Role of imaging biomarkers in mutation-driven non-small cell lung cancer
2020-09-14
Dexter P Mendoza,Subba R Digumarthy,Division of Thoracic Imaging and Intervention,Department of Radiology,Massachusetts General Hospital,Boston,MA 02114,United States
Zofia Piotrowska,Massachusetts General Hospital Cancer Center and Department of Medicine,Massachusetts General Hospital,Boston,MA 02114,United States
Jochen K Lennerz,Center for Integrated Diagnostics,Department of Pathology,Massachusetts General Hospital,Boston,MA 02114,United States
Abstract
Key words:Non-small cell lung cancer;Imaging biomarker;Targeted therapy;Oncogenic mutations;Radiomics;Metastatic pattern
INTRODUCTION
Lung cancer results in millions of deaths annually and is the leading cause of cancerrelated deaths worldwide[1].Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancers,and more than half of NSCLC are metastatic at the time of diagnosis[2].The prognosis in cases of metastatic NSCLC remains dismal despite advances in treatment,with five-year survival rates of approximately 5%[2].
Genotyping studies have revealed genetic heterogeneity in NSCLC and identified several key driver mutations,many of which have been found to be targetable or potentially targetable[3].Mutations with currently approved targeted therapies(Table 1) includeEGFRmutations,ALKrearrangements,ROS1 rearrangements,BRAFmutations,andNTRKgene fusions[4].There are other driver mutations in NSCLC for which targeted therapies are under investigation in clinical trials or available as offlabel use of agents approved for other indications.These include mutations involving rearranged during transfection proto-oncogene (RET),MET,human epidermal growth factor receptor 2 (HER2),andKRASgenes.
In certain patient subgroups,targeted therapy can improve outcomes,making the detection of these mutations an important step in developing personalized treatment strategies.Several (phenotypic) biomarkers have been reported to suggest the presence of specific mutations in NSCLC and to predict responsiveness to certain targeted therapies.The fundamental principle of these biomarkers is that their presence may be indicative of a specific underlying driver mutation in NSCLC and these biomarkers include clinical,pathologic,as well as imaging features.
MOLECULAR TESTING PLATFORMS
Given the success of targeted therapy in certain molecular subsets of patients,screening for driver mutations has become an essential step in the evaluation of patients with newly diagnosed NSCLCs.Current guidelines,including those from the College of American Pathologists,International Association for the Study of Lung Cancer,and Association of Molecular Pathologists,now recommend assessment for the presence of driver mutations in patients with advanced NSCLC,specifically in those with adenocarcinoma[4,5].
While screening for driver mutations has been widely adopted in clinical practice,no standard screening platform has been established.The optimal testing platform would be accurate,cost-effective,and with a fast turnaround time.The methods currently available offer these features to varying degrees.Their sensitivities may also depend on the mutation being assessed.As such,no single platform has emerged as the optimal testing method for all (Table 2).
The techniques most commonly employed in molecular analysis of tumor tissue include direct gene sequencing,allele-specific sequencing by polymerase chain reaction (PCR),next generation sequencing (NGS),fluorescence in situ hybridization(FISH) and evaluation of protein expression by immunohistochemistry (IHC).All of these techniques require tissue samples.Direct gene sequencing (i.e.,Sanger sequencing) was one of the first methods used to perform genotyping but has largely been replaced since by the other methods as it requires a higher tumor cellularity in tissue samples and is more prone to false negative results.
In allele-specific tissue testing,raw DNA is amplified using PCR and is then analyzed for specific abnormalities.Amplification with PCR allows for greater sensitivity and allows for testing of more than one abnormality at a time (i.e.,multiplex testing).Its main drawback is that is can only test for predefined abnormalities and is unable to detect new mutations.
FISH testing can be used to detect gene rearrangements,amplifications,or deletions.It is highly sensitive in detecting rearrangements in theALK,ROS1,andRETgenes as well asMETamplification andNTRKfusion[6,7].Tissue IHC has also been found to be highly sensitive and specific in detectingALKandROS1rearrangements by detecting expression of abnormal proteins,but it has not been as helpful in the detection of other mutations[8,9].It is also routinely used in determiningPD-L1expressivity in tumor cells[10].

