二氧化铈表面构建固体“受阻”Lewis酸碱对用于小分子活化
2020-09-28张赛张铭凯瞿永泉
张赛,张铭凯,瞿永泉
西安交通大学化学工程与技术学院,前沿科学技术研究院,西安 710054
1 Introduction
Transition metals, especially for the versatility ofdblock metals, have been exclusively associated with the activation of small molecules. However, this phenomenon has been altered since the discovery of the frustrated Lewis pairs (FLP), the combinations of a sterically hindered Lewis acid and Lewis base that are prevented from forming a class Lewis acid-base adduct(Fig. 1)1. The first breakthrough in this filed was that the FLP could reversibly activate hydrogen molecule reported in 2006 by Prof. Douglas W. Stephan2. Afterwards, much attention has been devoted to the field of FLP, due to its promising potentials for the activation of small molecules. To date, homogeneous FLP catalysts with unquenched Lewis acidic (electrophilic) and Lewis basic (nucleophilic) sites results in the ability to both accept and donate electrons simultaneously, which has been proved to active H2, CO2, SO2, N2, nitrogen oxides, andπ-bonds3–5.However, the industrial application of homogeneous FLP catalysts are generally restricted by the difficulties in product purification and catalyst recovery. Therefore, the development of heterogeneous catalysts with FLP sites is extremely expected owing to their advantages in separation and recovery.
Similar to homogeneous FLP sites, the solid FLPs consist of the Lewis acid-base pair that can interact with each other, but are prevented from the formation of chemical bond. Specifically, the prerequisite for the formation of solid FLP sites is that the solid surface should contain both Lewis acid sites and Lewis base sites at the same time6. Moreover, an appropriate distance between Lewis acid and base sites need to be maintained to achieve the best FLP function, that is to say, the distance should be kept neither too close resulting in the formation of the class Lewis acid-base pair, nor too long failing to activate the reactants6.Nevertheless, different from the homogeneous catalysts, the construction of heterogeneous FLP sites with the optimized distance requires precise synthesis and the regulation of the surface properties of nanomaterials. To the best of our knowledge, it is still a great challenge to create the effective heterogeneous FLP sites.

Fig. 1 Scheme of (a) class Lewis acid-base pairs and(b) frustrated Lewis pairs.
In principle, the combination of a Lewis acid and Lewis base,in which at least one of them is solid, can easily form heterogeneous FLP sites. For example, Au powder as Lewis acid combining with molecular Lewis base can successfully exhibited the FLP-like hydrogenation behavior7. In addition, functional polymer, molecules, and zeolites can also work as Lewis base site, presenting the FLP-like catalytic propertiesviacombining with Lewis acid of B(C6F5)38,9. However, these heterogeneous systems will form large amount of undesired class Lewis acidbase pairs. Therefore, more and more efforts focus on the construction of FLP sites on one solid surface. The graphene synthesized by chemical exfoliation has been firstly reported as the efficient heterogeneous FLP catalysts for hydrogen activation10. However, the catalytic activity is limited by the random existence of Lewis acid and base sites on the surface of graphene. Recently, heterogeneous FLP sites based on metal organic frameworks (MOFs) exhibit the capability for H2and CO2activation, due to their well-defined chemical composition and structure11–13. Unsatisfactorily, mass transfer is restrained by narrow channels of MOFs, which impedes the extensive developments and applications of these catalysts. Heteroatom doping provides another feasible approach for construction of heterogeneous FLPs. For instance, B/Al-doped 2-dimensions phosphorenes (B/Al as the Lewis acid site, P as the Lewis base sites) can facilitate the hydrogenation of small unsaturated molecules14. Moreover, In2O3–x(OH) nanocrystals can activate CO2 and H2viathe FLP sites, which are consist of InOH as Lewis base sites and In as Lewis acid sites15,16. Therefore, there are still very limited successful examples reported as efficient heterogeneous FLP sites.
Recently, we reported a new strategy by regulating the surface defects to construct heterogeneous FLP sites on the surface of CeO2, which could strongly activate H2 and CO2 for the subsequent hydrogenation and CO2transformation, respectively.In this perspective, the principles of the formation of FLP sites on CeO2was firstly discussed. Afterwards, the catalytic activation of two important small molecules (H2and CO2) on such interfacial FLP sites was demonstrated experimentally and theoretically. In addition, their potentials for the activation of methane were also included. After these discussions, the challenges and further perspectives on the design of heterogeneous FLP sites as well as their potential applications were also demonstrated.
