Preparation of large-area isotopic magnesium targets for the 25Mg(p,γ)26Al experiment at JUNA
2020-09-24ChenChenYunJuLiHaoZhangZhiHongLi
Chen Chen· Yun-Ju Li · Hao Zhang · Zhi-Hong Li,3
Abstract To study the 25Mg(p,γ)26Al reaction at the Jinping Underground Nuclear Astrophysics laboratory, a large-area 25Mg target with a uniform thickness is needed.A rotating unit is used to ensure the uniformity of the target thickness during evaporation. After many attempts, 19 targets with diameters of 40 mm and a non-uniformity of 8.4% were prepared simultaneously. The rate of material utilization was approximately 4.7 times higher than that obtained using a conventional evaporation method.
Keywords Vacuum evaporation · Substrate rotation ·Uniformity · Material utilization
1 Introduction
At the energies of interest for nuclear astrophysics, the cross sections of reactions involving charged particles decrease approximately exponentially as the energy decreases [1]. Thus, a low background and high beam intensity in the laboratory are essential for accurate direct measurement at stellar energies, which is an international frontier in nuclear astrophysics. The underground China JinPing Laboratory has the thickest rock shield in the world [2–4]. The Jinping Underground Nuclear Astrophysics (JUNA) laboratory is under construction and will be equipped with a high-current 400 kV accelerator that will be able to produce proton and helium beams with intensities of 10 mA and 5 mA, respectively, which are approximately 10 times those of the 400 kV accelerator at the Laboratory for Underground Nuclear Astrophysics(LUNA) [5].Thus,JUNA will enable pioneering studies of key nuclear astrophysics reactions.
The most important reaction in nuclear astrophysics,12C(α,γ)16O, plays an important role in stellar evolution and is referred to as the Holy Grail reaction in nuclear astrophysics [6–9].13C(α,n)16O is the key neutron source reaction for the stellar s-process [10–13].19F(p,α)16O and25Mg(p,γ)26Al are the keys to understanding the massive amounts of19F and26Al in the universe [14–19]. The reaction rates of these four reactions still have rather large uncertainties, which limit our understanding of nucleosynthesis and stellar evolution. Thus, these four reactions have been selected as the first four scientific targets of the JUNA project [20].
The25Mg(p,γ)26Al reaction was chosen as the day-one experiment of JUNA. Its astrophysical reaction rates are dominated by the 58, 92, 190, and 304 keV resonances.Many studies have been performed since the 1970s [21–35],and direct measurements have reached only as low as 192 keV.The strength of the 92.2 keV resonance was measured with a large error by LUNA in 2012 [5,36].Our ultimate goal is to measure the strength of the 58 and 92 keV resonances of25Mg(p,γ)26Al.
To increase the lifetime of the25Mg target, a rotating target and beam scanning technology have been developed,and the diameter of the effective beam spot will be enlarged to 40 mm. Therefore, the experiments require a large-area reaction target.
Isotopic25Mg targets can typically be prepared by evaporation [37]or sputtering [38].It is difficult to produce a largearea target with a uniform thickness using conventional methods,and the utilization efficiency of the isotopic material is quite low. In addition, because experiments at an underground laboratory usually last several months, hundreds of targets are needed for one successful experiment. Target preparation to meet this requirement is also a challenge.
In this work, the rotary method is used to improve conventional evaporation equipment based on the Monte Carlo simulation. After many trials with a natural magnesium material,a large-area magnesium target with uniform thickness was produced by a rotation–evaporation method.
