Diagnostic value of liquid-based cytology and smear cytology in pancreatic endoscopic ultrasound-guided fine needle aspiration:A meta-analysis
2020-09-14
Hang-Hai Pan,Fei Zhao,Yu Zhang,Department of Gastroenterology,Zhejiang Provincial People's Hospital,People's Hospital of Hangzhou Medical College,Hangzhou 310014,Zhejiang Province,China
Xin-Xin Zhou,Department of Gastroenterology,The First Affiliated Hospital,College of Medicine,Zhejiang University,Hangzhou 310003,Zhejiang Province,China
Hui-Yan Chen,School of Laboratory Medicine and Life Sciences,Wenzhou Medical University,Wenzhou 325027,Zhejiang Province,China
Abstract
BACKGROUND
Smear cytology (SC) using endoscopic ultrasound-guided fine needle aspiration(EUS-FNA) is the established and traditional choice for diagnosing pancreatic lesions.Liquid-based cytology (LBC) is a novel alternative cytological method,however,the comparative diagnostic efficacy of LBC remains inconclusive.
AIM
To examine the diagnostic efficacy of LBC and SC for pancreatic specimens obtained through EUS-FNA via a systematic review and meta-analysis.
METHODS
A systematic literature search was performed using PubMed,EMBASE,the Cochrane Library,and Web of Science.The numbers of true positives,false positives,true negatives,and false negatives for each cytological test (LBC and CS) were extracted from the included studies.The pooled sensitivity and specificity and the area under the summary receiver operating characteristic curve(AUC) were calculated,and the AUC was compared by Tukey's multiple comparisons test.The quality of the included studies was assessed using the Quality Assessment of Diagnostic Accuracy Studies II tool.
RESULTS
A total of 1656 patients in eight studies were included.The pooled sensitivity and specificity and the AUC for LBC were 0.76 (95%CI:0.72-0.79),1.00 (95%CI:0.98-1.00),and 0.9174,respectively,for diagnosing pancreatic lesions.The pooled estimates for SC were as follows:Sensitivity,0.68 (95%CI:0.64-0.71);specificity,0.99 (95%CI:0.96-100.00);and AUC,0.9714.Similarly,the corresponding values for LBC combined with SC were 0.87 (95%CI:0.84-0.90),0.99 (95%CI:0.96-1.00),and 0.9894.Tukey’s multiple comparisons test was used to compare the sensitivities and AUCs of the three diagnostic methods;statistically significant differences were found between the three methods,and LBC combined with SC was superior to both LBC (P <0.05) and SC (P <0.05).The pooled sensitivity and AUC did not change significantly in the sensitivity analysis.
CONCLUSION
LBC may be sensitive than SC in the cytological diagnosis of pancreatic lesions,however,the superior diagnostic performance of their combination emphasizes their integrated usage in the clinical evaluation of pancreatic lesions.
Key words:Liquid-based cytology;Smear cytology;Pancreatic lesions;Endoscopic ultrasound-guided fine needle aspiration;Cytological diagnosis;ROC curve
INTRODUCTION
Pancreatic cancer is a highly lethal disease,and early detection and treatment are key to improve the survival and restrain the progression in these patients[1].In recent years,endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) has brought great improvement to the preoperative diagnosis of pancreatic lesions,but its diagnostic performance is affected by a variety of factors,including tumor size,location,and characteristics[2].
EUS-FNA is used to obtain tissues and cell specimens for cytopathological examination,while different cytological methods have a certain impact on diagnostic accuracy.Smear cytology (SC) has been recognized as the standard cytological diagnostic method for the establishment of an initial pancreatic lesion diagnosis and treatment plan.As a standard method for any EUS-FNA procedure,SC has its technical limitations of blood contamination and dry artifacts in the process that can obscure the cytological features and interfere with diagnosis[3].Liquid-based cytology(LBC) is an innovative slide-making technique that was developed to better preserve and display cell morphology and structure,and to produce representative standardized smears through automated processes.LBC was initially applied in cervical cancer screening[4],and it has gradually been accepted as the cytological diagnostic tool for non-gynecologic specimens as well as in pancreatic lesions[5,6].However,the quality of samples,the shape of cell clusters,the adequacy of samples,and the nature of background are prerequisites for an accurate diagnosis.