2 Theoretical analysis of the FLP sites on the surface of CeO2
Generally, ceria (CeO2) possesses the both surface Lewis acid sites (Ce cation) and Lewis base sites (O anion)17. On the ideal CeO2surface, the Ce cation and O anion form the class Lewis acid-base adjuncts (Ce―O bond), delivering no FLP activity.Meanwhile, the surface Lewis acid and Lewis base sites can be maintained at their lattice position due to the rigid crystal structure. Fortunately, the un-bonded surface Lewis acid sites and/or Lewis base sites can be easily created due to the reversible Ce3+/Ce4+redox pair associated with the lattice defects of oxygen vacancy. With those precedents in mind, a possible FLP site might be constructed near the surface oxygen defects on the surface of CeO2.
Generally, uniform CeO2rods, cubes and octahedral are enclosed by the (110), (100) and (111) crystal planes,respectively. Stabilities of the CeO2(111), (100) and (110)surfaces were calculated theoretically following the order of(111) > (110) > (100). Since the solid FLP active sites require a relatively fixed Lewis acid and base sites, the CeO2(100) surface may not be suitable for the construction of FLP sites. Meanwhile,the solid FLP sites cannot be realized on the CeO2(111) surface by removing surface oxygen atoms. Actually, different from the theoretical result, we have proved that CeO2(110) surface exhibited the highest possibility for the construction of FLP sites rather than CeO2(111) with mismatched spatial configuration and CeO2(100) with the lowest oxygen vacancy formation energy. The analysis with more details could be found in our previous reports18. As shown in Fig. 2a, the surface Ce and O on ideal CeO2(110) surface form Lewis acid-base adjuncts, along with interaction between electrons (Fig. 2d). After introducing an oxygen vacancy (removal of the OIbatom, Fig. 2e), the Lewis acid CeI(CeII) atom and Lewis base OIIcatom are independent with a distance of 4.72 Å (d(CeI, OIIc) = 4.72 Å, 1 Å = 0.1 nm)after structural relaxation. Although the unbonded CeI-OIIc falls in the domain of heterogeneous FLP sites, the presence of OIc,OIaand OIdwill affect the electronic interaction of Lewis acid(CeI/CeII) and Lewis base (OIc), hindering small-molecule activation (Fig. 2e). When the OIais removed, the OIcand OIdwill not migrate from the DFT calculation (Fig. 2c). Obviously,the CeI/CeII and OIIc are independent without affected by other Lewis acid or base sites. More importantly, a unique Lewis acid site will form through combining the two adjacent surface Ce sites (CeIand CeII, Fig. 2f). On this occasion, the Lewis acid site(CeI, CeII) and Lewis base site (OIIc) will construct as the FLP sites. The distance of CeI,CeII-OIIcis 3.9 Å, which is smaller than those of CeI-OIIc and CeII-OIIc, indicating the stronger charge contraction of FLP sites and consequently resulting in the higher capability to activate small molecules. Thus, the catalytic site of CeI,CeII-OIIcsatisfies both spatial and electronic matching for the activation of the third parties (small molecules), analogy to the homogeneous FLP catalysts. Therefore, the heterogeneous FLP sites could be realized by regulating the surface oxygen defects.Particularly, CeO2 with abundant surface defects is more likely to form the novel FLP sites.
3 The critical point for constructing of FLP sites on the surface of CeO2
Based on the theoretical analysis, the FLP sites can be constructed most possibly on the CeO2(110) surface with plenty of surface defects, owing to the high possibility to form the desired oxygen defect clusters. Thus, the critical points for constructing FLP sites on the surface of CeO2are that (1) the CeO2should have (110) facets mainly exposed; (2) the CeO2should have a boundary of surface defects to ensure the formation of oxygen defect clusters; (3) the CeO2 should be stable enough to allow the existence of oxygen defect clusters.
