The Metabolism of Methylene Blue and Its Derivatives in Japanese Eel (Anguilla Japonica)
2020-09-28WANGYaliHUANGXuanyunDUBingtanLVXinmeiSUNJingZHENGRuizhouandHUKun
WANG Yali, HUANG Xuanyun, DU Bingtan, LV Xinmei,SUN Jing, ZHENG Ruizhou, and HU Kun, *
The Metabolism of Methylene Blue and Its Derivatives in Japanese Eel ()
WANG Yali1), 2), 3), #, HUANG Xuanyun4), #, DU Bingtan1), 2), 3), LV Xinmei1), 2), 3),SUN Jing1), 2), 3), ZHENG Ruizhou1), 2), 3), and HU Kun1), 2), 3), *
1),201306,2),,201306,3),201306,4),,200090,
Methylene blue (MB) is commonly used in aquaculture as a fungicide and antidotes. This study was designed to explore the pharmacokinetics of MB in Japanese eel () immersed in 10mgL−1and 20mgL−1MB for 0.5h. The concentrations of MB and its derivatives in the blood, liver, kidney, skin and muscle were determined by HPLC after immersion.The results showed that the changes of drug concentrations in Japanese eel were basically the same in different dose groups, showing a general trend of increasing at first and then decreasing, but the peak time was slightly different.The peak concentration of the drug was positively correlated with the dose level. The peak concentrations of MB in MB (20mgL−1) group, MB, azure A and azure B in the tissues of Japanese eel were significantly higher than those in MB (10mgL−1) group.Moreover, MB, azure A and azure B remained for a long time and could still be detected at 64 days, and azure C was not detected in tissues.
methylene blue;; thiazine dye;aquaculture
1 Introduction
Eels are the important warm-water fish species, and their main breeding areas are in several European countries in- cluding as Italy, Spain, Germany, Denmark and the Netherlands and Southeast Asian countries including Japan, Ma- laysia, and China (Lee, 2003; Lv, 2018). Japanese eel () is an important commercially cultured eel species in China (Cao, 2011). There is a complex life cycle in eels, and they can be affected by diseases at any period of their life cycle, especially bacterial diseases. For decades, outbreaks of infectious diseases have led to declines in eel production and quality (Dezful, 2009; Guan., 2011; Joh., 2011). Currently, farmers treat the cultured fish with antibiotics such as oxytetracycline and florfenicol. However, the cost of antibiotics is too high for farmers in many underdeveloped and developing countries. In addition, the use of antibiotics may be harmful to the environment and human health; drug resistance may develop in fish, and the antibiotics may be transferred to other aquatic organisms (Harikrishnan, 2010; Lee, 2018).
Methylene blue is a tricyclic phenothiazine dye (Wain- wright and Amaral, 2005). Its derivatives are azure B (AZB), azure A (AZA) and azure C (AZC) (Munns., 1992; Atamna., 1996). In aquaculture, MB is used as a dis- infectant to prevent and treat saprolegniasis and parasitic diseases (Reyns., 2014). The antifungal effect of MB allows it to reduce fish mortality during long-distance transport (Kim., 2014). MB can also be used as an antidote to nitrites in aquaculture. However, MB and its derivatives can cause toxic side effects in animals. The United States doesn’t allow MB to be used in food fish farming, and the maximum allowable residue limit in Japan is 10μgkg−1(Meissner., 2005). The pharmacokinetics of MB has been mainly studied in mammals such as rats, sheep and humans (Peter., 2000; Pruthi., 2011). In aquaculture, most studies have focused on the detection of MB residues in aquatic products (Xu., 2012). There is no regulation regarding the limits for MB residues in China. Since a high concentration of MB can lead to animal poisoning and death, and reports on the metabolic regularity of MB in aquatic animal tissues are rare, studies on the elimination of MB and its derivative in animals are highly needed.
Japanese eels () were used in the study to investigate the metabolism of MB residues to provide the theoretical basis for strengthening the management and application of the drug.
2 Materials and Methods
2.1 Experimental Animal
Healthy Japanese eel individuals with an average body weight of 50g±5.2g were purchased from Tian-fa Fish Factory, Guangzhou, Guangdong Province. The fish were divided into two groups, approximately 250 each. They were placed in an aquarium (1m×0.8m×0.5m) and fill- ed with fully aerated tap water. During the cycle feeding process, the water of aquarium was continuously aerated and filtered, and the residual bait was removed immediately. Every day, about one quarter of the water in aquarium was replaced with fresh water. The animals were fed at 6:00 AM and 6:00 PM, and the feed is 2%–5% of the weight of Japanese eel. Before the experiment, the two groups of fish were acclimated to the breeding environment for 21d.