Table1 Targetable genotypes in non-small cell lung cancer with Food and Drug Administration-approved targeted therapies
Finally,NGS is an automated platform that,like allele-specific sequencing,can simultaneously test for multiple genetic abnormalities.It is highly sensitive in the detection ofEGFR,HER2,METex14,BRAF,andKRASmutations[6,11].It can also detectALK,ROS1,andRETrearrangements,but with lower sensitivity[6,11],and identify novel mutations.The main drawback is cost,as NGS demands advanced bioinformatics systems,fast and complex data processing,and large data storage requirements.Another potential challenge of NGS is the detection of novel variants and mutations of indeterminate significance.
CLINICOPATHOLOGIC BIOMARKERS IN MUTATED NSCLC
Although testing for several driver mutations is now standard of care in the management of advanced NSCLC,significant disparities in compliance with recommended exist around the world,and even within the United States[12,13].The identification of clinical,pathologic,and imaging biomarkers has the potential to improve compliance and mitigate these disparities.Moreover,identification of these biomarkers has the potential to lower cost by helping to identify the patients who may benefit the most from molecular testing and by assisting in in the selection of the most appropriate testing algorithm.
Several clinicopathologic features have been associated with the presence of certain mutations (Table 3).EGFRmutations are the first molecular alterations in lung cancer shown to confer sensitivity to specific targeted therapies.EGFRmutations are identified in approximately 15% of lung adenocarcinomas in the