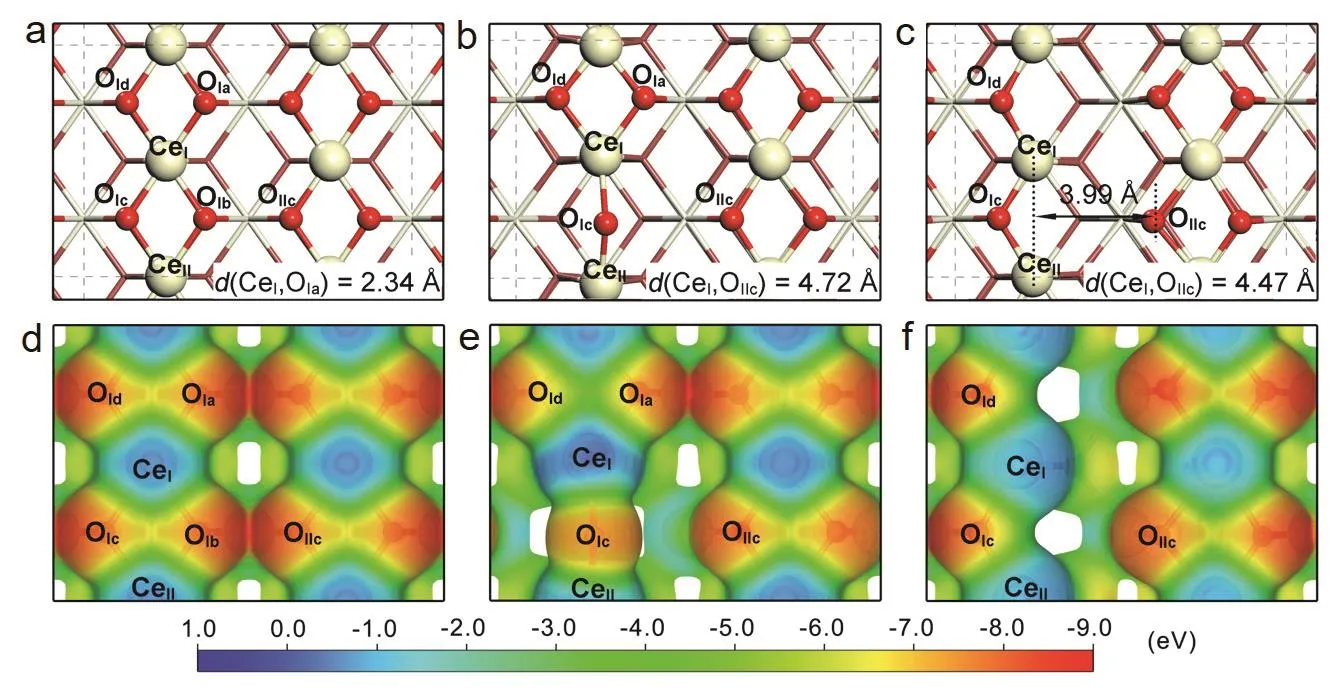
Fig. 2 Schematic images of concept for design of solid frustrated Lewis pairs in CeO2 crystal structure.
To realize this purpose, porous nanorods of CeO2(PN-CeO2)with mainly exposed (110) and (100) facets were synthesized through a two-step hydrothermal process, as shown in Fig. 3a19,20.Typically, the CeO2/Ce(OH)3precursor could be obtained during the first step of hydrothermal process at 100 °C, and the coexistence of CeO2and Ce(OH)3could be confirmed from the powder X-ray diffraction (XRD, Fig. 3c). The second step of hydrothermal process was used to transform the Ce(OH)3 into Ce2O3, and subsequently to CeO2at 160 °C, along with the sequential morphological change from the non-porous precursor to porous nanorods (Fig. 3d). As shown in Fig. 3e, only CeO2phase could be detected from the XRD analysis.
The surface area of PN-CeO2is 141 m2∙g−1obtained from Brunauer-Emmett-Teller (BET) analysis. Previous reports have indicated that the nanorods of CeO2preferentially grow along[110] direction, enclosed by (220) and (200) planes, which could be confirmed from the High-resolution TEM image (Fig. 3d)where two kinds of lattice fringes of 0.19 nm and 0.275 nm can be attracted to the inter-planar spacing of (220) and (200),respectively. Obviously, the crystal faces of obtained PN-CeO2meet the requirement of crystal faces for constructing the heterogeneous FLP sites. More importantly, the obtained PNCeO2generally exhibited a higher surface Ce3+fraction of more than 30% analyzed from X-ray photoelectron spectroscopy(XPS) of the Ce element, indicating the existence of abundant surface oxygen defects (Fig. 3f). In addition, the apparent peak at 600 cm−1in the Raman spectroscopic study could further support the presence of abundant surface oxygen defects (Fig.3g). Therefore, it is possible to construct the heterogeneous FLP sites on PN-CeO2with the plentiful surface oxygen defects.