Several studies have evaluated the diagnostic efficacy of LBC and SC in pancreatic EUS-FNA cytology by comparing the key differences between the two cytological diagnostic techniques[3,7-14].However,these studies have reported conflicting results that might be attributable to the diversity of the subject population,subtle differences in detailed procedures,and the infancy of application of this technology in this specialized field.Moreover,many of these studies have been designed and conducted under pressure to some extent to favor a certain LBC product,potentially leading to biased results,and this should not be neglected.In fact,the diagnostic efficacy of LBC compared with SC in some previous prospective studies is still controversial.Although several investigators now agree that application of LBC performed for pancreatic EUS-FNA specimens is acceptable,to what extent we can trust the results of LBC and whether it is feasible to use LBC alone or whether LBC should be applied in combination with SC are some aspects that remain unclear.
Few studies have compared the diagnostic value of LBC with that of SC for pancreatic cell specimens obtainedviaEUS-FNA,and to our knowledge,there are no meta-analyses on this topic.Accordingly,we performed a systematic review and metaanalysis of the comparative studies of LBP and CS that were conducted in pancreatic EUS-FNA,to draw a statistically convincing conclusion on the comparative diagnostic accuracy and practicability of SC and LBC in pancreatic lesions.
MATERIALS AND METHODS
Literature search
We performed a systematic literature search of articles in PubMed,Cochrane Library,Web of Science,and EMBASE (January 1990 to February 2020) containing quantitative data and manually searched the reference lists of retrieved articles.There were seven LBC methods included in the search:ThinPrep,SurePath (also known as AutoCyte PREP),Liqui-PREP (LGM-International,FL,USA),CellPrepPlus (Biodyne,Seongnam,Korea),Cell &Tech (Cell &Tech Bio,Seoul,Korea),EasyPrep (YD Diagnostics Corp.,Seoul,Korea),and HuroPath (formerly known as E-Prep,CelltraZone,Seoul,Korea)[5].The queries used were:((((((((""Pancreas""[Mesh]) OR Pancreatic)) AND(((((((((((""liquid-based preparation"") OR ""liquid-based cytology"") OR ThinPrep) OR SurePath) OR ""AutoCyte PREP"") OR ""Liqui-Prep"") OR CellPrepPlus) OR ""Cell &Tech"") OR EasyPrep) OR ""E-Prep"")))) AND ((""Endoscopic Ultrasound-Guided Fine Needle Aspiration""[Mesh]) OR EUS-FNA))" for Pubmed,[Pancreas] OR (pancreatic)AND [“Endoscopic Ultrasound-Guided Fine Needle Aspiration”] OR (EUS-FNA)AND ("liquid-based preparation") OR ("liquid-based cytology") OR (ThinPrep) OR(SurePath) OR ("AutoCyte PREP") OR ("Liqui-Prep") OR (CellPrepPlus) OR ("Cell &Tech") OR (EasyPrep) OR ("E-Prep") for Cochrane Library,'eus fna' OR 'eus-guided fna' OR 'endoscopic ultrasound guided fine needle biopsy'/exp AND 'liquid-based preparation' OR 'liquid-based cytology' OR thinprep OR surepath OR 'autocyte prep'OR 'liqui-prep' OR cellprepplus OR 'cell &tech' OR easyprep OR 'e-prep' AND pancreatic OR 'pancreas'/exp for Embase,TOPIC:(Pancreas) OR TOPIC:(Pancreatic)OR/AND TOPIC:("Endoscopic Ultrasound-Guided Fine Needle Aspiration") OR TOPIC:(EUS-FNA) AND TOPIC:("liquid-based preparation") OR TOPIC:("liquidbased cytology") OR TOPIC:(ThinPrep) OR TOPIC:(SurePath) OR TOPIC:("AutoCyte PREP") OR TOPIC:("Liqui-Prep") OR TOPIC:(CellPrepPlus) OR TOPIC:("Cell &Tech") OR TOPIC:(EasyPrep) OR TOPIC:("E-Prep") for Web of Science.All similar possible word variations were also searched.The attained records were retrieved and managed with EndNote X 9.0 (Bld 10136,Thomson Reuters,New York,NY,United States).