2 Theoretical analysis and rotation unit
The conventional evaporation method is illustrated schematically in Fig. 1a, where the target substrate is located above the evaporation source.For a point source or small-area source,the thickness of the target at a distance s from the center is given by

where h is the height of the target above the source. The thickness distributions for several typical h values are shown in Fig. 2a; as h increases, the uniformity of the thickness in the central region increases. To ensure target uniformity, the thickness at the edge of the target d(s)should be close to the center thickness d(0). We consider d(s)/d(0)>90%; then h >4.3 s, according to Eq. (1). To prepare a target with a diameter of 40 mm, h should be at least 86 mm. The effective rate of material utilization will be
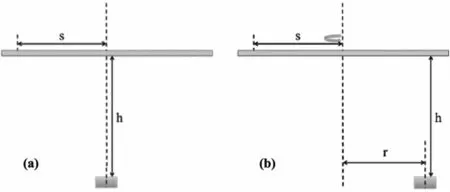
Fig. 1 Schematic diagram of target preparation by conventional evaporation method (a) and rotation–evaporation method (b)
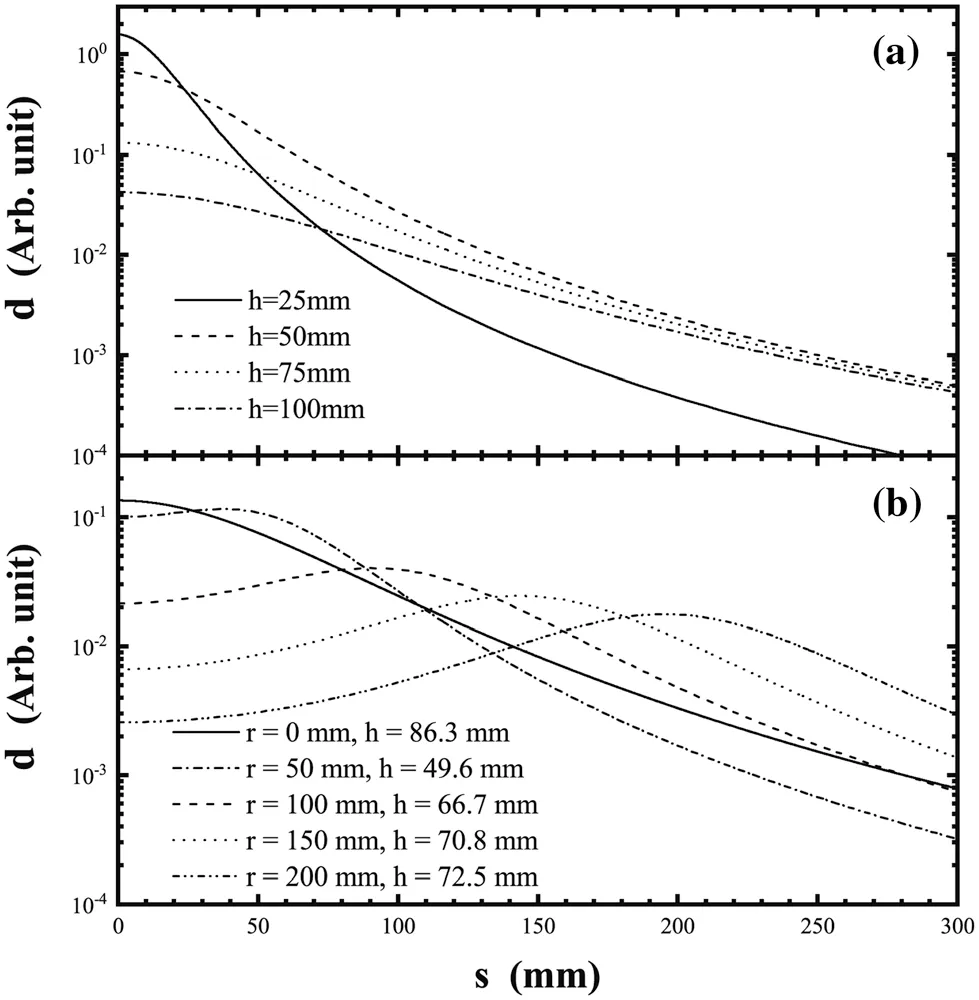
Fig. 2 Calculated thickness distributions for several typical h values for conventional method (a) and thickness distributions for several typical r values and the corresponding h values for the rotation method (b)
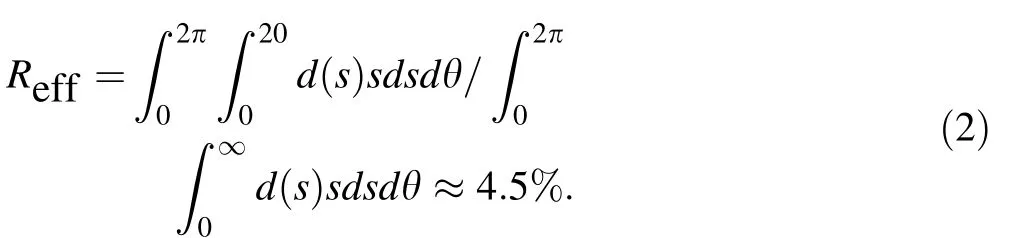
Thus, the conventional evaporation method will result in considerable waste of the target material, and the cost will be very high, especially for expensive isotopic targets.