Fig. 3 (a) Schematic of the synthesis of the PN-CeO2. Reproduced from Ref. 19 with the permission from The Royal Society of Chemistry.TEM images of CeO2/Ce(OH)3 precursor obtained (b) at room temperature and (c) after hydrothermal under low pressure. (d) TEM image of PN-CeO2. (e) XRD analysis of precursor and PN-CeO2. (f) XPS and (g) Raman analysis of PN-CeO2. Reproduced from Ref. 18 with the permission from Springer Nature.
4 Solid FLP sites for small-molecule activation
4.1 Hydrogen activation
Hydrogenation of styrene was selected as the model reaction to expose the possible hydrogen activation by FLP sites. As shown in Fig. 4a, the styrene could be completely hydrogenated to ethylbenzene after 14 h catalyzed by PN-CeO2 with FLP sites under optimized reaction conditions, revealing the successful hydrogen activation. In order to prove the necessity of the coexistence of Lewis acid sites and Lewis base sites, the hydrogenation reactions were also performed in the presence of small molecular Lewis base pyridine and Lewis acid pyrrole,which could block the Lewis acid and base sites of PN-CeO2,respectively. As expected, the catalytic activity of FLP sites for the hydrogenation was greatly reduced even under trace amount of Lewis base pyridine or Lewis acid pyrrole (Fig. 4b).Therefore, the experimental results under the blockage of either Lewis base sites or Lewis acid sites of PN-CeO2revealed that the coexistence of Lewis acid and base sites are essential to activate H2for the subsequent hydrogenation.
From the theoretical analysis, the concentration of surface defects is a critical factor for the construction of FLP sites on the surface of CeO2. To confirm the role of surface defects,hydrogenation of styrene was also catalyzed by other CeO2nanomaterials with various quantities of surface defects,including nanorods of CeO2(NR-CeO2), nanocubes of CeO2(NC-CeO2), nanoparticles of CeO2 (NP-CeO2). Meanwhile, the PN-CeO2 catalysts were also calcined under air at 300 °C (PNCeO2-300) and 500 °C (PN-CeO2-500) to further reduce the number of surface defects. The concentrations of surface defects of different samples were summarized in Fig. 4c. As shown in Fig. 4d, the positive correlation between the surface Ce3+fraction and the catalytic activity reveals that the hydrogenation activity of CeO2 is determined by the density of surface defects.The higher concentration of surface defects is, the higher possibility the FLP sites could be constructed, resulting in the enhanced hydrogenation activity.
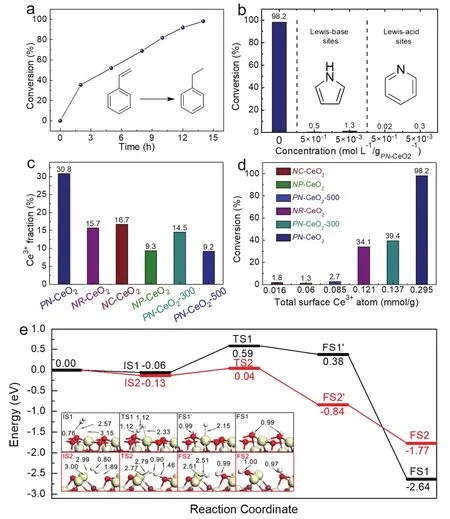
Fig. 4 (a) Time course of styrene conversion catalysed by PN-CeO2. (b) Influences of molecular Lewis-base or Lewis-acid on the catalytic activity of PN-CeO2 for hydrogenation of styrene. (c) Catalytic activity of various nanoceria for hydrogenation of styrene. (d) Effects of the total surface Ce3+atom number for hydrogenation of styrene. (e) Energy profiles for H2 dissociation on ideal CeO2(110) in black curve and CeO2(110) with two oxygen vacancies in red curves. The optimized structures of initial states (IS), transition states (TS) and final states (FS) are labeled with bond distance (in Å).The zero energy reference corresponds for the sum energy of H2 in the gas phase and the corresponding clean CeO2 surfaces.