Study selection
We included those comparative test accuracy studies in which all participants received both LBC and SC tests for pancreatic tissue collected by EUS-FNA that were followed by verification of the disease status with the reference standard.Studies in which participants were matched in a 1:1 ratio to control factors that might influence the diagnostic performance were also included.Further,those studies in which sufficient data were reported to calculate true positive (TP),false positive (FP),false negative(FN),and true negative (TN) were included.However,conference papers and duplicate published studies that fulfilled the above two criteria were excluded.This systematic review was performed in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA)[15].
Data extraction
Data on study-related information was extracted and cross-checked by two authors independently (Zhang Y and Pan HH).If there was a discrepancy in opinions,it was discussed with other authors to achieve a consistent result.The extracted data included the name of the first author,year of publication,demographics of the population,methods of cytological techniques,and outcomes.The numbers of TPs,FPs,TNs,and FNs for each cytological test (LBC and CS) were the main statistics extracted from the studies.We computed sensitivity [TP/(TP + FN)] and specificity[TN/ (TN+ FP)] for each technique separately.
Quality assessment
The quality of the included studies was independently assessed by two authors independently (Zhou XX and Zhao F) uses the Quality Assessment of Diagnostic Accuracy Studies II (QUADAS-II) tool[16].
Statistical analysis
The original data of each study (TP,FP,TN,and FN) were integrated by meta-analysis,and the pooled sensitivity,pooled specificity,and 95% confidence intervals (CIs) of LBC,SC,and LBC combined with SC tests were calculated using the DerSimonian Laird random effect model[17].The heterogeneity of pooled sensitivity and specificity was calculated by theI2statistic,and a high degree of heterogeneity was set atI2>50%[18].The Mose's constant linear model[19]was used to perform the summary receiver operating characteristic (SROC) curve analysis and Cochrane’sQ* test[18]was used to evaluate the accuracy of diagnostic tests (LBC,SC,and the combined test) in the diagnosis of pancreatic lesions.When heterogeneity was present,the Spearman correlation coefficient andPvalue or heterogeneity ratio caused by the threshold effect were calculated.The leave-one-out method was used for the sensitivity analysis[20].Tukey’s multiple comparisons test[21]was calculated to compare the area under the SROC curve (AUC) and pooled sensitivity with the significance set atP<0.05.The statistical software used for the diagnostic accuracy test was Meta-Disc 1.4.Revman5.3 was used to evaluate the quality of the included studies and the sample inadequacy of LBC and SC test.
RESULTS
Study selection
A total of 150 articles were initially searched,of which nine[3,7-14]seemed to meet the inclusion criteria.We excluded one study published in February 2020,in which the information to construct a 2 × 2 table was insufficient[14].Thus,eight studies[3,7-13]with a total of 1656 patients were ultimately eligible for the meta-analysis.
A total of 150 articles were initially searched,of which 142 were excluded because:(1) 60 were duplicate;(2) 43 were excluded by title and abstract;and (3) 39 were excluded by full-text review.One study published in February 2020 lacked the data to construct a 2 × 2 table,so it was also excluded[14].A PRISMA flow diagram is shown in Figure 1.
Description of studies and qualitative analysis
Our study was restricted to cross-sectional outcomes such as sensitivity and specificity,and the screening tests were compared to a gold standard (clinical outcome and histology).General information of the included studies is presented in Table 1.These studies were published from 2010 to 2019.Of the eight studies[3,7-13],two were retrospective[7,8]and six were prospective[3,9-13].Six studies[3,7,8,11-13]had both SC and LBC performed on the same population while two paired studies[9,11]used matched participants.Among different types of LBC that were included,ThinPrep was used in four studies[3,8,12,13],followed by SurePath in three studies[7,9,11],and CellprepPlus in one study[10].The reference standard,in the majority,was clinical and/or histological findings and only one study used histology alone[13].According to the reference standard,the definitions of positive and negative outcomes are malignancy and benign,respectively.The time of follow-up for all the eight studies was longer than 6 mo on average unless diagnosis was confirmed by histology,the patient was lost to follow-up,or the patient died before the stipulated period.The TP,FP,FN,TN,and heterogeneity-analysis information are extracted in Table 2.The QUADAS-II quality assessment for each study is presented in Figure 2.
