Mechanisms and anatomical risk factors of pneumothorax after Bevacizumab use:A case report
2020-09-14
Yuri Ozaki,Akiyo Yoshimura,Masataka Sawaki,Masaya Hattori,Naomi Gondo,Haruru Kotani,Yayoi Adachi,Ayumi Kataoka,Kayoko Sugino,Nanae Horisawa,Yuka Endo,Kazuki Nozawa,Shoko Sakamoto,Hiroji Iwata,Department of Breast Oncology,Aichi Cancer Center Hospital,Nagoya 464-8681,Japan
Abstract
Key words:Breast cancer;Lung metastasis;Bevacizumab;Adverse event;Pneumothorax;Case report
INTRODUCTION
Bevacizumab (BV) is a monoclonal humanized antibody targeting vascular endothelial growth factor (VEGF).BV exerts its high antitumor effect by suppressing tumor angiogenesis and improving the delivery of chemotherapeutic drugs through normalization of the vascular plexus in tumors[1-4].BV also synergizes with chemotherapeutic agents to promote tumor regression.However,BV-containing combination therapies can induce severe adverse events such as gastrointestinal perforation[5-7],hemorrhage[8,9],delayed wound healing[10],and pneumothorax[11-14].Although there have been few reports of pneumothorax,the risk factors and molecular mechanisms that underlie the onset of pneumothorax are not clear[11,14].Here,we report a case of pneumothorax associated with a combination therapy of BV with chemotherapy,and discuss the molecular mechanisms and risk factors that were associated with this serious adverse event.
CASE PRESENTATION
Chief complaints
A 45-year-old female who was diagnosed with lung metastases of breast cancer and received combination therapy with Bevacizumab and chemotherapy developed dyspnea.
History of present illness
The case is a 45-year-old female.She visited our hospital because of an abnormality in her mammography and was diagnosed with left breast cancer (cStageT3N1M0).Pathological analysis of a core needle biopsy sample indicated a triple negative subtype (estrogen receptor-negative,progesterone receptor-negative and human epidermal growth factor receptor 2-negative).We planned 4 cycles of 5-fluorouracil +epirubicin + cyclophosphamide (FEC) followed by weekly paclitaxel (PTX) given 12 times as neoadjuvant chemotherapy.However neoadjuvant chemotherapy was stopped because of progression during weekly PTX;a left mastectomy and axillary lymph node dissection was then performed.The patient received postoperative chemotherapy (2 cycles of FEC followed by 8 cycles of capecitabine),but bilateral lung metastases were detected after the 5thcycle of capecitabine following a chest computed tomography (CT) that was performed due to her complaint of a persistent cough.
Some masses were on the pleura and one of the masses developed surrounding a bronchus at the right lower lobe (Figure 1 and 2).We made a diagnosis of multiple lung metastases of breast cancer.BV + PTX therapy was started due to its expected high antitumor effect.One cycle was 28 days,and BV was administered at 10 mg/kg on days 1 and 8 and PTX was administered at 80 mg/m2on days 1,8 and 15.The patient noticed dyspnea before cycle 3,day 1.
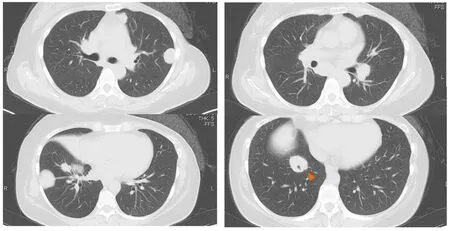
Figure1 Chest computed tomography showing multiple bilateral masses.
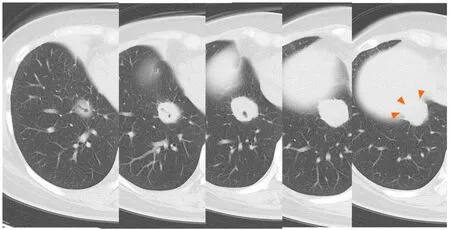
Figure2 The mass around the bronchi is on the pleura above the diaphragm (arrows).
Physical examination upon admission
She was tachypnea.The oxygen saturation at atmospheric pressure was 95%.
Laboratory examinations
There were no noticeable abnormalities.
