Lenvatinib for large hepatocellular carcinomas with portal trunk invasion:Two case reports
2020-09-15
Satoshi Komiyama,Kazushi Numata,Satoshi Moriya,Hiroyuki Fukuda,Makoto Chuma,Gastroenterological Center,Yokohama City University Medical Center,Yokohama,Kanagawa 2320024,Japan
Shin Maeda,Department of Gastroenterology,Yokohama City University Graduate School of Medicine,Yokohama,Kanagawa 2360004,Japan
Abstract
Key words:Hepatocellular carcinoma;Lenvatinib;Modified Response Evaluation Criteria in Solid Tumors;Main portal vein tumor thrombus;High tumor burden;Case report
INTRODUCTION
According to GLOBOCAN 2018,liver cancer is the sixth most commonly diagnosed cancer around the world,and ranks fourth as a cause of death from cancer,with about 841000 newly diagnosed cases and 782000 deaths reported worldwide annually[1].The SHARP trial demonstrated the efficacy of first-line systemic chemotherapy with sorafenib in Child-Pugh class A (CP-A) patients with primary advanced hepatocellular carcinoma (HCC) and Barcelona Clinic Liver Cancer (BCLC) stage B/C[2].The REFLECT trial showed the non-inferiority of lenvatinib to sorafenib in terms of the duration of survival[3];however,the trial also showed that lenvatinib was significantly superior to sorafenib in terms of the progression-free survival and overall response rate (ORR) in the trial;therefore,lenvatinib is often administered in preference to sorafenib as first-line chemotherapy for patients with advanced HCC who are not suitable candidates for locoregional treatment.
Patients with BCLC stage C HCC have heterogeneous background factors.In a retrospective study of BCLC stage C HCC patients treated with various therapeutic regimens,a serum Alpha-Fetoprotein (AFP) level of>200 ng/mL,tumor diameter of>50 mm,and presence of macrovascular invasion prior to the start of treatment were identified as poor prognostic factors[4].In another retrospective analysis of patients with BCLC stage C HCC treated with various therapeutic regimens,a tumor diameter of ≥ 100 mm,presence of a tumor thrombus in the main portal vein (Vp4),presence of distant metastasis,and poor residual liver function were identified as independent poor prognostic factors[5].In addition,a subgroup analysis of the SHARP trial also identified portal vein invasion and extrahepatic metastasis as poor prognostic factors in patients with HCC.However,according to both the SHARP trial and one retrospective analysis,treatment with sorafenib improved the overall survival,as compared to placebo or no treatment,in advanced CP-A HCC patients with a tumor thrombus in the main portal vein and/or extrahepatic metastasis,both of which correspond to BCLC stage C disease[2,6].
On the other hand,presence of a tumor thrombus in the main portal vein and a high tumor burden (tumor occupancy>50% of the total liver volume) were listed as exclusion criteria in the REFLECT trial.Therefore,the American Association for the study of Liver Disease guideline and European Association for the Study of the Liver guideline recommend that advanced HCC patients with a tumor thrombus in the main portal vein and/or a high tumor burden be excluded from the indications for lenvatinib administration[7,8].Thus,sorafenib is often considered to be the agent of first choice for the treatment of patients with primary CP-A HCC with a tumor thrombus in the main portal vein and/or a high tumor burden.However,the National Comprehensive Cancer Network guideline recommends lenvatinib as the first-line chemotherapy for CP-A HCC patients,without limitations imposed by the background factors[9].While the safety of lenvatinib in advanced BCLC stage C HCC patients with portal vein invasion remains unclear,there is a case report of long-term disease control having been achieved with lenvatinib administration,after controlling for adverse events,in an HCC patient with portal vein invasion[10].
Herein,we report two patients with advanced BCLC stage C HCC,both of whom had a tumor thrombus in the main portal vein,a high tumor burden,and wellpreserved liver function,in whom lenvatinib administration caused necrosis of the main portal vein tumor thrombus,and yielded good treatment outcomes,without the occurrence any adverse events necessitating treatment discontinuation.