United States but have been reported in up to approximately 60% of Asian cases[14].Affected patients tend to be younger with minimal or absent history of smoking[15].Several generations of tyrosine kinase inhibitors (TKI) have been approved as first-line treatment in advancedEGFR-mutant NSCLC[16-22].
ALKgene rearrangements,most commonly resulting in fusion ofALKtoechinoderm microtubule-associated protein-like 4 (EML4),are reported in approximately 5% of NSCLC[23,24].Similar toEGFR-mutant NSCLC,ALK-positive NSCLCs are more common in younger patients with minimal or no smoking history[25,26].SeveralALK-targeted TKIs have been shown to be highly effective in treatingALK-positive NSCLC and are now Food and Drug Administration-approved[27-32].
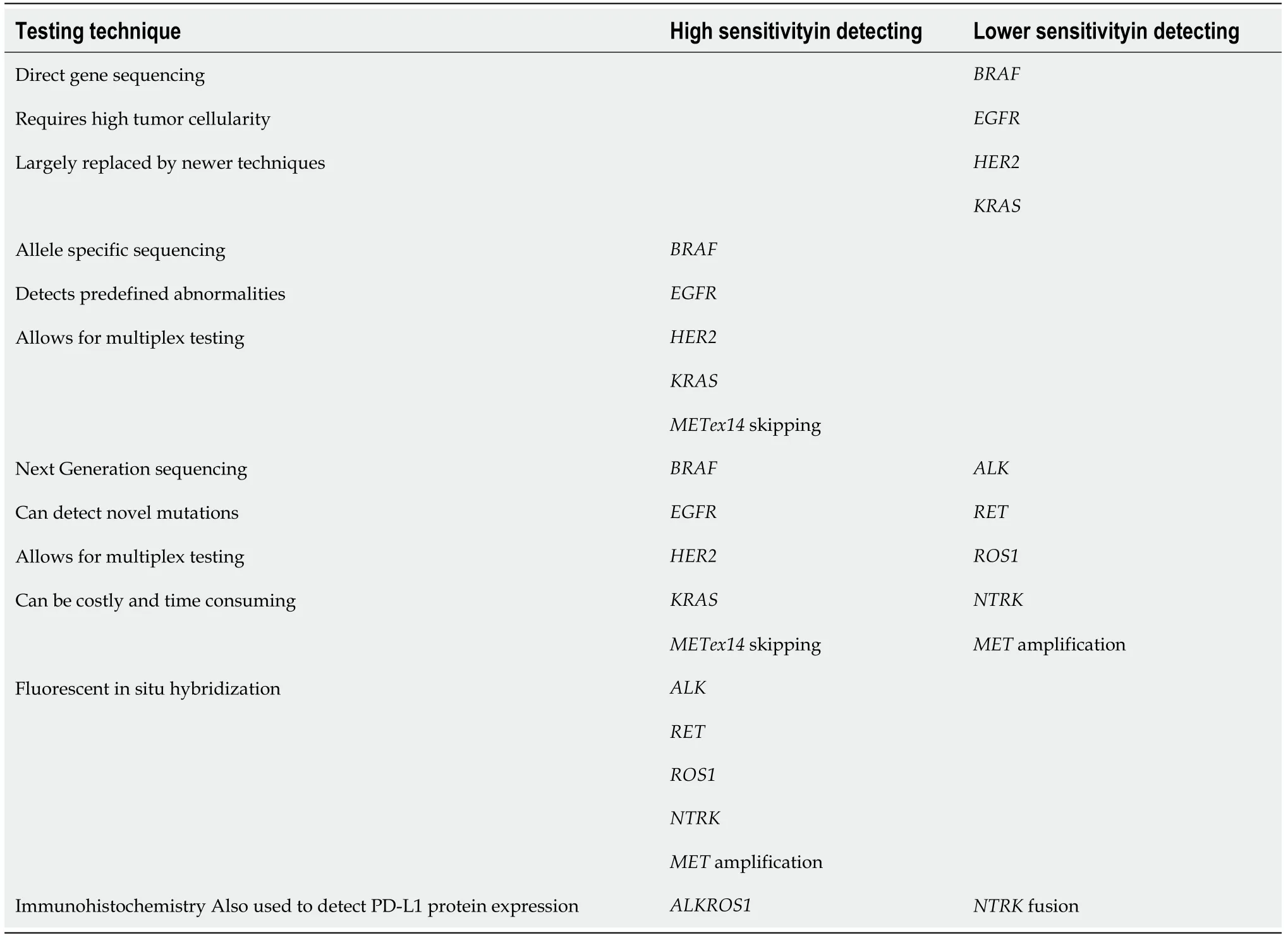
Table2 Commonly used platforms for detection of mutations in non-small cell lung cancer
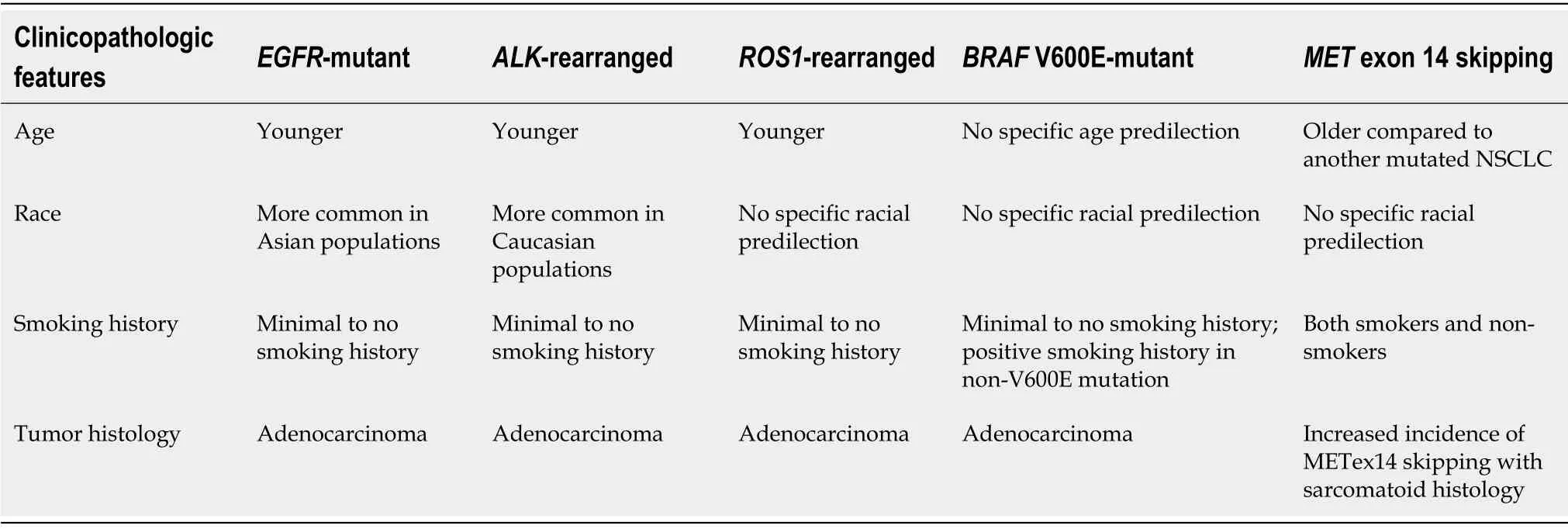
Table3 Summary of reported clinicopathologic biomarkers in select molecular genotypes in non-small cell lung cancer1
ROS1rearrangements,most commonly genetic translocations betweenROS1andCD74,represent another targetable driver alteration identified in 1%-2% of NSCLC[33,34].Similar toEGFRmutations andALKrearrangements,ROS1rearrangements are also associated with younger age,little to no smoking history,and adenocarcinoma cell type[33,34].ALKandROStyrosine kinase domains share a high degree of homology,makingROS1-positive NSCLC highly sensitive to crizotinib[33].Entrectinib,a tropomyosin receptor kinase (TRK)/ROS1inhibitor,has also been found to be effective and has been approved for the treatment of advancedROS1-positive NSCLC[4].