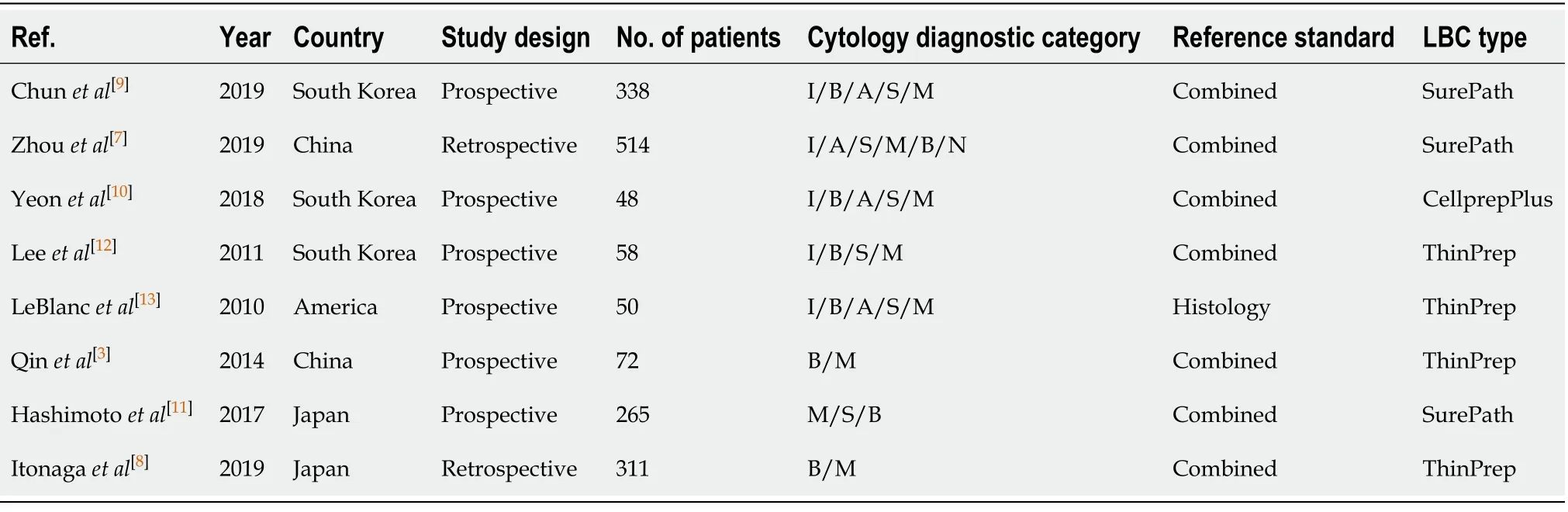
Table1 General characteristics of included studies
Sample inadequacy of LBC and SC
Five studies[7,9,10,12,13]investigated the proportion of inadequate samples obtained by EUS-FNA cytology.Sample inadequacy using the case data of the comparative studies of 839 LBC cases and 839 SC cases out of 1008 patients is summarized in Table 3.The results showed that there was no statistically significant difference in the proportion of inadequate smears between SC and LBC (odds ratio = 1.71;95%CI:0.50-5.81)(Figure 3).
Diagnostic performance
In total,1656 patients in eight studies[3,7-13]were evaluated through LBC and/or SC to diagnose pancreatic tissues obtained by EUS-FNA.The pooled values for LBC were as follows:Sensitivity,0.76 (95%CI:0.72-0.79),I2= 80.0%;specificity,1.00 (95%CI:0.98-1.00),I2= 0.00.The AUC was 0.9174 (Figure 4).The pooled values for SC were as follows:Sensitivity,0.68 (95%CI:0.64-0.71),I2= 93.1%;specificity,0.99 (95%CI:0.96-100.00),I2= 0.00.The AUC was 0.9714 (Figure 5).Four studies involving 931 patients[7,8,10,12]reported the diagnostic value of LBC combined with SC for pancreatic lesions.The included studies reported sufficient data to examine the diagnostic performance of the combinational method.The pooled values for LBC combined with SC were as follows:Sensitivity,0.87 (95%CI:0.84-0.90),I2= 77.8%;specificity,0.99(95%CI:0.96-1.00),I2= 0.00.The AUC was 0.9894 (Figure 6).The corresponding SROC curve for each test is presented in Figure 7.The SROC using these data showed a higher curve for combined LBC and SC,showing a difference in specificity and sensitivity between the three methods in pancreatic EUS-FNA (Figure 7).Tukey's multiple comparisons test was used to compare the sensitivities and AUCs of the three diagnostic methods;statistically significant differences in both sensitivities and AUCs were found between LBC and SC (P<0.05),and LBC combined with SC was superior to both LBC (P<0.05) and SC alone (P<0.05).