CASE PRESENTATION
Chief complaints
Case 1 was a 53-year-old man who had visited previous hospital with a 2-mo history of right hypochondrial pain,and case 2 presented with the chief complaint of lower abdominal pain.
History of present illness
Case 1 presented with the complaint of right back pain that did not improve even after rest,and case 2 presented with prolonged lower abdominal pain that persisted even after treatment with medicines for enteritis given by the previous doctor.
History of past illness
Case 1 had never been diagnosed as having hepatitis until he visited our hospital.Case 2 was diagnosed as having chronic hepatitis C as she was being evaluated for enteritis at previous hospital.Other than the above,both patients denied having any history of diabetes or surgery,being on any medications or having any food allergies.Their vaccination history was unknown.
Personal and family history
Both patients had a history of smoking,and case 1 gave a history of drinking for more than 30 years.Neither patient had any family history of liver disease,history of blood transfusion/blood product use,or any recent history of use of hepatotoxic drugs.
Physical examination upon admission
The ECOG-PS of both patients was 1 and their Karnofsky Performance Scale score was 90.Physical examination revealed no yellow staining of the skin or sclera in either patient.Abdominal examination revealed a flat abdomen and mild tenderness in the upper abdomen in case 1,and mild tenderness in the lower abdomen in case 2.
Laboratory examinations
Blood tests at our hospital in case 1 revealed positive results for anti-HBs antigen and anti-HBe antibody,and a negative result for anti-HBe antigen,and the patient was diagnosed as having chronic hepatitis B.The serum HBV DNA titer was 5.0 log IU/mL.Other laboratory test results were as follows:Serum total bilirubin 17.10 μmol/L,serum albumin 40 g/L,serum alanine aminotransferase 405 U/L,serum aspartate aminotransferase 86 U/L,serum alkaline phosphatase 566 U/L,serum alpha-fetoprotein 6487 ng/mL,and serum protein induced by Vitamin K absence or antagonists-II 11900 mAU/mL.The laboratory examination revealed no significant abnormalities other than the above.His residual liver function was classified as CP-A(5 points) and modified Albumin-Bilirubin (mALBI) grade 2A (-2.59 points)[11,12].
Laboratory tests at our hospital in case 2 revealed the following:Serum total bilirubin 22.23 μmol/L,serum albumin 39 g/L,serum alanine aminotransferase 44 U/L,serum aspartate aminotransferase 121 U/L,serum alkaline phosphatase 318 U/L,serum alpha-fetoprotein 18.4 ng/mL,and serum protein induced by Vitamin K absence or antagonists-II 3110 mAU/mL.The serum test for anti-HCV antigen was positive,and the HCV RNA titer was 5.8 log IU/mL.The laboratory examination revealed no significant abnormalities other than the above.Her residual liver function was classified as CP-A (6 points) and mALBI grade 2A (-2.43 points)[11,12].
Imaging examinations
Case 1:Abdominal ultrasonography at the previous hospital showed multiple tumors in both hepatic lobes.Dynamic contrast-enhanced computed tomography (CT) at our hospital revealed scattered small nodules in both lung fields and multiple tumors in both hepatic lobes.The tumor located in the right hepatic lobe measured 130 mm in maximum diameter.This tumor was associated with a tumor thrombus in the main portal vein (Vp4) (Figure1A) and inferior vena cava (Vv3) (Figure2A),and a high tumor burden.The main portal vein was completely obstructed,and there were no apparent collateral veins.Dynamic contrast-enhanced CT showed early enhancement and subsequent washout of the entire hepatic tumor.