To support the abovementioned conclusion, the dissociation of hydrogen was also performed through DFT calculations,investigated on the ideal CeO2(110) and CeO2(110) with FLP sites. Compared with ideal CeO2(110), the energy barrier of hydrogen dissociation on FLP sites is obviously lower than depicted energy profile (Fig. 4e), indicating the superior capacity for the H2activation. Despite two hydrogen atoms are both adsorbed at surface oxygen in two configurations, the FS2 is more active than the FS1, facilitating the subsequent hydrogenation reaction. Thus, the DFT calculation results also confirms that the FLP sites on the surface of CeO2 exhibit an enhanced capability of H2 dissociation, improving the catalytic activity for the styrene hydrogenation.
4.2 CO2 activation
The constructed FLP sites on the surface of PN-CeO2also exhibited the strong capacity for CO2activation. It has been reported that CO2 can be activatedviaLewis base sites of FLP through C atom and Lewis acid sites of FLP through one of the O atoms, respectively. Due to the unique spatial structure, the FLP sites on PN-CeO2exhibited a novel interaction with CO2viathe Lewis base site of lattice O with the C atom and two adjacent Lewis acid Ce3+sites bonding with both O atoms,forming a carbonate-like stable adsorption configuration (Fig. 5)21.Meanwhile, CO2 molecule bonded with FLP sites can be activated more effectively than that adsorbed on the CeO2surface without FLP sites achieved from the elongated C=O bond and decreased O―C―O angle, as shown in Fig. 5.Moreover, the O atom of CO2on FLPs also exhibited the most negative charge (−2.13e), resulting in the strongest ability of nucleophilic addition reaction.
Afterwards, the activated CO2was used for the selective tandem transformation of olefins (styrene as the model molecule) and CO2into cyclic carbonates. To highlight the catalytic performance of FLP sites, the nanocubes of CeO2(NCCeO2) and nanooctahedra of CeO2 (NO-CeO2) without FLP sites were also selected as the catalysts for the tandem transformation.As shown in Fig. 6a, NR-CeO2with FLP sites exhibited the highest catalytic activity and selectivity to phenylethylene carbonates compared with NO-CeO2and NC-CeO2catalysts under the same reaction conditions. Even though normalizing the activity to their surface area, NR-CeO2 still delivered the highest conversion of styrene. Therefore, the FLP sites on NR-CeO2is critical for effective CO2activation and tandem reaction. When the defective PN-CeO2was used as the catalysts, the both catalytic activity and selectivity were further improved under the identical conditions.
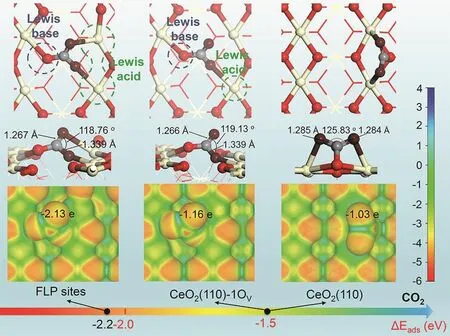
Fig. 5 Adsorption and activation of CO2 on CeO2(110).
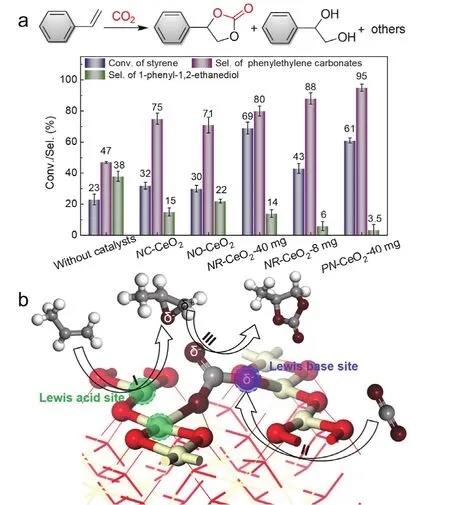
Fig. 6 (a) Catalytic performance of various CeO2 catalysts for the selective tandem transformation of olefins and CO2 into cyclic carbonates. (b) Proposed catalytic process for the tandem transformation of olefins (propylene) and CO2 into cyclic carbonates on FLPs.
Previous reports have proved that the Ce3+sites exhibited the peroxidase-like activity for the styrene epoxidation. Combining with the FLP sites for the CO2activation, the catalytic process of tandem transformation could be achieved (Fig. 6b). Firstly, the olefin can be oxidized into epoxide on the Lewis acidic Ce3+sites. Then CO2 molecule is adsorbed and activated on the FLP sites. Next, the generated epoxide approaches and attacks the activated CO2molecule, releasing the final product cyclic carbonate. Therefore, such CO2activation by FLP sites can be applied in other scenarios with the CO2as the fundamental reactant.