Sensitivity analysis
Sensitivity analysis was performed by removing one study at a time to assess the impact of a single study on this meta-analysis.Table 4 shows the pooled sensitivity and AUC calculated after removing each study.It was observed that the pooled sensitivity and AUC did not change significantly in this analysis,suggesting that the results of this analysis were not dependent on a certain study.Thus,the results concluded by our meta-analysis with the full set of studies are reliable.
Heterogeneity analysis
Significant heterogeneity existed among the included studies.Estimation of the Spearman’s correlation coefficient andP-value for the three test methods were as follows:LBC (coef.= 0.342,P= 0.452),SC (coef.= 0.464,P= 0.294),and LBC combined with SC (coef.= 0.800,P= 0.200).These results indicated the absence of the threshold effect.The sources of potential heterogeneity in the sensitivity and specificity were not detected by univariate regression analysis due to the limited number of included studies.
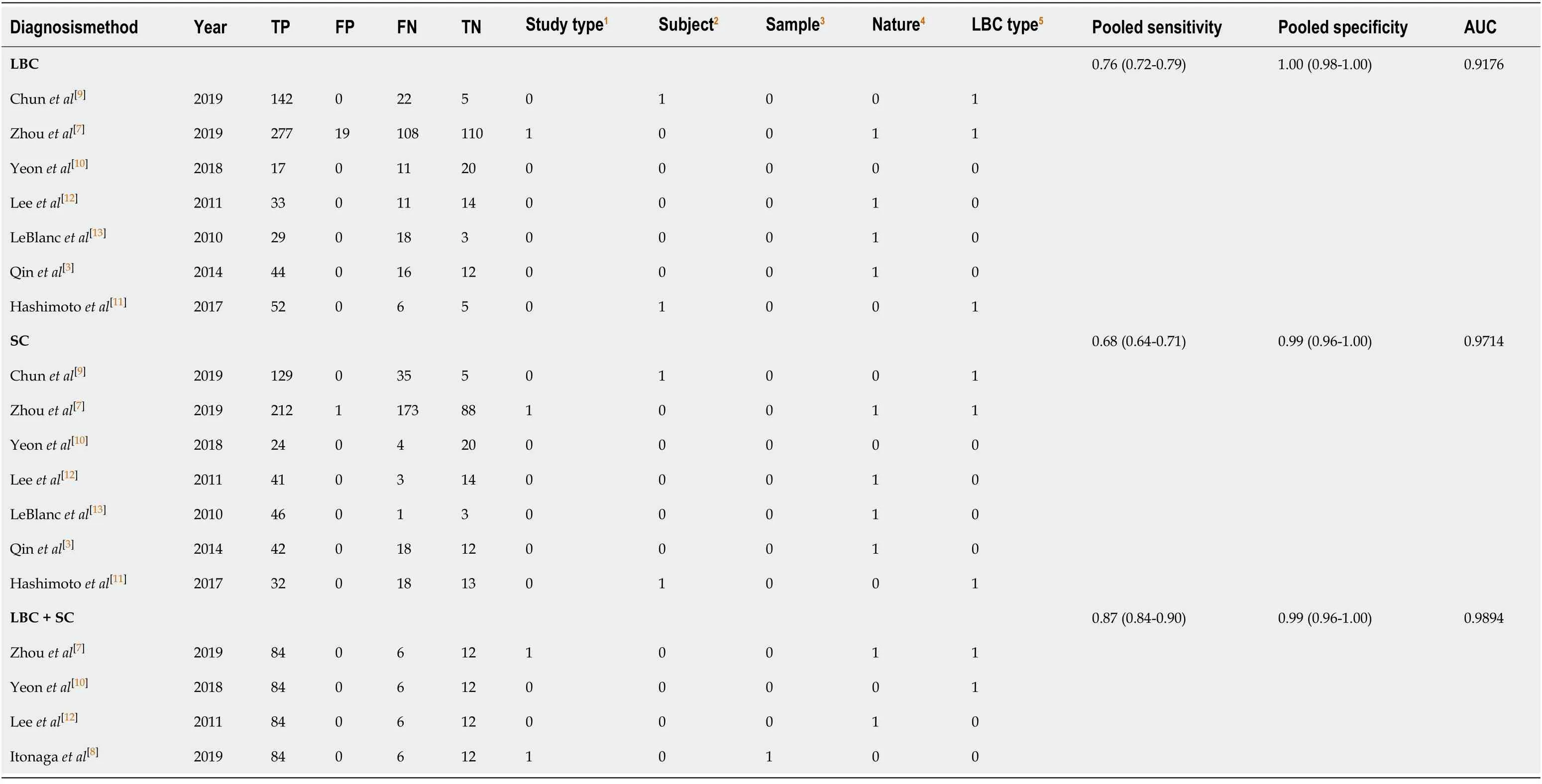
Table2 Summary of results of liquid-based cytology,smear cytology,and the combination test in included studies
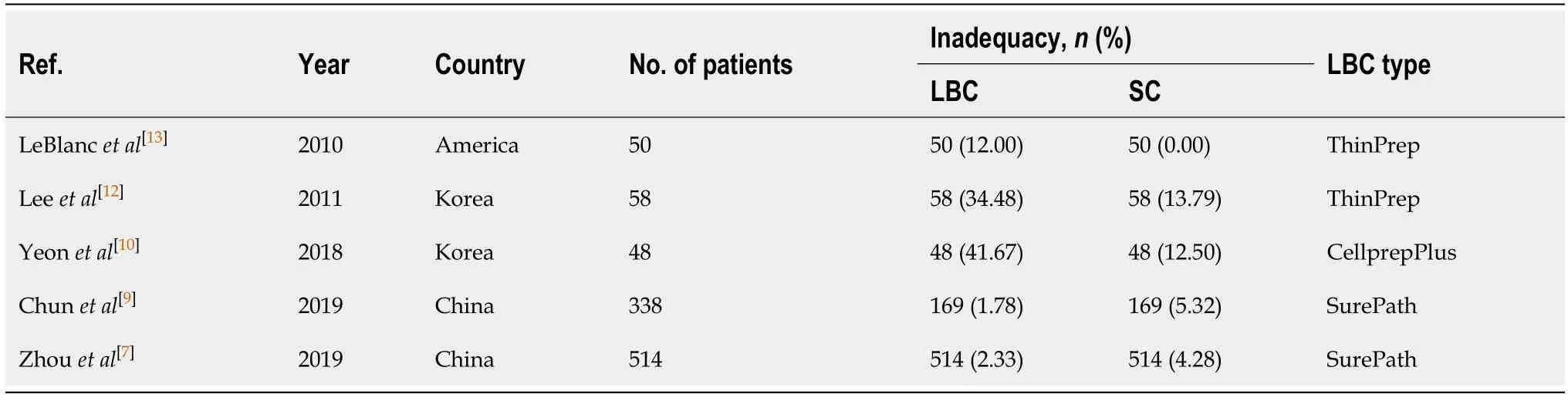
Table3 Difference in sample inadequacy between liquid-based cytology and smear cytology

Table4 Influence of each study on outcome of meta-analysis
DISCUSSION
SC has been usefully employed in many fields as a screening test for malignant lesions.However,SC has its disadvantages of cell overlaps due to the non-uniform smear,an insufficient number of cells,interference by inflammatory cells and blood cells,and inadequate specimens from dryness[10].LBC is a monolayer preparation technique that is applied in the screening of various type of cancers,such as thyroid cancer,lung cancer,and malignant breast lesions[6].According to different processing methods,LBC can be classified into two categories:The precipitation methods (ThinPrep,CellprepPlus,and E-Prep) and the filtration methods (SurePath and Liqui-Prep).The advantages of LBC are that it improves slide quality (including background,cell dispersion,and reducing the confounding cells),eliminates the need for smearing skill,and provides aided methods or further detection after cell morphology interpretation.Additionally,the automatic specimen processing and staining of LBC is another advantage[22].However,the disadvantage is that morphological changes of cells and destruction of architectural features may be caused by treatment with LBC[22].