2.2 Chemicals and Reagents
The following chemicals and reagents were used: me- thylene blue (MB), azure A, azure B, and azure C stan- dards (Sigma-Aldrich, USA); acetonitrile (chromatogra- phic purity); hydroxylamine hydrochloride, p-toluenesul- fonic acid, ammonium acetate, n-hexane, sodium chloride, dichloromethane (analytically pure); microporous mem- branes (0.22μm; Pall, Whatman, USA); and ultrapure water.
2.3 Drug Management
The standard stock solution of methylene blue (100μgmL−1): 10.0000mg±0.0001mg of MB standard was dissolved in acetonitrile, then diluted to 100mL in a volumetric flask, and finally stored at −18℃ in a refrigerator.
P-toluene sulfonic acid solution (0.5molL−1): 0.475g±0.001g p-toluene sulfonic acid was diluted to 500-mL volumetric by distilled water.
Ammonium acetate buffer (0.1molL−1): 3.855g±0.001gammonium acetate was dissolved in a 500-mL volumetric flask, and the acidity of the solution was adjusted to pH 4.5 with sodium hydroxide solution (0.1molL−1).
2.4 Single Administration of Methylene Blue and Sample Collection
The two groups of Japanese eels were stopped feeding 48 hours before the experiment, and 8 fish were randomly selected from each group as blank controls. The first group was immersed in 10mgmL−1of MB for 30min, and the second group was immersed with 20mgmL−1of MB for 30 min. After immersing, replace the water in the tank and replace it once a day. The temperature was maintained to vary between 22℃ and 28℃, and pH was main- tained to be approximately 7.0. After the MB was placed, three fish were taken from each group at different time points (as shown in Tables 1 and 2), blood, muscle, liver, skin and kidney of Japanese eels were collected. Blood was added to a centrifuge tube containing an anticoagulant (2mg ammonium oxalate) and blended until homo- genous. After 5min of Centrifugation (1000rmin−1, 20℃), transfer supernatant to 2mL centrifuge tube and freeze. Other samples were stored at −80℃.
2.5 Sample Pretreatment
Blood (1mL) was placed in a 15mL centrifuge tube and p-toluenesulfonic acid-ammonium acetate buffer (3mL) was added. The samples were vortex-mixed for 30min. Then,8mL acetonitrile was added to the tissue and the samples were vortex-mixed for 3min. The tubes were centrifuged for 5min at 3500rmin−1. The supernatant was poured into another 50mL centrifuge tube. Residual material was vor- tex-mixed with another 4mL acetonitrile for 1min follow- ed by centrifugation for 5min at 3500rmin−1. Combined supernatants were vortex-mixed with 10mL supersaturated N-hexane 3 min followed by centrifugation for 5min at 3500rmin−1. The lower organic layer was collected and was added 20% diethylene glycol (2mL) and dichlorome- thane (10mL). The tubes were centrifuged for 5min at 3500rmin−1. Then, the lower layer was evaporated to dry-ness, was constant volume to 1.5mL, and filtered througha 0.45mm.
After grinding the muscle, liver, skin and kidney, each sample is weighed at 5g and placed in a 50mL centrifuge tube. 10% hydroxylammonium chloride (4mL) and p-tolue- nesulfonic acid-ammonium acetate buffer (4mL) were added into tube. The samples were vortex-mixed for 30min. Then, 20mL acetonitrile was added to the tissue and the samples were vortex-mixed for 3min. The tubes were centrifuged for 5min at 3500rmin−1. The supernatant was poured into another 50mL centrifuge tube. Residual ma- terial was vortex-mixed with another 10mL acetonitrile for 1min followed by centrifugation for 5min at 3500rmin−1. Combined supernatants were vortex-mixed with 25mL supersaturated N-hexane 3 min followed by centrifugation for 5min at 3500rmin−1. The lower organic layer was collected and was added sodium chloride solution (200gL−1, v/v) and dichloromethane (10mL). The tubes were centrifuged for 5min at 3500rmin−1. Then, the lower layer was evaporated to dryness, was constant volume to 1.5mL, and filtered through a 0.45mm.
2.6 Chromatographic Condition
Chromatographic analysis was performed using the Agi- lent 1100 HPLC system (Agilent Technologies, USA) coupled with a fluorescence detector (detection wavelength 650nm). Separation was performed on an XDB-C18 co- lumn (4.6mm×150mm internal diameter, 5-μm particle size; Agilent Technologies) using the mobile phase (acetonitrile: ammonium acetate, 0.5molL−1; 40:60, v/v) at a flow rate of 1.0mLmin−1. The injection volume was 50μL and the column temperature was maintained at 35℃.