Case 2:Dynamic contrast-enhanced CT revealed a small nodule in the upper lobe of the left lung and left adrenal gland,and multiple tumors in both the hepatic lobes.A tumor located in the right hepatic lobe measured 190 mm in maximum diameter.This tumor was associated with a tumor thrombus in the main portal vein (Vp4) (Figure3A) and inferior vena cava (Vv3) (Figure4A),and a high tumor burden.The main portal vein was almost completely obstructed,and there were no apparent collateral veins.Dynamic contrast-enhanced CT showed early enhancement and subsequent washout of the entire hepatic tumor.
FINAL DIAGNOSIS
Both patients were diagnosed as having HCC with a tumor thrombus in the main portal vein and intrahepatic and distant metastases;furthermore,the HCC in both patients was classified as BCLC stage C,and cT4N0M1,stage IVB,according to the UICC classification 8thedition.
TREATMENT
According to the American Association for the study of Liver Disease guideline,we initiated both patients on systemic chemotherapy.
Case 1
Informed consent for treatment was obtained from the patient and his relatives.His body weight at his first visit to our department was 56 kg.We started the patient on lenvatinib 8 mg/d.At one week after the start of lenvatinib administration,he developed high fever (body temperature 40 °C).Dynamic contrast-enhanced CT was performed at 2 wk after the start of lenvatinib administration to determine if the fever represented an adverse event or was associated with tumor necrosis (Figures 1B and 2B).There were no interval changes in the size or number of the HCCs,however,decreased hypervascularity of the HCC associated with the tumor thrombus in both the main portal vein and the inferior vena cava was observed in the arterial-phase images (Figures 1B and 2B).Furthermore,the serum AFP level had decreased to 3259 ng/mL.Based on the findings,we regarded the fever as having arisen from tumor necrosis and decided to continue the lenvatinib administration without any dose reduction.At 8 wk after the start of lenvatinib administration,the maximum tumor response was rated as partial response (PR) according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) (Figures 1C and 2C)[13].During treatment,the serum AFP level continued to decline,and the mALBI score continued to improve from 2 wk onward after the start of lenvatinib administration (Figure5).At 14 wk after the start of lenvatinib administration,the portal vein tumor thrombus decreased in size and collateral veins around the portal vein could be clearly visualized on dynamic contrast-enhanced CT (Figure1D).There were no apparent hepatic encephalopathy episodes or adverse events other than grade 2 fever(Common Terminology Criteria for Adverse Events version 4.0 [CTCAE v4.0]).
Case 2
Informed consent was obtained from the patient and her relatives.The body weight of the patient at her first visit to our department was 45 kg.She showed evidence of a high tumor burden,and we started her on treatment with lenvatinib 4 mg/d.At one week after the start of lenvatinib administration,she developed high fever (grade 1)and she recovered without specific therapy.Dynamic contrast-enhanced CT was performed at 4 wk after the start of lenvatinib administration,which revealed moderate shrinkage of the HCCs,and marked decrease in the hypervascularity of the largest liver tumor and tumor thrombus in the inferior vena cava in the arterial-phase images (Figures 3B and 4B).At that point,the response was rated as PR according to mRECIST[13].Furthermore,the serum AFP level decreased to within normal limits at 2 wk after the start of lenvatinib administration.The mALBI score worsened once after the start of treatment,but recovered and,in fact,improved by 5 wk after the start of treatment (Figure6).In response to this,we increased the lenvatinib dose to 8 mg/d.At 9 wk after the start of lenvatinib administration,the response was rated as PR according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1).At the same time,both the portal vein tumor thrombus and inferior vena cava thrombus decreased in size,and collateral veins around the portal vein could be clearly visualized (Figures 3C,D and 4C).