4.3 CH4 activation
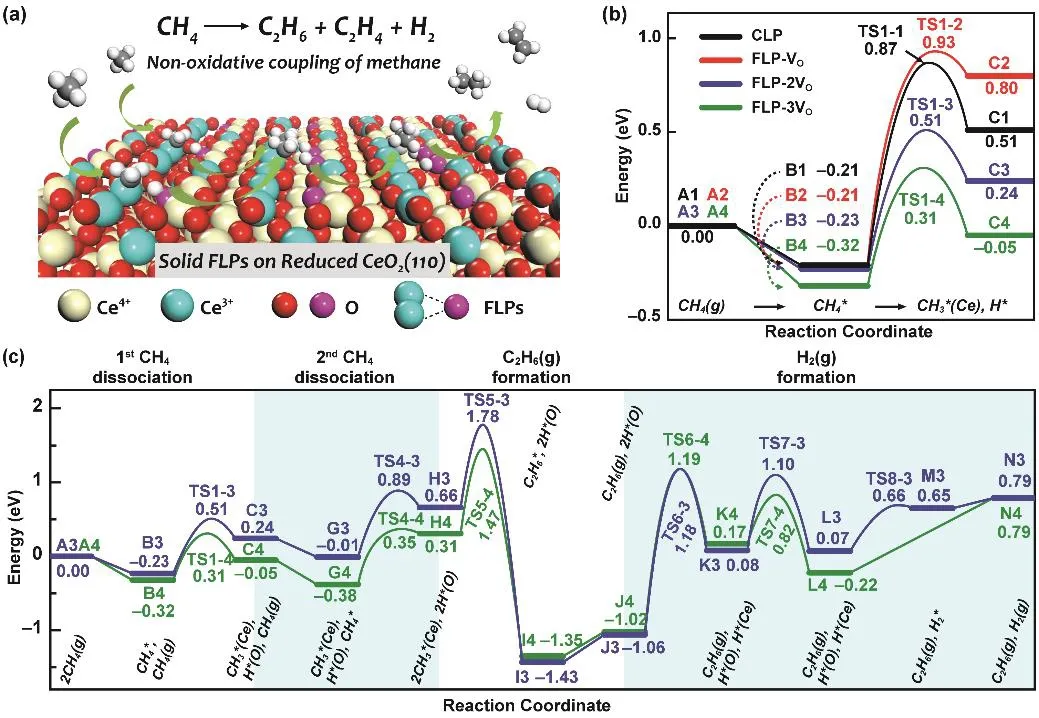
Fig. 7 (a) Schematic of nonoxidatvie coupling of methane to generate ethane, ethylene and hydrogen on FLPs.(b) Potential energy diagram of methane dissociation occurring on CLP sites (black curve) and FLP sites (red, blue and green curves).(c) Potential energy diagram of nonoxidative coupling of methane to ethane at FLP-2VO sites (blue curve) and FLP-3VO sites (green curve).The zero energy reference corresponds for the sum of energies of CH4(g), and the corresponding clean CeO2(110) surfaces.
Very recently, the FLP sites on CeO2(110) were predicted theoretically to activate methane and directly convert methane into ethane, ethylene and hydrogen in a nonoxidative route as shown in Fig. 7a22. As shown in Fig. 7b, the activation of methane was explored at classical Lewis pair (CLP) sites on stoichiometric CeO2(110) and at FLP sites on reduced CeO2(110) with one (FLP-VO), two (FLP-2VO) and three (FLP-3VO) oxygen vacancies. Due to enhanced acidity and basicity of FLP sites, the reaction barrier of methane dissociation decreased from 1.08 eV on CLP sites to 0.63 eV on FLP-3VO. Further calculations indicated that transformation of an individual methyl group at FLP-2VO and FLP-3VO sites was largely impeded due to the high barriers, which creates the possibility for on-surface conversion of methyl groups directly to C2products. Fig. 7c shows the whole pathways of coupling two methane into ethane and hydrogen. The critical C−C coupling of methyl groups at FLP sites has a reaction barrier of ~1.1 eV,much lower than that of methyl group desorption and gas-phase C−C coupling23. Although the formation of hydrogen to regenerate FLP sites seems to be difficult, it can be overcome at high temperatures. Overall, this study uncovers a possible strategy for nonoxidative coupling of methane into valuable hydrocarbons on FLPs-contained oxide catalyst and may provide guidance for experimental design of FLP catalysts for methane activation.