Mutations in theBRAFgene,which are present in 2%-4% of NSCLC,have emerged as another possible target in the treatment of NSCLC[35,36].BRAF is a protein kinase,which,when constitutively activated by a mutation,can lead to increased cell proliferation and survival,decreased cell death,and oncogenesis through the RAS/MAPK pathway[36,37].Several subtypes ofBRAFmutations exist and are typically classified as either V600E or non-V600E[36-38].Unlike mutations involvingEGFR,ALK,andROS1,those with activating non-V600EBRAFmutations are typically current or previous smokers,although those withV600Emutations are typically also less likely to have a history of smoking[36,39,40].Combination treatment withBRAFandMEKinhibitors,dabrafenib and trametinib,has been approved for advanced NSCLC withBRAFV600E mutations[41].
Fusions involving one of three TRK (NTRKfusions) are seen in less than 1% of NSCLC and has not been shown to have a predilection based on gender,age,smoking history,or histology[42].Two TRK inhibitors,larotrectinib and entrectinib,have shown efficacy against NSCLC harboringNTRKfusions and have been approved in advanced cases[43,44].
RETfusions are detected in 1%-2% of NSCLC and are more commonly seen in patients with no significant smoking history[45,46].Multi-targeted TKIs such as cabozantinib and vandetanib have been found to have anti-RETactivity[47,48].Subsequently,highly potent,RET-selective TKIs,pralsetinib (BLU-667) and selpercatinib (LOXO-292),have shown promising preliminary safety and efficacy profiles in patients with advanced solid tumors harboringRETalterations and are under investigation in the treatment ofRET-positive NSCLC[49,50].