2.7 Pharmacokinetic Analysis
Pharmacokinetic analysis was performed using DAS 3.0 program. The following pharmacokinetic parameters were determined by classical compartmental analysis of the plas- ma and tissue concentration-time data:max(peak concentration),1/2α(half-life of the absorption rate constant),1/2β(half-life of the elimination rate constant), AUC(0–t)(area under the concentration-time curve from time zero to), and/(total body clearance).
2.8 Statistical Analysis
Statistical analysis was performed using Microsoft Excel 2013 to describe the elimination of methylene blue, azure A, and azure B.
3 Results
3.1 Analytical Method Validation
The standard calibration curves for methylene blue and its derivatives were all linear in the range of 1–10000μgL−1, yielding a correlation coefficient of 0.998. The linear equations determined for MB, AZB, AZA, and AZC were=0.3736−0.4511,=0.0455−1.1833,=0.0442−0.1672, and=0.1124−2.3458, respectively. The recov- ery rates of MB, AZB, AZA and AZC were 80.8%84.7%, 82.3%86.2%, 80.4%85.4%, and 83.6%87.2%, respectively. The inter-day and intra-day coefficients of variation were below 10% and the detection limit was 1.0μgkg−1.
3.2 Elimination of Methylene Blue and Its Derivatives in Japanese Eel Tissues
After analysis, residues of MB and its derivatives were not found in eels in the control group. MB, azure A and azure B were detected in the blood, muscle, liver, skin and kidney of eels in both experimental groups. But azure C was not detected in eels in both experimental groups. Elimination of MB, azure A and azure B in eels is shown in Figs.1–3. The elimination of MB, azure A and azure B are long in all tissues and can be detected after 64h. Although the concentrations of MB were different in both groups, the rules of elimination were similar, except that the higher concentration of MB can take longer time to eliminate. According to Tables 1–3, the elimination half- life (1/2z) of the MB in the first group of eel tissues were 4.955h (Blood), 0.81h (Liver), 1.126h (Kidney), 1.22h (Skin), 1.026h (Muscle), respectively. MB is most rapidly eliminated in the liver. And azure A and azure B is also most rapidly eliminated in the liver in Tables 1–3. The same situation obtained for the second group and the first group. The results showed that the liver is the main me- tabolic organ of MB and its derivatives.
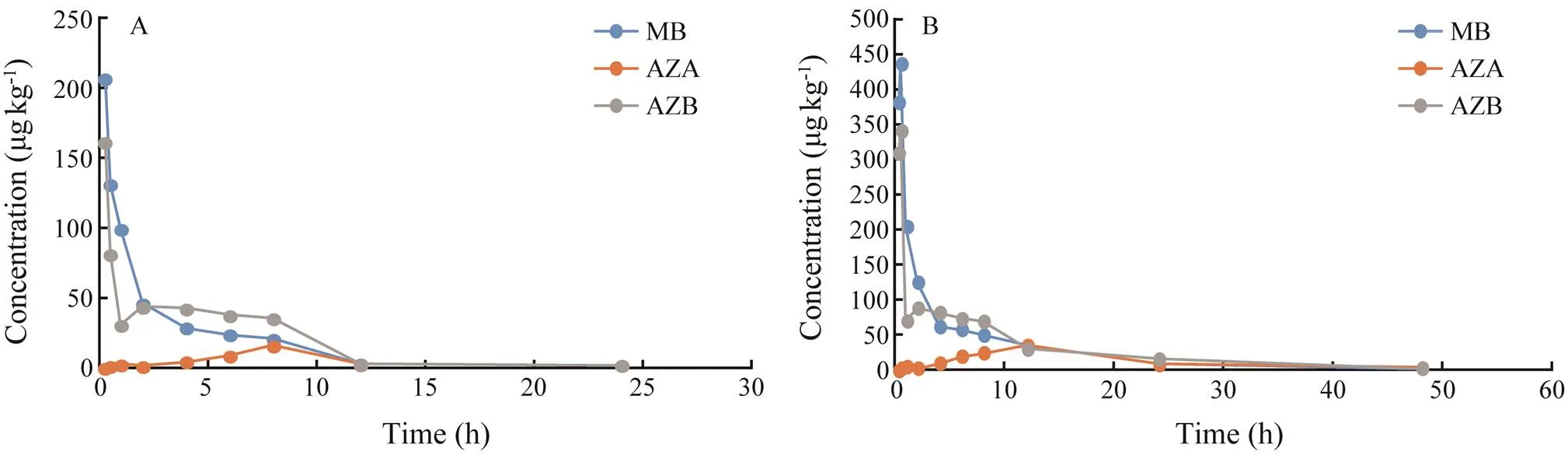
Fig.1 The metabolism of methylene blue and its derivatives in blood of Japanese eel. A, 10mgL−1 Group; B, 20mgL−1 Group.
