on lipid for biodiesel production., 33:209-217.
Naumann, T., Çebi, Z., Podola, B., and Melkonian, M., 2013. Growing microalgae as aquaculture feeds on twin-layers: A novel solid-state photobioreactor., 25(5): 1413-1420.
Ooms, M. D., Dinh, C. T., Sargent, E. H., and Sinton, D., 2016. Photon management for augmented photosynthesis., 7: 12699-12699.
Schnurr, P. J., and Allen, D. G., 2015. Factors affecting algae biofilm growth and lipid production: A review., 52: 418-429.
Schnurr, P. J., Espie, G. S., and Allen, D. G., 2013. Algae biofilm growth and the potential to stimulate lipid accumulation through nutrient starvation., 136: 337-344.
Shen, Y., Wang, S., Ho, S. H., Xie, Y., and Chen, J., 2018. En- hancing lipid production in attached culture of a thermoto- lerant microalgasp. F51 using light-related strate- gies., 129: 119-128.
Singh, S., 2011. Methodology for membrane fabric selection for pilot-bioreactor. Master thesis. Ohio University, Athens.
Toninelli, A. E., Wang, J., Liu, M., Wu, H., and Liu, T., 2016. Scenedesmus dimorphus biofilm: Photoefficiency and biomass production under intermittent lighting., 6: 32305.
Wang, H., Gao, L., Chen, L., Guo, F., and Liu, T., 2013. Integra- tion process of biodiesel production from filamentous oleagi- nous microalgae., 142: 39-44.
Wang, H., Gao, L., Zhou, W., and Liu, T., 2016. Growth and pal- mitoleic acid accumulation of filamentous oleaginous micro- algaeat varying temperatures and light re- gimes., 39(10): 1589-1595.
Wang, H., Ji, B., Wang, J., Guo, F., Zhou, W., Gao, L., and Liu, T. Z., 2014. Growth and biochemical composition of filamen- tous microalgaesp. as potential biofuel feedstock., 37(12): 2607-2613.
Wang, J., Liu, W., and Liu, T., 2017. Biofilm based attached cul- tivation technology for microalgal biorefineries–A review., 244: 1245-1253.
Zhang, L., Chen, L., Wang, J., Chen, Y., Gao, X., Zhang, Z.,and Liu, T., 2015. Attached cultivation for improving the biomass productivity of., 181: 136-142.
Zheng, Y., Huang, Y., Liao, Q., Zhu, X., Fu, Q., and Xia, A., 2016. Effects of wettability on the growth ofbiofilm attached on glass surface coated with polytetrafluo- roethylene emulsion.,41 (46): 21728-21735.
. E-mail: wanghui@qibebt.ac.cn
June 12, 2019;
October 8, 2019;
November 27, 2019
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Diffusion Characteristics of Swells in the North Indian Ocean
- Analysis of the Dynamic System of Wave Glider with a Towed Body
- Provenance and Tectonic Implications of Paleozoic Strata in the South Yellow Sea Basin, China–Revealed from the Borehole CSDP-2
- Ecological Risk of Heavy Metals in Sediment Around Techeng Island Special Marine Reserves in Zhanjiang Bay
- Geochemical and Grain-Sized Implications for Provenance Variations of the Central Yellow Sea Muddy Area Since the Middle Holocene
- Grain-Size Distribution of Surface Sediments in the Bohai Sea and the Northern Yellow Sea: Sediment Supply and Hydrodynamics