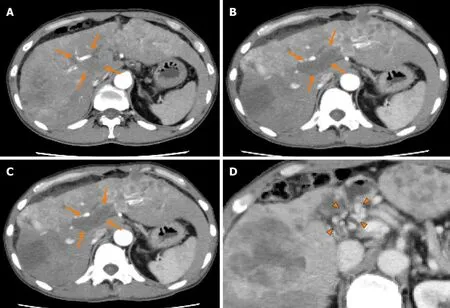
Figure1 Dynamic contrast-enhanced computed tomography findings of the tumor thrombus in the main portal vein in case 1.A:Arterial-phase computed tomography (CT) image prior to the start of lenvatinib administration showing marked vascularity of the hepatocellular carcinomas (HCCs),and a tumor thrombus in the main portal vein (arrows);B:Arterial-phase CT image at 2 wk after the start of lenvatinib administration showing a marked decrease of the vascularity of both the HCCs and the tumor thrombus in the main portal vein (arrows);C:Arterial-phase CT image at 8 wk after the start of lenvatinib administration showing a continuous decrease of the vascularity of both the HCCs and the tumor thrombus in the main portal vein,and decrease of the size of the tumor thrombus in the main portal vein(arrows);and D:Portal-phase CT image at 14 wk after the start of lenvatinib administration showing collateral veins around the main portal vein (arrowheads).
OUTCOME AND FOLLOW-UP
Case 1
The serum AFP level increased,and the mALBI score worsened at 6 mo after the start of lenvatinib administration.Dynamic contrast-enhanced CT after 6 mo of lenvatinib administration revealed the appearance of new intrahepatic metastases and enlargement of both the primary liver lesion and the pulmonary nodules,with the emergence of ascites around the liver,and the treatment response was rated as progressive disease according to mRECIST (Figure7A and 7B).After informed consent was obtained from the patient and his relatives,the treatment was switched to regorafenib at the starting dose of 120 mg/d.At 2 wk after the start of regorafenib administration,the patient reported reduced dietary intake,which was suspected as being due to progression of the HCC.Thereafter,the patient desired to receive treatment at his native place and the treatment at our hospital ended at 8 mo after the start of lenvatinib administration.
Case 2
The dose of lenvatinib was reduced to 4 mg/d at 19 wk after the start of lenvatinib administration because the patient developed grade 1 thrombocytopenia.At 21 wk after the start of lenvatinib administration,the patient developed grade 2 hyperthyroidism.Thiamazole treatment was started and the patient recovered by 21 wk after the start of treatment.The treatment response of PR according to mRECIST and RECIST v1.1 was maintained until 80 wk after the start of lenvatinib administration (Figure8A and B).
DISCUSSION
Although our patients had primary advanced HCC with a high tumor burden and a tumor thrombus in the main portal vein (Vp4),lenvatinib was successfully administered without the appearance of any adverse events necessitating treatment discontinuation.Moreover,the therapeutic responses were rated as PR according to mRECIST,and the antitumor effect was sustained for a long time,with declining serum AFP levels.Thus,even in patients with advanced HCC with a high tumor burden and a tumor thrombus in the main portal vein (Vp4),if the liver function is well-preserved,lenvatinib can be safe and effective.