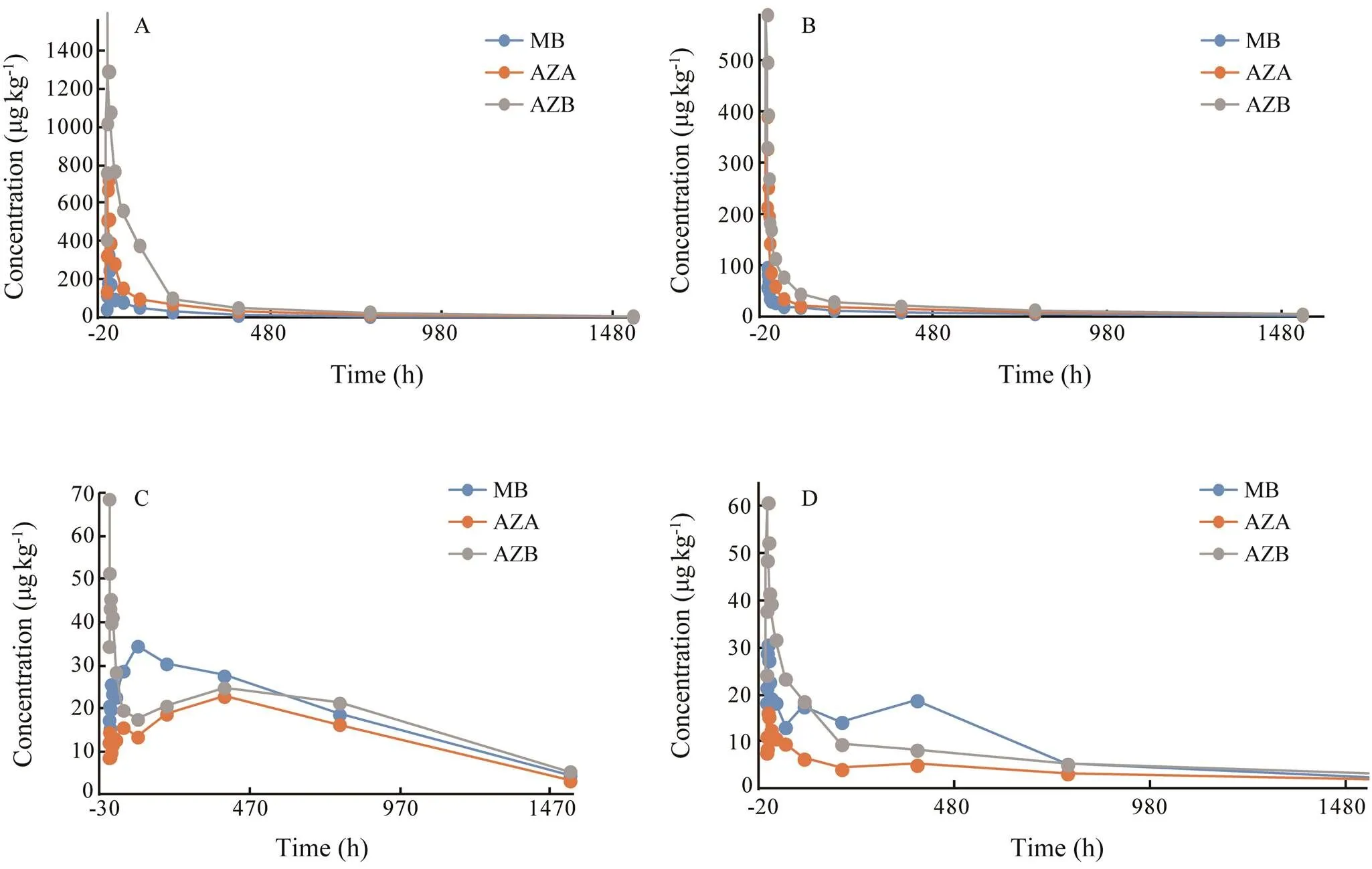
Fig.2 Elimination of methylene blue and its derivatives in liver (A), kidney (B), muscle (C), and skin (D) of Japanese eel after immersion in 10mgL−1 methylene blue.