To increase the material utilization rate,the evaporation equipment is improved by adopting a rotation method. As shown in Fig. 1b, the target substrate is placed on a rotary disk at a height h, and the evaporation source is fixed at a distance r from the central axis of rotation. The target thickness deposited at the position s is [39]

For each evaporation source position r,the height h can be adjusted so that the edge thickness reaches 90% of the maximum thickness.In this step,several typical values of r are selected, and the corresponding heights h are determined by a Monte Carlo method with the random of the position s.The thickness distributions are shown in Fig. 2b.Relatively uniform targets with a diameter of 40 mm can be prepared using a suitable setup.
Different numbers of targets can be arranged in a circular region with different center positions s and prepared simultaneously. Table 1 lists the maximum material utilization rates for the preparation of different numbers of targets using the rotation method, along with the corresponding h and r values. According to the fitting curve in Fig. 3,the utilization rate reaches a maximum at an r value of approximately 60 mm and decreases slowly thereafter with increasing r.However,as r increases,more targets can be placed on the rotating frame simultaneously. Thus, the material utilization rate can be controlled at 20% for 50 mm<r<200 mm, which is approximately 4.5 times that obtained using the conventional evaporation method.
The rotation unit is designed according to the size of our target preparation equipment, as shown in Fig. 4. The central rotation axis is sealed with a magnetic fluid [40,41]at the top of the chamber, and the rotation speed can be adjusted from 0 to 150 RPM. The rotating copper disk(φ 360 mm) can be cooled by internal circulating water. A maximum of 19 substrates can be placed in an annular area of the rotating target frame (φ 290 mm). The evaporation source is installed 155 mm from the central axis and 70 mm below the rotating target frame on the basis of the calculation.