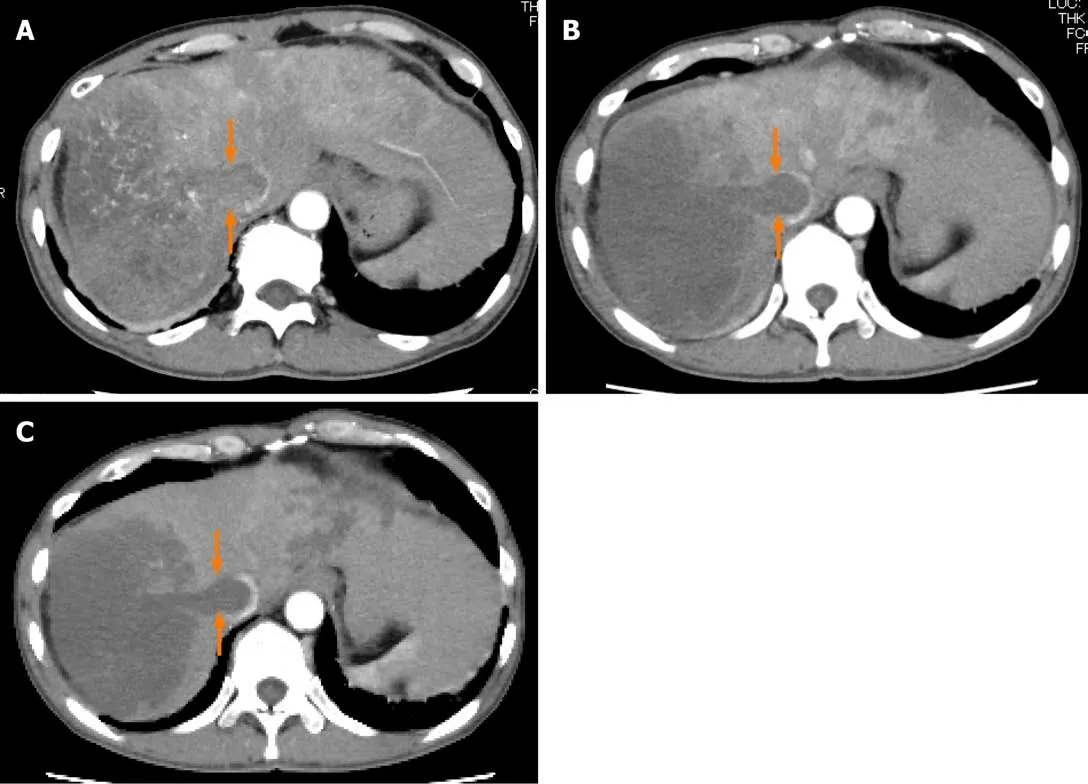
Figure2 Dynamic contrast-enhanced computed tomography findings of a tumor thrombus in the inferior vena cava in case 1.A:Arterial-phase computed tomography (CT) image prior to the start of lenvatinib administration showing marked vascularity of the tumor thrombus in the inferior vena cava (arrows);B:Arterialphase CT image at 2 wk after the start of lenvatinib administration showing the tumor thrombus in the inferior vena cava (arrows);and C:Arterial-phase CT image at 8 wk after the start of lenvatinib administration showing the tumor thrombus in the inferior vena cava (arrows).
Patients with BCLC stage C HCC have heterogeneous background factors,and a high serum AFP level,large tumor size,presence of macrovascular invasion,presence of distant metastasis and poor residual liver function prior to the start of treatment are reported as poor prognostic factors in BCLC stage C HCC patients[5-8].Although both our patients had multiple poor prognostic factors,including a tumor diameter of ≥100 mm,tumor thrombus in the main portal vein,and distant metastasis,and case 1 had a high serum concentration of AFP,we elected to treat the patients with lenvatinib,because in the REFLECT trial,lenvatinib treatment was shown to yield a superior progression-free survival as compared sorafenib in patients with wellpreserved liver function.In our cases,the residual liver function was classified as CPA and mALBI grade 2A;the mALBI grade is calculated from the serum bilirubin and albumin levels and has been proposed as a new liver function indicator in addition to the Child-Pugh score.In a clinical trial of sorafenib for patients with advanced HCC with well-preserved liver function (Child-Pugh score 5-7) who were unsuitable candidates for loco-regional therapy[14],a subgroup analysis revealed a significant difference in the overall survival and a different tendency in decline of the residual liver function after sorafenib administration between patients classified as mALBI grade 1-2A and 2B before the start of treatment[15].Therefore,it was proposed that the eligibility judgment for sorafenib administration be made by the mALBI grade.In addition,lenvatinib administration has been reported to yield significantly higher response rates and lower treatment discontinuation due to adverse events in advanced HCC patients with Child-Pugh score 5 and ALBI grade 1 than in other groups[16].It is expected that the mALBI grade could also become established as a reliable basis for determining the eligibility for treatment with lenvatinib.