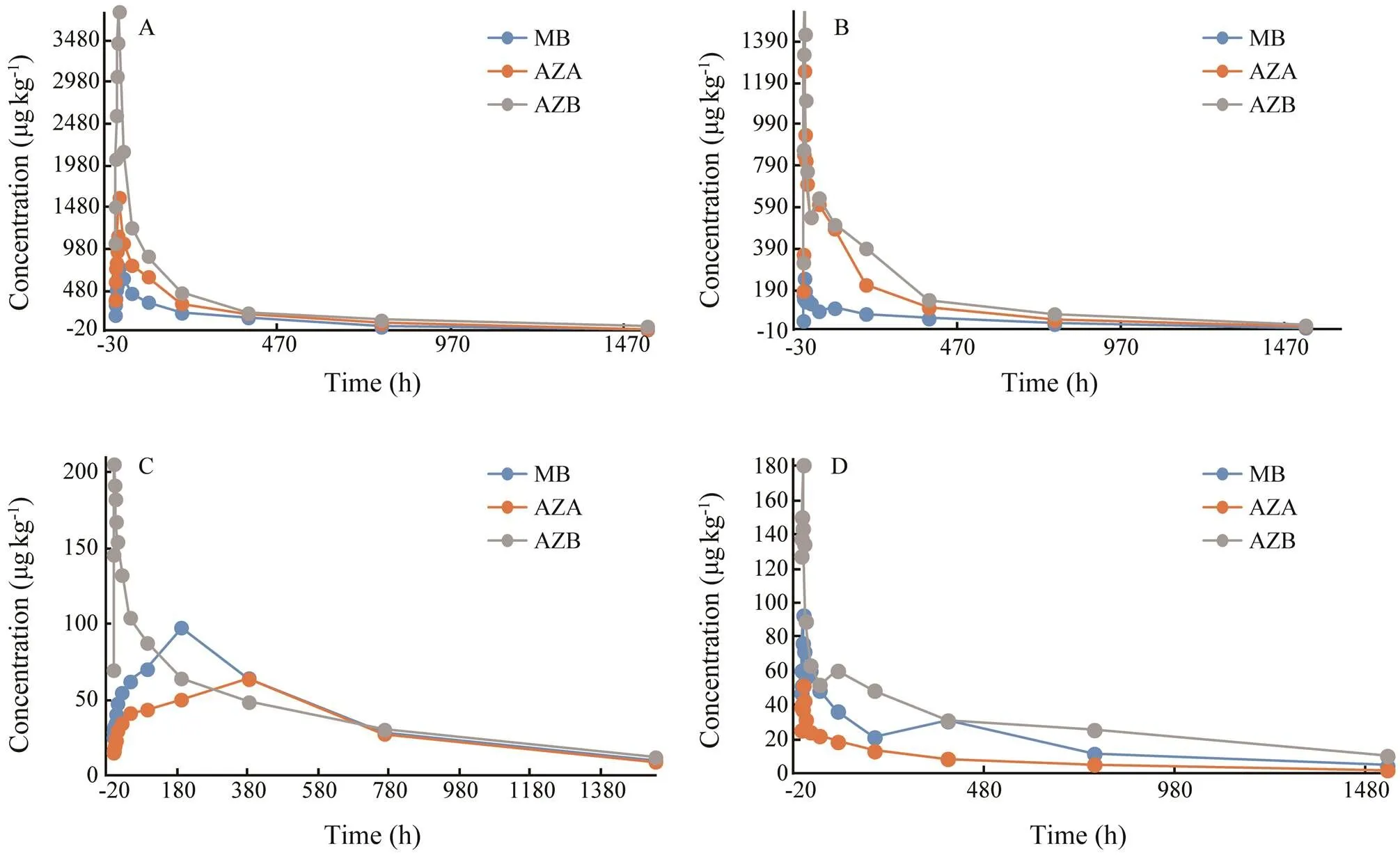
Fig.3 Elimination of MB and its derivatives in liver (A), kidney (B), muscle (C), and skin (D) of Japanese eel after immersion with 20mgL−1 methylene blue.