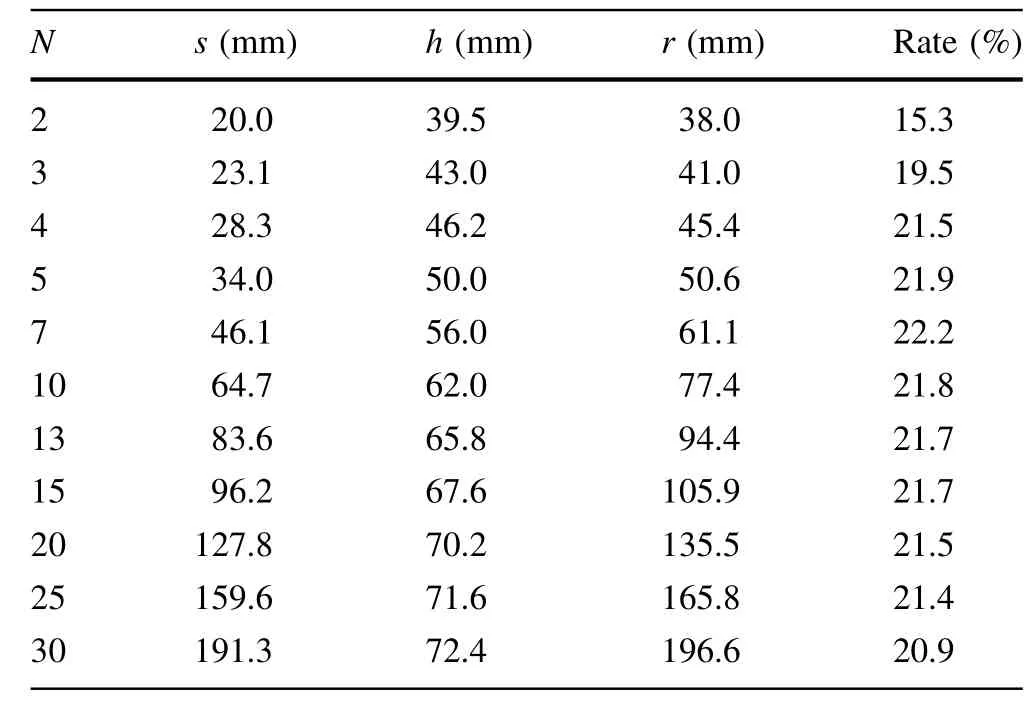
Table 1 Maximum material utilization rates (Rate) for various numbers of targets (N) arranged in a circular region at the center position (s) and prepared simultaneously

Fig. 3 Rate of material utilization for rotation–evaporation method with different source positions

Fig. 4 Schematic diagram of the rotation unit; the target frame is used to hold the substrate under the rotating disk
3 Preparation and uniformity measurement
Pure copper was chosen as the substrate in the25Mg(p,γ)26Al reaction experiment owing to its desirable heat conductivity and machinability. The substrate has a diameter of 50 mm and thickness of 3 mm.However,to test the preparation method, a substrate with a thickness of 0.1 mm was used so that the target could be weighed using a more precise balance. The surface of the substrate was first polished using different grades of sandpaper and a precision auto lapping and polishing machine. Next, the substrate was degreased using a water-soluble detergent;it was then deoxidized and passivated using 1%hydrochloric and citric acid.
A cuboid tantalum crucible with a size of 10 mm×10 mm×5 mm was used as the evaporation source; it was covered with a 10 mm×10 mm tantalum net with an array of 0.1 mm holes,as shown in Fig. 5a,to prevent spluttering of the boiling magnesium.

Fig. 5 Photographs of tantalum net (a), an arrangement of small pieces of copper substrate (b), and the Mg target (c)
First,the conventional preparation method was tested at an h value of 86 mm. Natural metallic magnesium (50 mg)was evaporated, and the average deposition thickness was 200 ± 11 μg/cm2for a diameter of 40 mm. The value was determined by weighing the substrate before and after evaporation using an analytical balance with a precision of 0.01 mg; the error was determined by repeated weighing.The material utilization rate was 5.0% ± 0.3%.
In the rotation–evaporation test, 100 mg of natural metallic magnesium was used, and 13 substrates were arranged according to the parameters in Table 1 to prepare 13 targets simultaneously. The thickness of the targets is shown in Fig. 6 (solid circles);the material utilization rate is 23.4%±0.7%,which is approximately 4.7 times higher than that obtained using the conventional method. The uncertainty arises mainly from the weighing process and the variation among the targets.
To determine the uniformity of the target thickness, 10 pieces of copper with a size of 7 mm×7 mm×0.1 mm were placed side by side in two rows, as shown in Fig. 5b,which were arranged along the tangential and radial directions. The thickness of the deposited magnesium was determined by weighing the targets using an analytical balance with a precision of 0.001 mg. The results are shown in Fig. 7, along with the fitting results based on Eq. (2).The results of weighing are in fair agreement with the results of the theoretical calculations. The standard deviations in the radial and tangential directions are 3.6%and 2.5%, and the non-uniformity is found to be 8.4% and 5.8% (full width at half-maximum), respectively.