Imaging examination
A right pneumothorax was diagnosed following a chest X-ray.The coronal plane CT revealed one solid mass replaced by a cavity that passed through the bronchus in the right lower lobe (Figure 3).The cavity eventually ruptured the pleura and made the bronchopleural fistula that led to this pneumothorax (Figure 4).
FINAL DIAGNOSIS
We diagnosed pneumothorax due to bronchopleural fistula that is associated with rapid necrosis of the lung metastasis.
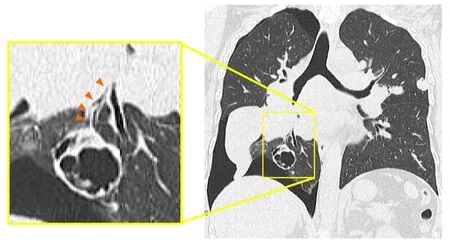
Figure3 Coronal plane chest computed tomography showing a cavity replaced by a solid mass which was connected to the bronchi in the right lower lobe.
TREATMENT
The patient was admitted to our hospital for management of the pneumothorax.Thoracic cavity drainage using an intercostal catheter was performed.Supplemental oxygen was not required.On the 2ndday,the leakage disappeared.On the 4th,the right lung was fully expanded on the X-ray and the drain was clamped.On the 6th,the drain was withdrawn.On the 7th,the patient was discharged from our hospital on recovery.
OUTCOME AND FOLLOW-UP
No recurrence of pneumothorax was observed following release from hospital.The continuance of chemotherapy was made possible by changing the regimen (irinotecan,gemcitabine + carboplatin).The patient died due to breast cancer 23 mo after her first treatment,which was 9 mo from the occurrence of the pneumothorax.
DISCUSSION
BV is associated with the occurrence of several adverse events,such as gastrointestinal perforation[5-7],hemorrhage[8,9],delayed wound healing[10]and pneumothorax[11-14].Although there have been few reports of pneumothorax,the risk factors and molecular mechanisms that underlie the onset of pneumothorax are not clear[11,14].
A more effective delivery of chemotherapeutic agents during BV treatment may explain the onset of gastrointestinal perforation in colon cancer[5].This is because the drugs induced rapid necrosis in the tumor,which infiltrates the serosa.Fatal hemoptysis in patients with lung squamous carcinoma has been reported in a phase II trial[9].This suggests that centrally located lung tumors close to major blood vessels might be linked to severe pulmonary hemorrhage,since they may lead to collapsed vessels.And,as for breast cancer,a case was reported in which axillary arterial bleeding occurred after BV chemotherapy induced axillary metastatic lymph node necrosis[8].
BV promotes tumor regression by normalizing the vascular plexus in tumors,which is associated with improved delivery of chemotherapeutic drugs.This causes necrosis of tumors that lie close to important organs,and leads to severe adverse events[1-4].Another adverse effect associated with BV is delayed wound healing,as it inhibits the physiological endothelial repair processes mediated by VEGF[10].
The response of tumors to BV chemotherapy can to some extent explain why the solid mass in this case developed a cavity.The tumor surrounded the bronchi,and therefore induction of rapid necrosis and discharge of breakdown productsviathe bronchi would lead to cavity formation.This may be unique to this particular case,where only one solid mass surrounded the bronchi and became cavitary.
Another anatomical feature of this case is that the mass was on the pleura,and was therefore also susceptible to necrosis in this location.The cavity most likely ruptured the pleura,creating the bronchopleural fistula that led to this pneumothorax.The delayed wound healing caused by BV may also be associated with fistula formation.
CONCLUSION
Patients with multiple lung metastases surrounding the bronchi and on the pleura,should be monitored for pneumothorax,an adverse event that can be induced by the rapid necrosis due to Bevacizumab-containing combination chemotherapies.
杂志排行
World Journal of Clinical Oncology的其它文章
- Complete response in anaplastic lymphoma kinase–rearranged oncocytic thyroid cancer:A case report and review of literature
- Is there a role for treatment-oriented surgery in liver metastases from gastric cancer?
- Why natural killer cells in triple negative breast cancer?
- Circulating cell-free nucleic acids as prognostic and therapy predictive tools for metastatic castrate-resistant prostate cancer
- MUTYH:Not just polyposis
- Role of imaging biomarkers in mutation-driven non-small cell lung cancer