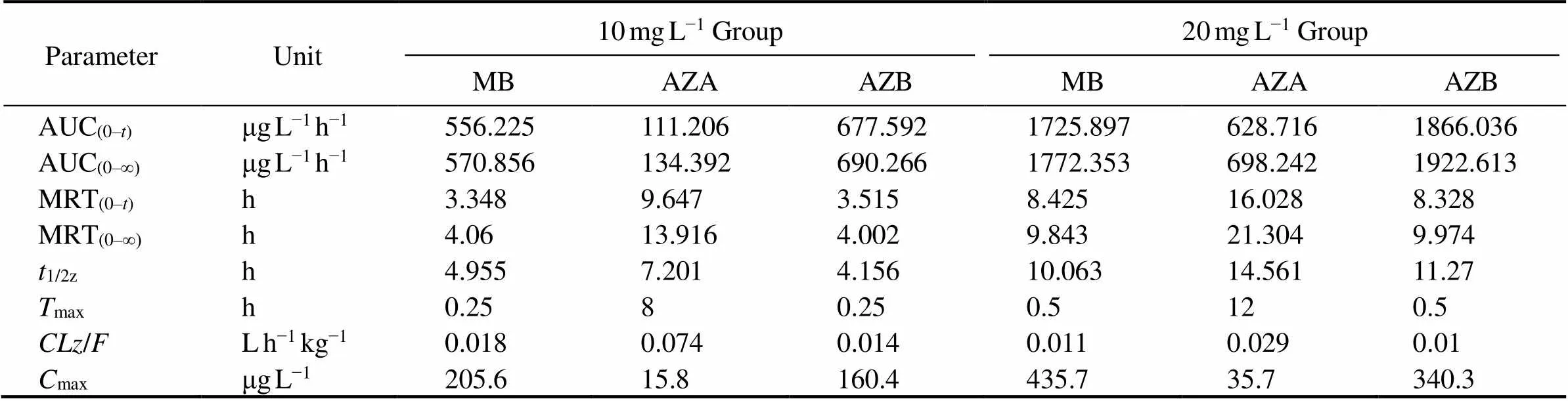
Table 1 Pharmacokinetic parameters of methylene blue and its derivatives in blood of Japanese eel
Notes: AUC(0–t)and AUC(0–∞)are areas under the concentration-time curve; MRT(0–t)and MRT(0–∞)are mean residue times;1/2zis the elimination half-life of the drug;maxis the time of maximum concentration;/is the overall clearance rate; andmaxis maximum concentration in tissues.
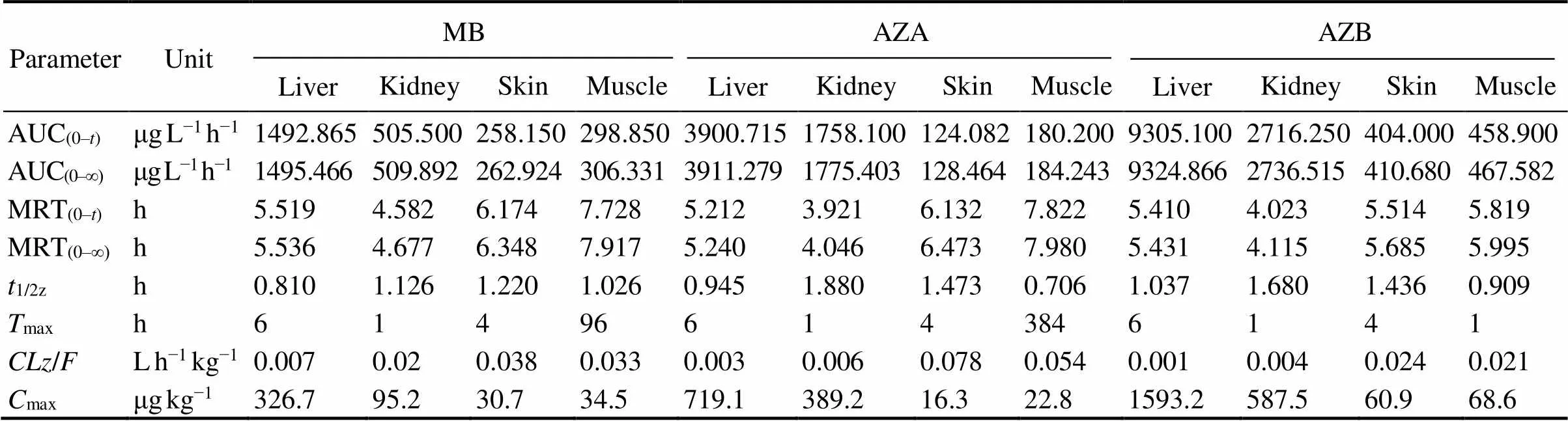
Table 2 Pharmacokinetic parameters of methylene blue and its derivatives in liver, kidney, skin, and muscle of Japanese eel after immersion in 10mgL−1 methylene blue
Notes: The definitions of parameters were the same as those of Table 1.