Theoretically, the target thickness should be proportional to the amount of material evaporated. Hence, additional preparation processes using different amounts of material were tested at the position corresponding to the maximum target number. The substrate for the experiment with a thickness of 3 mm was used; a photograph of the target is shown in Fig. 5c. The test results are shown in Fig. 8; they are consistent with a linear relationship. The deviation may be caused by the variations in material loss with increasing temperature and in the vacuum stability,as well as other uncontrollable factors. Thus, the target thickness can be controlled roughly by adjusting the amount of material evaporated.

Fig.6 Thickness of 13 targets prepared simultaneously.Solid circles represent the values of each target based on the weight, and the shaded area represents the average thickness with ±1σ confidence intervals

Fig. 7 Deposition thickness of magnesium on copper sheets in the radial and tangential directions.Solid circles are the values according to the measured weight, and the solid lines are the fitting results

Fig. 8 Measured relationship between the amount of magnesium evaporated and the thickness of the target, with linear fitting
4 Target preservation and calibration
Magnesium targets are easily oxidized, and the oxygen content of the target can directly affect the experimental results, especially the incident energy for direct measurement at low energies of astrophysical interest. Thus, it is important to avoid exposing the targets to the atmosphere.
To avoid the effects of the above factors, the target manufacturing equipment should integrate the functions of the rotating unit, a glove box, and equipment for target preparation by evaporation, electron sputtering,magnetron sputtering, and even ion implantation [42].
After magnesium evaporation, we placed the targets under a protective nitrogen or argon gas during storage and transportation, and operated in the glove box to fix the target on the accelerator experiment terminal.
To calibrate the oxygen content of the target, the 326 keV resonance in the26Mg(p,γ)27Al reaction was measured at the 400 kV JUNA accelerator using an HPGe detector, in agreement with the LUNA experimental procedure [43]. The yield data are shown in Fig. 9, and the ratio of oxygen to magnesium is calibrated at 0.14,which is comparable to the value of 0.12 for the magnesium target used in the LUNA experiment [43].
Finally, 19 isotopic25Mg targets were successfully produced simultaneously and used to measure the 189 keV resonance of the25Mg(p,γ)26Al reaction at the 4π bismuth germanate (BGO) array at Ep = 215 keV. The γ-ray spectrum is shown in Fig. 10. The 189 keV resonance clearly appears at approximately 6.5 MeV, and the 143 keV resonance of the18O(p,γ)19F reaction also appears at approximately 8.1 MeV. On the basis of the known strengths of these two resonances [5, 44], the ratio of oxygen to magnesium is determined to be approximately 0.15. More details on the measurement of the 189 keV resonance will be published elsewhere.