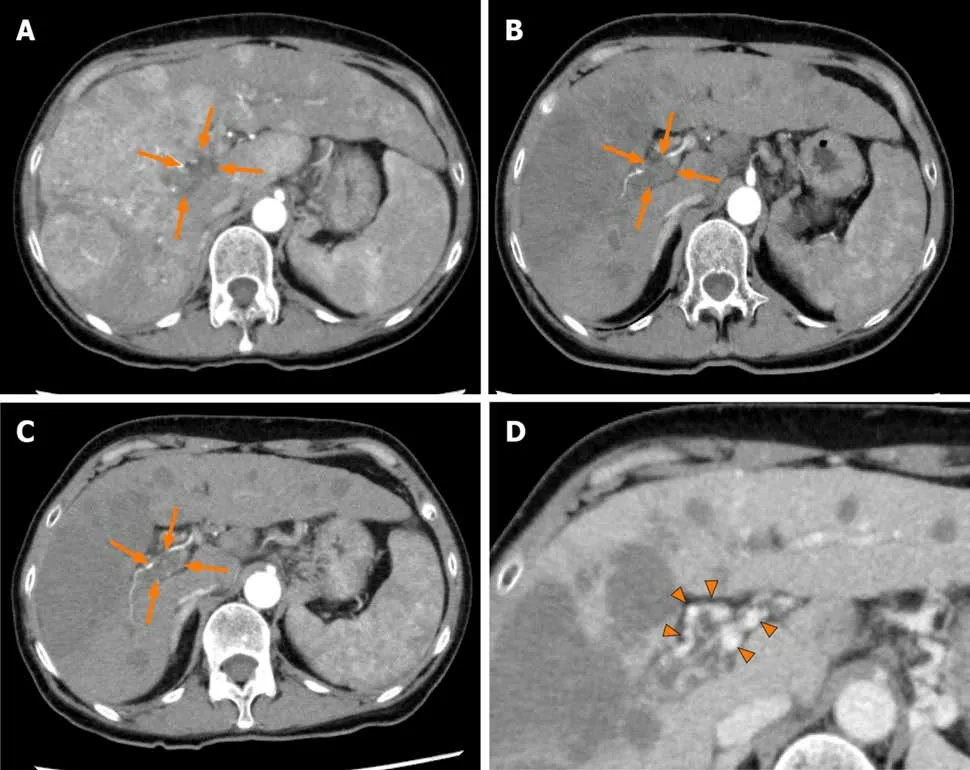
Figure3 Dynamic contrast-enhanced computed tomography findings of the tumor thrombus in the main portal vein in case 2.A:Arterial-phase computed tomography (CT) image prior to the start of lenvatinib administration showing marked vascularity of the hepatocellular carcinomas,and a tumor thrombus in the main portal vein (arrows);B:Arterial-phase CT image at 4 wk after the start of lenvatinib administration showing a marked decrease of the vascularity of the hepatocellular carcinomas and decrease of the size of the tumor thrombus in the main portal vein (arrows);C:Arterial-phase CT image at 9 wk after the start of lenvatinib administration showing a continuous decrease of the vascularity of both the hepatocellular carcinomas and the size of the tumor thrombus in the main portal vein(arrows);and D:Portal-phase CT image at 9 wk after the start of lenvatinib administration showing collateral veins around the main portal vein (arrowheads).
On the other hand,our case 1 developed grade 2 fever one week after the start of lenvatinib administration.To determine if the fever arose as an adverse event,such as drug allergy,or from tumor necrosis,dynamic contrast-enhanced CT was performed;based on the results of this imaging examination,we determined tumor necrosis as the cause of the fever and followed up the patient about once a week.In addition,as both the patients had macrovascular invasion,we were concerned about hepatic dysfunction occurring due to decreased hepatic blood flow,which however,did not occur.One possible reason for this could be that the collateral veins around the portal vein,called cavernous transformation of the portal vein,maintained the hepatic blood flow.Cavernous transformation of the portal vein has been reported in HCC patients with a tumor thrombus in the main portal vein and reported as collateral veins of the biliary and gastric branches of the portal vein[17].In advanced HCC patients with multiple poor prognostic factors,it is essential to pay attention to the possibility of emergence of adverse events and hepatic dysfunction after the initiation of lenvatinib treatment.