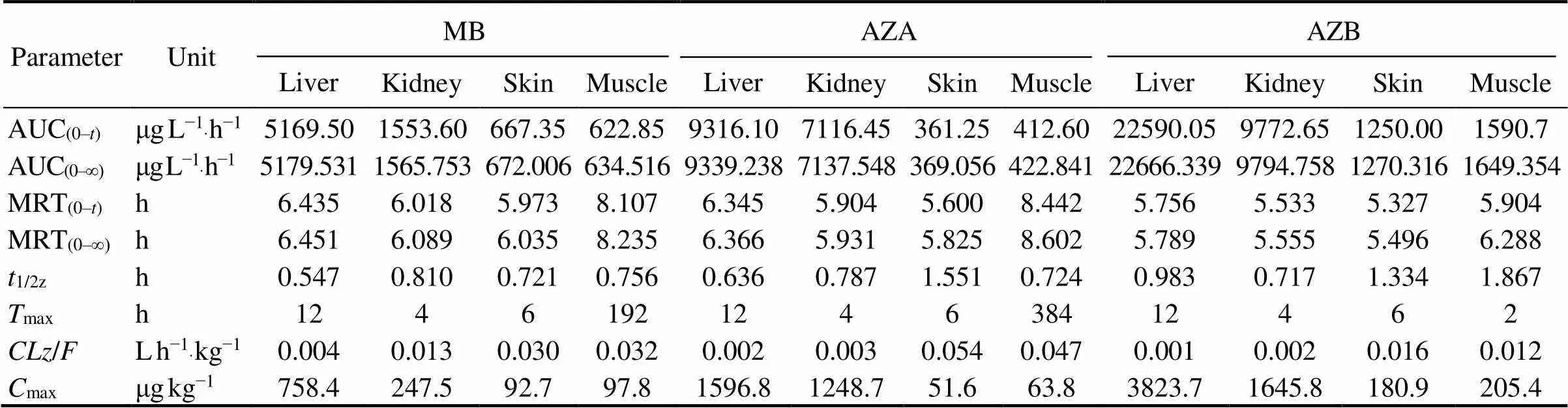
Table 3 Pharmacokinetic parameters of methylene blue and its derivatives in liver, kidney, skin, and muscle of Japanese eel after immersion in 20mgL−1 methylene blue
Notes: The definitions of all parameters are the same as those of Table 1.
3.3 Transformation of Methylene Blue into Azure A and Azure B in Japanese Eel Blood
According to Tables 1–3, MB and azure B had the shortest time (max) of maximum concentration in eel blood in both experimental groups. The peak concentration of azure C appeared for a long time, and the level of azure C is low in blood. The result suggested that the transformation of MB into the azure B was rapid after it entered the blood by immersed, but the transformation of azure B into the azure A was long.
4 Discussion
The classic pharmacokinetics model is the atrioventri- cular model. Unfortunately, the atrioventricular model has some defects. It does not adequately consider some of the physiological factors involved in drug metabolism, and it is not applicable for all drug metabolism in body (Wang, 2011). Therefore, the parameters of methylene blue and its derivatives in the tissues of Japanese eel were ana- lyzed with non-compartment method in this experiment. This method does not need to consider the applicability of models. It uses the area under the drug-time curve as the main calculation basis to analyze the process of drug absorption, distribution, biotransformation and excretion (Guo, 2014). At present, the analysis of drug concentration in the organism is mainly based on non-atrioventri- cular model, and it is widely used in relevant drug eva- luation departments (Wang, 2011).
Peak time (max) and peak concentration (max) are im- portant pharmacokinetic parameters which reflect the ab- sorption rate of drugs in the organism. In this study, MB and azure B in two experimental groups had the fastest peak time in blood (0.25h and 0.5h), indicating that MB was absorbed mainly through blood and dispersed to other tissues, and MB in the blood can be quickly converted into demethylated product, azure B. It also suggested that the MB is simultaneously enriched, transformed and excreted in Japanese eel. In both groups, the peak time of azure A was longer, and all the peak concentrations of azure A were lower than those of azure B, indicating that the amount of MB transformed into azure A was less and the absorption was slower. The combined values of AUC and MRT indicated that both MB and azure B in Japanese eel are rapidly absorbed, and the degree of absorption is high thus takes more time to be eliminated.
5 Conclusions
Currently, the detection method of methylene blue in China is available only for its residues in edible tissues, and no limit of residues has been defined. Our findings showed that the azure B and azure A are easily remained in Japanese eel, their elimination times are long and their contents are high. The total contents of methylene blue and its derivatives should be used as the detection bases of residues in aquatic products.
Acknowledgements
This study was supported by the National Key R&D Program of China (No. 2019YFD0900102), the Science and Technology Commission of Shanghai Municipality (Nos. 17050502100 and 18391901500), the National Natural Re- sources Platform of China, and Knowledge Service Plat- form of Shanghai Ocean University.
Atamna, H., Krugliak, M., Shalmiev, G., Deharo, E., Pescarmona, G., and Ginsburg, H., 1996. Mode of antimalarial effect of methylene blue and some of its analogues onin culture and their inhibition ofand., 51 (5): 693-700.
Cao, H. P., He, S., Wei, R. P., Marek, D., and Lu, L. Q., 2011.G1: A potential antagonistic bacterium against eel-pathogenic., 8: 1-7.