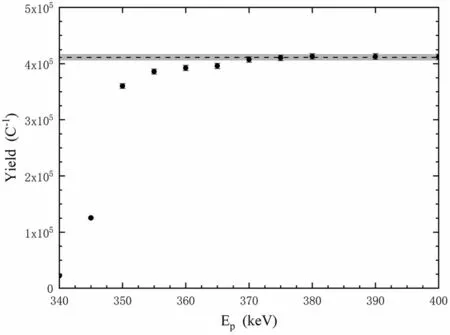
Fig. 9 Yield of 326 keV resonance for the 26Mg(p,γ)27Al reaction(solid circles) and fitting of the platform (dashed line). The shaded area represents the ±1σ confidence interval
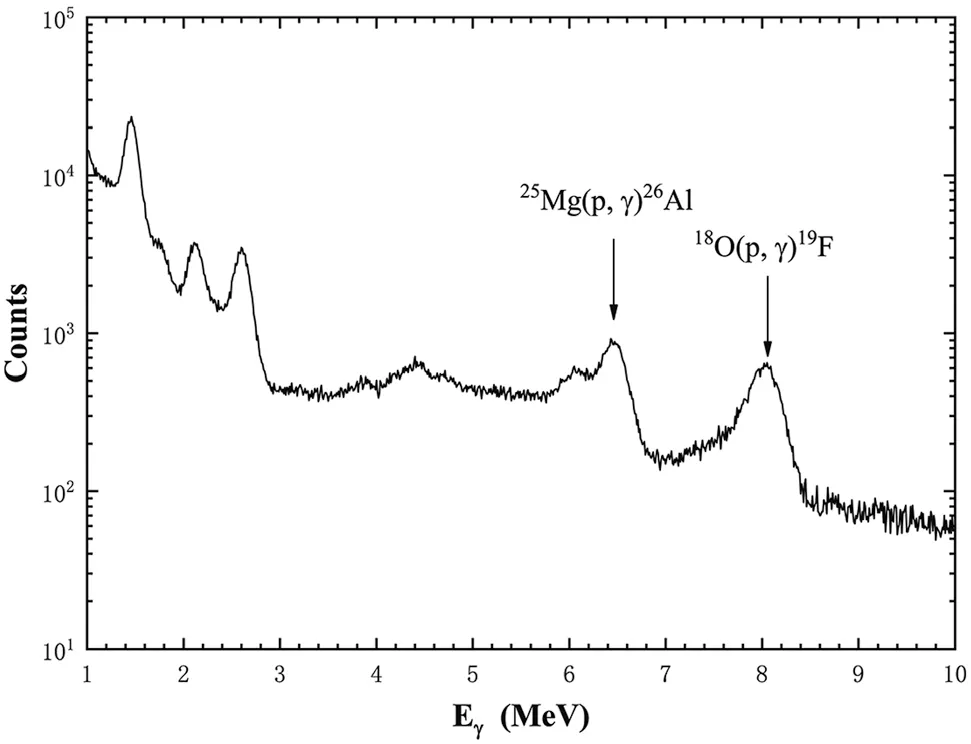
Fig.10 Spectrum of the 25Mg(p,γ)26Al reaction using 4π BGO array at Ep = 215 keV
In summary, the prepared25Mg isotope targets, which have a diameter of 40 mm, a non-uniformity of 8.4%, and an O/Mg ratio of approximately 0.15, completely meet the requirements for direct measurement in the upcoming25Mg(p,γ)26Al experiment at JUNA.
5 Summary
A rotation method was used to prepare large-area magnesium targets by evaporation.Up to 19 targets with a diameter of 40 mm and a thickness non-uniformity of 8.4%were prepared simultaneously. The rate of material utilization was 23%,which is approximately 4.7 times higher than that obtained using the conventional evaporation method. The rotation method greatly reduces the waste of the target material and can reduce the cost of the targets,especially those made of expensive isotopes.
After many attempts using natural magnesium,25Mg isotopic targets were also successfully produced and used in the day one experiment of direct measurement of the25Mg(p,γ)26Al reaction at JUNA. This preparation technology establishes a good foundation for planned experiments at a high current at JUNA.
In addition,the rotation method can be used not only for evaporation of other natural and isotopic materials,but also for target preparation by ion beam sputtering, magnetron sputtering, and ion implantation.
AcknowledgementsThe authors thank Prof. Qi-Wen Fan for his guidance in this work.
杂志排行
Nuclear Science and Techniques的其它文章
- Digitalization of inverting filter shaping circuit for nuclear pulse signals
- Development of a low-loss magnetic-coupling pickup for 166.6-MHz quarter-wave beta = 1 superconducting cavities
- Scheme for generating 1 nm X-ray beams carrying orbital angular momentum at the SXFEL
- Investigating the effect of entrance channel mass asymmetry on fusion reactions using the Skyrme energy density formalism
- High-precision and wide-range real-time neutron flux monitor system through multipoint linear calibration
- Correction to: Theoretical prediction of radiation-enhanced diffusion behavior in nickel under self-ion irradiation