TheMETproto-oncogene encodes a receptor tyrosine kinase,which plays a role in the RAS/MAPK,Rac/Rho,and PI3K/Akt signaling pathways,which mediate cellular growth,anti-apoptosis,and metastasis[51].METamplification and overexpression have been found in a wide variety of malignancies including lung cancer,both as a primary driving mutation and as an acquired resistance mechanism inEGFR-mutated NSCLC[52,53].METexon 14 (METex14) skipping represents a distinct subset ofMETmutations seen in up to 4% of NSCLC and is mutually exclusive of other driver mutations,includingEGFR,ALK,andROS1[3,53].METex14 skipping mutations tend to affect older patients compared toEGFRandALK[53-55].Although most tumors with METex14 skipping mutations are adenocarcinomas,there is increased incidence of the mutation in those with sarcomatoid histology[54].Crizotinib and cabozantinib have shown promise in treating the treatment of NSCLC harboring METex14 skipping mutations,and several clinical trials are currently underway investigating novelMETtargeted TKIs,including tepotinib and capmatinib[56,57].
HER2encodes anEGFRfamily receptor tyrosine kinase,with mutations inHER2gene detected in approximately 1%-3% of NSCLC[58,59].These mutations are more commonly seen in lung adenocarcinoma and are more common in nonsmokers and women[59].There is evidence showing thatHER2-mutated NSCLC may respond to trastuzumab-based regimens and ado-trastuzumab emtansine[58-60],and several clinical trials of novel TKIs targetingHER2are currently underway including poziotinib,TAK-788,pyrotinib and others.
Finally,activatingKRASmutations are the most commonly identified alterations in NSCLC,seen in up to 25% of lung adenocarcinomas[61].UnlikeEGFRandALKalterations,KRASmutations are generally seen in smokers.Several previous efforts to identify RAS-specific inhibitors have been unsuccessful.Currently,several agents are under investigation in the treatment of NSCLC withKRAS-G12C mutations,which accounts for approximately 12% ofKRASmutations in NSCLC[62].
IMAGING BIOMARKERS IN MUTATED NSCLC
There has been increasing awareness of the clinical features (e.g.,minimal to no history of smoking,Asian descent,etc.) that are associated with certain mutations in NSCLC,but the association of the imaging features and underlying driver mutations in NSCLC remains under-recognized.Emerging data suggest that there are differences among NSCLC harboring different targetable oncogenic driver mutations with respect to the imaging features of the primary tumor and patterns of metastases (Table 4,Figure 1).These features,when present,can potentially point to certain mutations.

Table4 Summary of reported imaging biomarkers in select molecular genotypes in non-small cell lung cancer1
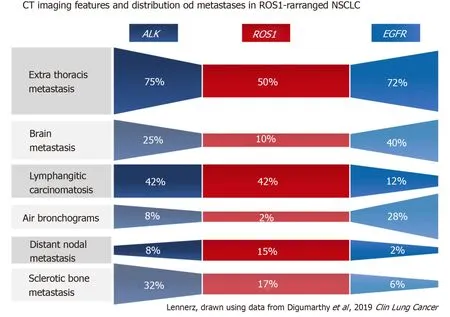
Figure1 Comparison of select primary tumor features and metastatic patterns among patients with non-small cell lung cancer with ALK,ROS1,or EGFR alterations[71].
PRIMARY TUMOR FEATURES
Several researchers have investigated the imaging features of the primary tumors in those with mutation-driven NSCLC.The most commonly investigated features are the tumor density,morphology,and location.
Overwhelmingly,most primary tumors in both mutated and non-mutated NSCLC are solid in density,including those with mutations involvingKRAS,EGFR,ALK,ROS1,RET,MET,BRAF,HER2,andKRAS[40,54,63-71].To date,the imaging features of NSCLC withNTRKfusions have not been studied,likely owing to their rarity.While most lung tumors are typically solid,several studies[63,64,66]have reported increased propensity of primary tumors inEGFR-mutant NSCLC to have a consolidative “pneumonic” appearance with ground-glass components,cavitations,and air-bronchograms (Figure 2A and 4A).This highlights the need for vigilance in the setting of non-resolving consolidations to prevent missed or delayed diagnosis in these patients.
Primary tumor location,particularly the tumor’s axial location (i.e.,central versus peripheral location),is another commonly investigated imaging feature.Increased tendency for peripheral rather than central locations has been reported in NSCLC withALKrearrangements (Figure 3A)[66],RETrearrangements[70,72]and METex14 skipping mutations[54].Two small studies have also suggested that the primary tumors inROS1-positive NSCLC tend to be peripheral[70,73],although a subsequent larger study failed to support these findings[71].