Dezfuli, B. S., Szekely, C., Giovinazzo, G., Hills, K., and Giari, L., 2009. Inflammatory response to parasitic helminths in the digestive tract of., 296: 1-2.
Guan, R., Xiong, J., Huang, W., and Guo, S., 2011. Enhancement of protective immunity in European eel () againstandby a recombinantouter membrane protein., 43: 79-88.
Guo, T., 2014.. People’s Military Medical Publishing House, Beijing, 36-39 (in Chinese).
Harikrishnan, R., Balasundaram, C., and Heo, M., 2010. Effect of probiotics enriched diet oninfected with lymphocystis disease virus (LCDV)., 29: 868-874.
Joh, S. J., Kim, M. J., Kwon, H. M., Ahn, E. H., Jang, H., and Kwon, J. H., 2011. Characterization ofisolated from farmcultured eels,, in the Re- public of Korea., 73 (1): 7-11.
Kim, S. J., Ha, D. J., and Koo, T. S., 2014. Simultaneous quantification of methylene blue and its major metabolite, azure B, in plasma by LC-MS/MS and its application for a pharmacokinetic study., 28 (4): 518-524.
Lee, S. H., Lee, Y. K., Katya, K., Park, J. K., and Bai, S. C., 2018. Natural dietary additive yellow loess as potential antibiotic replacer in Japanese eel,: Effects on growth, immune responses, serological characteristics and disease resistance against., 24 (3): 1034-1040.
Lee, W. C., Chen, Y. H., Lee, Y. C., and Liao, C., 2003. The com- petitiveness of the eel aquaculture in Taiwan, Japan, and Chi- na., 221: 115-124.
Lv, X. M., Yang, X. L., and Xie, X. Y., 2018. Comparative transcriptome analysis oflivers following exposure to methylene blue., 49 (3): 1232- 1241.
Meissner, P. E., Mandi, G., Witte, S., Coulibaly, B., Mansmann, U., Rengelshausen, J., Schiek, W., Jahn, A., Sanon, M., and Tap- soba, T., 2005. Safety of the methylene blue plus chloroquine combination in the treatment of uncomplicated falciparum ma- laria in young children of Burkina Faso., 4:45.
Munns, R. K., Holland, D. C., Roybal, J. E., Meyer, J. G., Hurl- but, J. A., and Long, A. R., 1992. Liquid-chromatographic de- termination of methylene blue and its metabolites in milk., 75 (5): 796-800.
Peter, C., Hongwan, D., Kupfer, A., and Lauterburg, B. H., 2000. Pharmacokinetics and organ distribution of intravenous and oral methylene blue., 56 (3): 247-250.
Pruthi, S., Haakenson, C., Brost, B. C., Bryant, K., Reid, J. M., Singh, R., Netzel, B., Boughey, J. C., and Degnim, A. C., 2011. Phar-macokinetics of methylene blue dye for lymphatic mapping in breast cancer-implications for use in pregnancy., 201 (1): 70-75.
Reyns, T., Belpaire, C., Geeraerts, C., and Van Loco, J., 2014. Multi-dye residue analysis of triarylmethane, xanthene, phenothiazine and phenoxazine dyes in fish tissues by ultra-per- formance liquid chromatography-tandem mass spectrometry., 953: 92-101.
Wainwright, M., and Amaral, L., 2005. The phenothiazinium chromophore and the evolution of antimalarial drugs., 10: 501-511.
Wang, J. G., Wang, Y. T., and Chen, C. F., 2011.. China Agriculture Press, Beijing, 84- 85(in Chinese).
Xu, Y. J., Tian, X. H., and Zhang, X. Z., 2012. Simultaneous de- termination of malachite green, crystal violet, methylene blue and the metabolite residues in aquatic products by ultra-per- formance liquid chromatography with electrospray ionization Tandem Mass Spectrometry., 50 (7): 591-597.
# The two authors contributed equally to this work.
. E-mail: khu@shou.edu.cn
March 9, 2019;
May 23, 2019;
March 6, 2020
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Diffusion Characteristics of Swells in the North Indian Ocean
- Analysis of the Dynamic System of Wave Glider with a Towed Body
- Provenance and Tectonic Implications of Paleozoic Strata in the South Yellow Sea Basin, China–Revealed from the Borehole CSDP-2
- Ecological Risk of Heavy Metals in Sediment Around Techeng Island Special Marine Reserves in Zhanjiang Bay
- Geochemical and Grain-Sized Implications for Provenance Variations of the Central Yellow Sea Muddy Area Since the Middle Holocene
- Grain-Size Distribution of Surface Sediments in the Bohai Sea and the Northern Yellow Sea: Sediment Supply and Hydrodynamics