We started case 1 on regorafenib treatment as second-line therapy after failure of lenvatinib treatment.Regorafenib is pharmacologically more potent than sorafenib due to differences in the molecular structure[18];therefore,we decided to administer regorafenib while paying attention to possible adverse effects.
In both our patients,dynamic contrast-enhanced CT showed decrease of the tumor hypervascularity as early as by 4 wk after the start of lenvatinib administration,and the therapeutic response of PR according to mRECIST was achieved within 8 wk after the start of lenvatinib administration;the antitumor effect was sustained for a long period,with declining serum AFP levels.Hiraokaet al[19]also reported an early therapeutic response as follows,from a study of lenvatinib treatment in patients with advanced unresectable hepatocellular carcinoma:The ORR was 40.7% and the disease control rate was 85.2%,according to mRECIST,at 4 wk after the start of lenvatinib administration,and the ORR and disease control rate in tyrosine-kinase inhibitornaïve patients were 50.0% and 87.5%,respectively[19].Shoet al[20]and Kuzuyaet al[21]reported that high early therapeutic response was obtained in advanced HCC patients treated with lenvatinib,including CP-B patients,which was set as an exclusion criterion in the REFLECT trial[20,21].It has been suggested that in cases where lenvatinib proves to be effective,the tumor hypervascularity begins to decrease and the treatment effect is obtained in the early period after the start of lenvatinib administration.In addition,in our case 1,the serum AFP level decreased by 50% by 2 wk after the start of lenvatinib administration.Hiraokaet al[22]reported that the serum AFP level decreased significantly in advanced HCC patients in which disease control was obtained by 4 wk after the start of lenvatinib administration[22].Kodamaet al[23]also reported a relationship between higher objective response based on imaging findings and early decline of the serum AFP levels[23].It is possible that early evaluation of the tumor hypervascularity and measurement of the serum AFP level after the start of lenvatinib administration represent predictors of the treatment response to the drug.
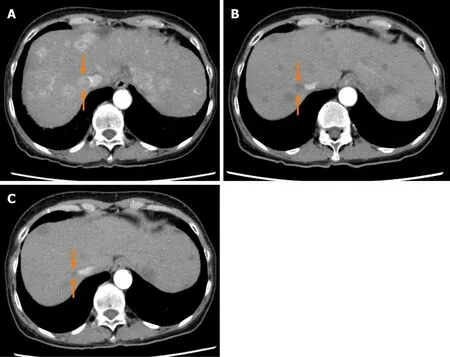
Figure4 Dynamic contrast-enhanced computed tomography findings of a tumor thrombus in the inferior vena cava in case 2.A:Arterial-phase computed tomography (CT) image prior to the start of lenvatinib administration showing a tumor thrombus in the inferior vena cava (arrows);B:Arterial-phase CT image at 4 wk after the start of lenvatinib administration showing a marked decrease in both the vascularity and the size of the tumor thrombus in the inferior vena cava (arrows);and C:Arterial-phase CT image at 9 wk after the start of lenvatinib administration showing a continuous decrease in both the vascularity and the size of the tumor thrombus in the inferior vena cava (arrows).
CONCLUSION
We report the cases of two advanced BCLCL stage C HCC patients with a tumor thrombus in the main portal vein and a high tumor burden,in whom lenvatinib treatment,administered with attention paid to the possible development of adverse events,was found to be effective and safe.Further accumulation of cases is necessary to endorse and establish the efficacy and safety of lenvatinib for advanced BCLC stage C HCC patients with a tumor thrombus in the main portal vein and a high tumor burden.