More recently,it has been reported that the primary tumors inALK-positive NSCLC are more likely to occur in the lower lobes,compared toEGFR-wild type andALK/EGFR-negative tumors[66].Most lung cancers develop in the upper lobes.Propensity for lung cancer development in the lower lobes has been reported in lung cancers developing in nonsmokers,although the presence or absence of an underlying driver mutation was not included in the study[74].It has also been suggested that lower lobe tumors may be associated with a worse prognosis,but the studies did not include NSCLC with targetable mutations[75,76].Tumor location may have implications with respect to accessibility for biopsy,surgery,or radiation therapy.
PATTERNS OF NODAL AND DISTANT METASTASES
Nodal metastasis
Nodal status is an important prognostic factor and determinant of treatment offered to patients with lung cancer.A number of studies have suggested that certain driver mutations may have increased predisposition for both intrathoracic and distant nodal metastases.In particular,several studies have reported increased frequency for extensive lymphadenopathy inALK-positive NSCLC (Figure 3C)[65,66,71,77].More recently,a similar predilection for intrathoracic and distant nodal metastases have been associated withROS1-positive NSCLC[71].The extensive lymphadenopathy seen inALK-positive andROS1-positive NSCLC can potentially be misinterpreted initially on imaging as either lymphoma or small cell lung cancer[65,71].
Lung metastases
Several studies have reported that there is increased frequency of diffuse “miliary” (i.e.,widespread disseminated) lung metastases inEGFR-mutant NSCLC (Figure 2A and 4A).Our group has previously reported up to a six-fold increased incidence of diffuse lung metastases inEGFR-mutant NSCLC compared toEGFR-wild type NSCLC[63].While diffuse lung metastases are typically associated with worse prognosis,the presence of anEGFRmutation and increased responsiveness to targeted therapy (Figure 2B) can potentially improve outcomes in these patients.In the setting of a dominant lung mass and diffuse “miliary” lung metastases,EGFR-mutant NSCLC should be suspected[63].
ALK-positive NSCLC,on the other hand,has been associated with lymphangitic carcinomatosis (Figure 3B and 4B) in comparison toEGFR-mutant NSCLC[65-67,78].More recently,ROS1-positive NSCLC has also been associated with predilection for lymphangitic carcinomatosis (Figure 5A)[71].On imaging,lymphangitic carcinomatosis is characterized by nodular thickening of the axial and peripheral,subpleural interstitium,with relative sparing of the intralobular interstitium[79].Lymphangitic carcinomatosis is associated with worse prognosis in various extrapulmonary malignancies,but its prognostic impact in the setting of primary lung malignancies remains unclear du to paucity of data[80].While it may appear intuitive to that lymphangitic carcinomatosis is suggestive of more advanced disease,a concurrent targetable mutation with eitherALKorROS1may improve outcomes in these patients(Figure 5C).
More recently,it has been suggested that NSCLC with METex14 skipping mutations may have increased frequency of multifocal,synchronous primary lung cancer at presentation (Figure 4C),which was observed in approximately 1 in 5 patients[54].The authors suggested that this multifocality may be secondary to synchronous adenocarcinomas with distinct splice site mutations,which has been previously described forMETex14-mutated primary lung adenocarcinomas[81].

Figure2 Primary tumor features and “miliary” type metastases in 66-year-old male non-smoker with EGFR-mutant non-small cell lung cancer.