5 Conclusion, challenge and prospects
What we summarized in this perspective is the surface regulation engineering that is linked to the construction of solid FLP sites. Although the direct combination of Lewis acid and base exhibits the potential for the formation of FLP sites, the precise surface regulation of nanomaterials at atom level, which plays significant roles in the accurate construction of FLP sites,needs further investigations. Moreover, the essential factors that influence the activation of small molecules should be deeply understood, owing to their regular spatial configuration. It was proved that the FLP sites on the surface of CeO2, which were constructedviacreation of surface oxygen defect clusters,exhibited the superior ability for H2 and CO2 activation for the subsequent hydrogenation and CO2 transformation, respectively.The regulation of CeO2surface could enlighten the following researches on the construction of catalysts with the solid FLP sites through the similar surface engineering in other oxides. In addition, another example on the formation of FLPs sites was achieved in the case of CoBOxnanosheets25, in which the surface hydroxyl groups function as typical Lewis base sites and the Co metal ions works as Lewis acid sites, further enriching the approaches for the design of solid FLP sites.
As a new established field, solid FLP catalysts exhibit great potentials for small-molecule activation in various advanced catalytic reactions. However, the main current challenge lies in how to construct FLP sites on various nanomaterials at a more precise level. The lack of efficient approaches to accurately construct FLP sites would limit their applications in various fields. Therefore, it can be expected that developing various FLP catalysts and/or exploring novel approaches for creating FLP sites on the nanomaterials with ordered structure could be the main research orientation in this field. Except for the metal oxides, molecular sieves and MOFs with ordered structure and abundant functional sites are also very promising as the platform for solid FLP sites, in which surface defects could offer a great potential for the construction of solid FLP sites as well.Meanwhile, doping of acid or base atom may be another feasible strategy to create FLP sites on the surface of nanomaterials. In addition, the hydroxyl group, commonly found on the surface of nanomaterials, can work as typical Lewis base sites, which could provide another feasible approach for constructing solid FLP sites through combining with surface Lewis acid sites.
At present, the applications of current solid FLP sites is only confined to the activation of H2and CO2. In order to extend the utilizations of solid FLP sites for more small-molecule activation, the Lewis acidity/basicity of FLP sites should be precisely controlled, which is greatly determined by their electronic structures. Until now, few effective approaches have been found to realize the precise control or adjustment of electronic structures of Lewis acid and base sites. However,electronic structure regulation could be realized due to their unique spatial configuration, and successful cases in homogeneous FLP sites has already be achieved. After solving the abovementioned problems, the solid FLP sites could even be used in the activation or dissociation of C―H bond, Si―H, H2O,N2, NO,etc., resulting in more comprehensive applications.
Finally, the short of effective technologies for the accurate characterization of FLP sites on the surface of nanomaterials could be another limitation. At this stage, DFT calculation is the main method to understand the spatial and electronic structure of FLP sitesviasimulation process, by which the activation process of small molecule is also simulated to understand the catalytic performance in hydrogenation and CO2 transformation.However, due to the absence of direct experimental evidence, the authenticity of computational results is debatable, which could bring lively scientific controversy in the solid FLP sites catalytic reactions. Consequently, it is extremely desired that advanced techniques can be developed to probe and track solid FLP sites.The obtained information would be extremely useful to understand the formation of surface FLP sites as well as to prove the explanation of their catalytic mechanism.
Despite the inherent challenges, the potential of solid FLP catalysts for the small-molecule activation is continually driving research in this field, particularly taking advantage of alternative transition metals. Recently, the strengths of Lewis acid and base sites have been preliminary analyzed by the Fourier transform infrared (FTIR) spectroscopy, temperature-programmed adsorption/desorption and calorimetric enthalpy of adsorption.The latest developed achievements can provide strong confidence for the future development and understand the solid FLP catalysts. In addition, more and more solid FLP catalysts with ordered spatial structure would further reduce the difficulties in structural analysis and mechanism studies. In general, the past and present progress in solid FLP can ultimately facilitate the development of surface chemistry. Meanwhile, the deep understanding of FLP sites for small-molecule activation also improves the rational design and preparation of nanocatalysts, and explores their possible applications in advanced synthetic chemistry and other utilizations.