Figure5 Time course of the serum alpha-fetoprotein and modified albumin-bilirubin score during chemotherapy in case 1.The serum alpha-fetoprotein level began to decrease soon after the start of lenvatinib administration and continued to decrease until 24 wk after the start of administration,after which it began to rise again,which possibly reflected the therapeutic response,according to the modified Response Evaluation Criteria in Solid Tumors,of progressive disease,at 6 mo after the start of lenvatinib administration.The modified albumin-bilirubin score improved,after initial worsening for up to 2 wk after the start of lenvatinib administration but showed peak worsening again at 14 wk after the start of lenvatinib administration.AFP:Alpha-fetoprotein;ALBI:Albumin-bilirubin.

Figure6 Time course of the serum alpha-fetoprotein and modified albumin-bilirubin score during chemotherapy in case 2.The serum alpha-fetoprotein level decreased to normal at 2 wk after the start of administration,with no apparent subsequent rise.The modified albumin-bilirubin score improved,after initial worsening for up to 2 wk after the start of lenvatinib administration but recovered to improve at 5 wk after the treatment.AFP:Alpha-fetoprotein;ALBI:Albumin-bilirubin.

Figure7 Dynamic contrast-enhanced computed tomography findings at 6 mo after lenvatinib administration in case 1.A,B:Arterial-phase computed tomography image at 6 mo after the start of lenvatinib administration showing newly developed intrahepatic metastases and a tumor growth of the hepatocellular carcinomas and no obvious change in the size of the tumor thrombus in the main portal vein (arrows) (A),and the emergence of ascites around the liver were observed (B).
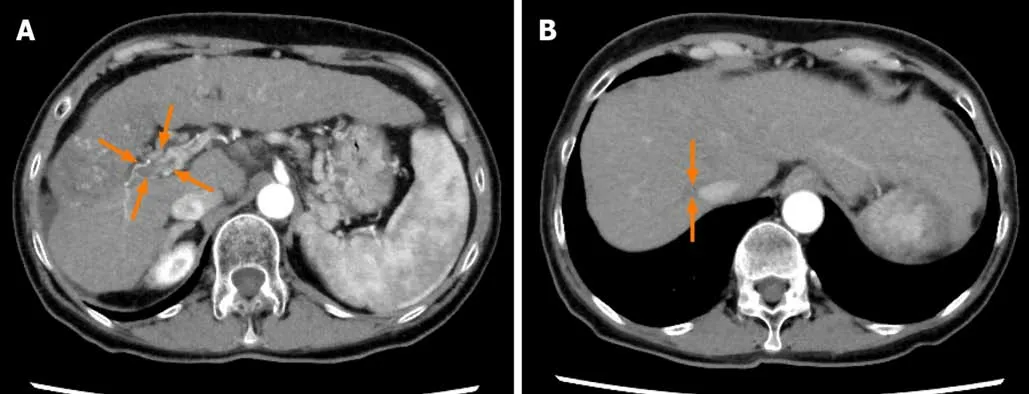
Figure8 Dynamic contrast-enhanced computed tomography findings at 80 wk after lenvatinib administration in case 2.A and B:Arterial-phase computed tomography image at 80 wk after the start of lenvatinib administration showing further decrease of the size of the hepatocellular carcinomas and the tumor thrombus in the main portal vein (arrows) (A) and further decrease of the size of the tumor thrombus in the inferior vena cava (arrows) (B).
杂志排行
World Journal of Clinical Cases的其它文章
- Assessment of diaphragmatic function by ultrasonography:Current approach and perspectives
- Computer navigation-assisted minimally invasive percutaneous screw placement for pelvic fractures
- Research on diagnosis-related group grouping of inpatient medical expenditure in colorectal cancer patients based on a decision tree model
- Evaluation of internal and shell stiffness in the differential diagnosis of breast non-mass lesions by shear wave elastography
- Real-time three-dimensional echocardiography predicts cardiotoxicity induced by postoperative chemotherapy in breast cancer patients
- Biopsy-proven acute phosphate nephropathy:A case report