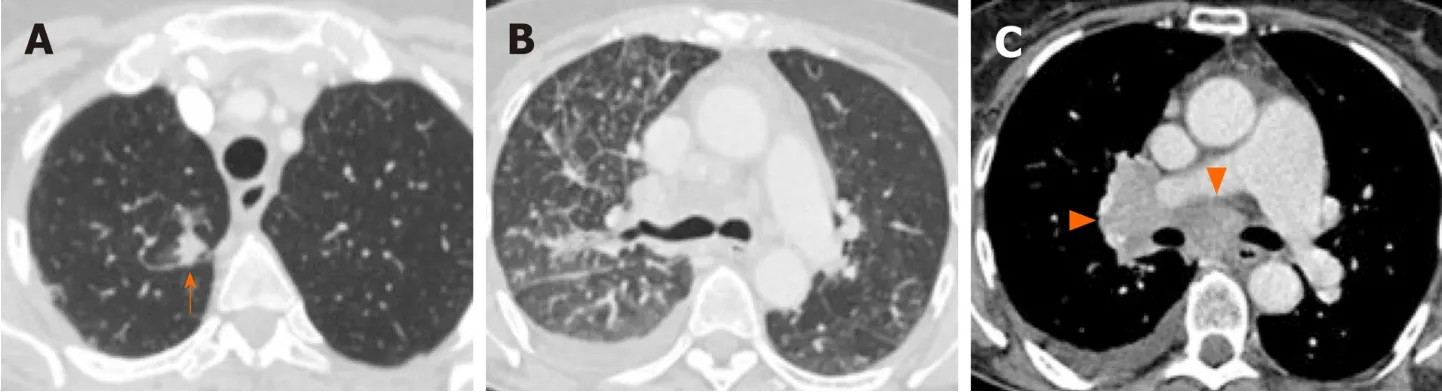
Figure3 Lymphangitic carcinomatosis,pleural metastasis and,extensive lymphadenopathy in 64-year old female non-smoker with ALKpositive non-small cell lung cancer.

Figure4 Imaging features of the primary lung tumor and patterns of lung metastases in non-small cell lung cancer with driver mutations.
Pleura and pericardial metastases
In addition to increased frequency of lymphangitic carcinomatosis,ALK-positive NSCLC has also been associated with increased frequencies of both pleural (Figure 3C)and pericardial metastases[65],andROS1-positive NSCLC has also been associated with pleural metastases (Figure 5B)[71].The mechanism behind these potential differences in metastatic tropisms among the different genotypes remains to be determined.

Figure5 Lymphangitic carcinomatosis and pleural metastases in 26-year old male non-smoker with ROS1-positive non-small cell lung cancer.
Brain metastases
The brain is a common site of metastasis in NSCLC,with over 20% of patients with advanced NSCLC having brain metastases at the time of diagnosis,and up to approximately 50% developing them within three years[82-84].Brain metastases present a unique challenge,as their treatment requires agents that can cross and can remain active beyond the blood-brain barrier.
Several studies have suggested potential differences in the frequencies of brain metastases across the different oncogenic drivers in NSCLC[85].NSCLC harboring alterations inEGFR,ALK,orROS1have been associated with increased frequencies of brain metastases[86-89].Some reports,however,show that there is significant overlap in the frequencies of brain metastases among the different mutation groups[90].Reported frequencies of brain metastasis at time of diagnosis of advanced disease range from 23%-41% inEGFR-mutant NSCLC[66,86,87],23%-42% inALK-positive NSCLC[66,86],and 9%-36% inROS1-positive NSCLC[71,88,90].Less data is available with respect to the frequencies of brain metastases in the other mutational subgroups.Incidence of 25%have been reported for bothRET-positive[91]andHER2-mutant NSCLC[92],21% for NSCLC with METex14 skipping mutations[54],and 10% forBRAF-mutant NSCLC.Ranges of reported incidences of brain metastases in the more common molecular subtypes are presented on Figure 6.Further investigation is necessary to determine if differences in tropism to the brain truly exist across the different oncogenic subsets in NSCLC and to determine the underlying mechanism resulting in differences.Nevertheless,the high incidences of brain metastases across several of these mutated tumors underscore the need for targeted agents that have robust CNS activity.
Bone metastases
The bones are a common site of metastasis in NSCLC and osseous metastases are seen in up to 40% of patients with advanced lung cancer[93].Bone metastases are a significant cause of morbidity in cancer patients as they can predispose to pathologic fractures,cause debilitating pain and severely reduce quality of life.
Osseous metastases from a variety of malignancies can be either predominantly lytic or predominantly sclerotic or osteoblastic in appearance.Many lytic lesions may also become sclerotic with treatment.Malignancies classically associated with sclerotic bone metastases are prostate cancer and small cell lung cancer.In general,bone metastases in NSCLC usually present as lytic lesions,with sclerotic metastases rarely seen prior to treatment[94,95].
A number of studies,however,have reported that there may be a predisposition to either lytic or sclerotic bone metastasis based on the presence of an underlying driver mutation in NSCLC.ALK-positive NSCLC,for instance,has been associated with sclerotic metastases (Figure 7).In a study comparing the imaging findings ofALKpositive NSCLC to those ofEGFR-mutant NSCLC,more than half of the patients with bone metastases in the setting ofALK-positive NSCLC had sclerotic bone metastases prior to any treatment.In contrast,sclerotic bone metastases were seen in only 1 of 6 patients withEGFR-mutant NSCLC[66].More recently,a different study comparing the imaging features ofROS1-positive NSCLC to those withALKorEGFRalterations,showed similar frequencies of bone metastases among the three mutational subgroups,but an increased frequency of sclerotic bone metastases in botROS1-positive NSCLC andALK-positive NSCLC compared toEGFR-mutant NSCLC[71].In contrast,a series presenting the clinicopathologic and imaging features of NSCLC with METex14 skipping mutations reported the bone metastases to be predominantly lytic in these patients[76].The morphology of bone metastases in the other molecular subgroups has yet to be reported.

Figure6 Range of reported incidences (%) of brain metastases in advanced mutated non-small cell lung cancer with driver mutation.

Figure7 Patterns of bone metastases in non-small cell lung cancer with driver mutations.
Other metastatic patterns
No specific imaging biomarker has yet to be identified to suggest the presence of an underlyingBRAFmutation in NSCLC[40,96].It has been suggested,however,that at the time of presentation,patients with lung cancer harboring the V600EBRAFmutation may be more likely to have intrathoracic metastases,particularly pleural metastases,while those with non-V600EBRAFmutations may be more likely to have intraabdominal metastases[40].
Finally,METex14 skipping mutations in NSCLC have recently been associated with increased incidence of oligometastatic disease[54].In the case series,the authors reported 4 patients that had only a single site of metastases (3 with adrenal metastasis and 1 one with soft tissue metastasis),although the findings have yet to be validated[54].Several studies,however,have reported better outcomes in patients with limited metastatic burden when managed with radical treatment with curative intent[97].
CONCLUSION
The mechanism behind the morphological differences of the primary tumor and the differences in metastatic tropisms among the molecular subgroups of NSCLC remain unclear.While none of the imaging features and metastatic tropisms we discussed can reliably predict the presence of specific genetic alterations in isolation and they are unlikely to replace molecular genotyping in directing the need for targeted therapy,these imaging biomarkers can indicate the presence of specific targetable mutations and can play an adjunctive role.These features can assist in the selection patients who may benefit from expedited pathways for molecular testing or repeat testing when the initial genotyping results are equivocal or discordant with the clinical and imaging presentation.Given the importance of initiating targeted therapy in patients with targetable mutations,it is imperative to use all biomarkers available – clinical,histopathologic,and radiologic – in detecting these mutations.
杂志排行
World Journal of Clinical Oncology的其它文章
- Mechanisms and anatomical risk factors of pneumothorax after Bevacizumab use:A case report
- Complete response in anaplastic lymphoma kinase–rearranged oncocytic thyroid cancer:A case report and review of literature
- Is there a role for treatment-oriented surgery in liver metastases from gastric cancer?
- Why natural killer cells in triple negative breast cancer?
- Circulating cell-free nucleic acids as prognostic and therapy predictive tools for metastatic castrate-resistant prostate cancer
- MUTYH:Not just polyposis