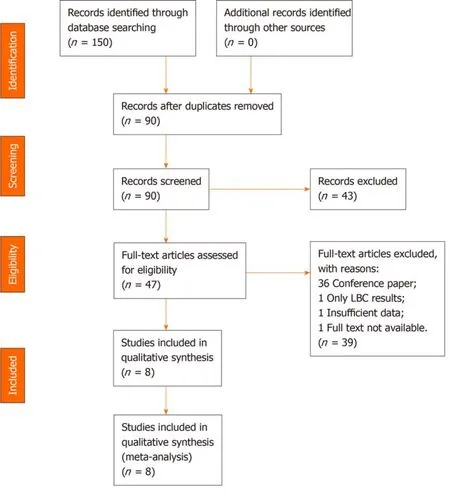
Figure1 Study identification,inclusion,and exclusion for meta-analysis.
Few studies[3,7-14]have been published that used LBC for pancreatic lesions,and to the best of our knowledge,no meta-analysis has been carried out to systematically evaluate the diagnostic performance of LBC and SC for cell specimens obtainedviaEUS-FNA.The present meta-analysis compared and evaluated the diagnostic outcomes of these two cytological methods.We report that the sensitivity of SC is lower than that of LBC,and a significant difference was found between the two methods.However,three studies showed that the diagnostic utility was relatively inferior in LBC,and we analyzed the reasons for the lower accuracy and sensitivity.First,it was attributed to the lack of adequate sample cells in LBC[10,12,13].The sample inadequacy in these studies was significantly higher than that in others.Eight studies[3,7,9-13]used independent samples,but the specimens were not equally distributed among each method.In these studies,more cell specimens were allocated to SC than LBC in the process of sample preparation.In the study conducted by Itonagaet al[8],LBC slides were prepared from cells remaining after SC slides were prepared,and therefore the performance of LBC might have been adversely affected.Second,pancreatic EUS-FNA sometimes obtains fewer cell specimens,and in the process of LBC production,more dilution or air-drying artifact is applied,which might have further caused cell dilution and lack of additional background information.Third,the different LBC processing approaches were another factor affecting the sensitivity.On a side note to the results of meta-analysis,it showed 70% sensitivity for ThinPrep and 78% sensitivity for SurePath for the histologic correlation (data not shown).Only one study[10]compared the methods using CellPlusPrep;thus,the CellPlusPrep category subgroup was not analyzed.In addition,the sample sizes of these three studies[10,12,13]are small,which may also lead to statistical bias.Although LBC may also have some drawbacks,the diagnostic performance of LBC in the differentiation of benign and malignant pancreatic lesions is still better than SC in our study.
We further analyzed the diagnostic value of LBC combined with SC in pancreatic lesions and obtained exciting results.The pooled sensitivity of the combinational method can reach 87%,which is significantly better than those of LBC and SC alone.The sensitivity and specificity of LBC combined with SC showed better results than those of LBC and SC alone (Figure 7).While both LBC and SC have their advantages and disadvantages,by combining the two methods,the sensitivity and accuracy are significantly improved.
Additionally,we compared the sample inadequacy between LBC and SC and observed no significant difference.There were only five studies[7,9,10,12,13]that reported the sample inadequacy of each method,and a high degree of statistical heterogeneity demonstrated by highI2value.Yeonet al[10]reported a much higher inadequacy rate of LBC than SC in 2019.This might be because the allocation of passes for each method was not standardized and favored SC.Therefore,the adequacy of LBC might be adversely affected.Apart from this study,we can see that the sample inadequacy of LBC was much higher than that of SC in the earlier decades,while it is getting better with time,particularly after the introduction of LBC for pancreatic EUS-FNA in recent years (Table 3).This must be due to a learning curve of the new technology.This trend suggests that the learning curve has reached a stage of maturity for the new technology.With the continuous progress of EUS-FNA technology,the advantages of LBC in cytological diagnosis may be further revealed.
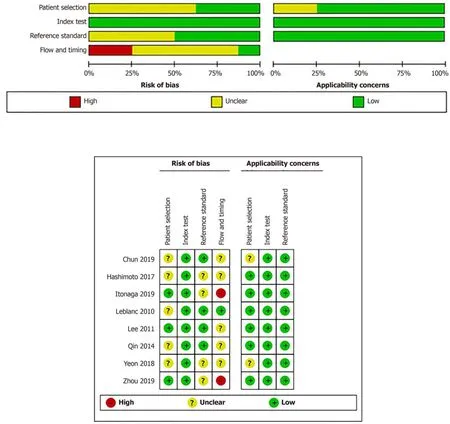
Figure2 Quality assessment of the included studies.
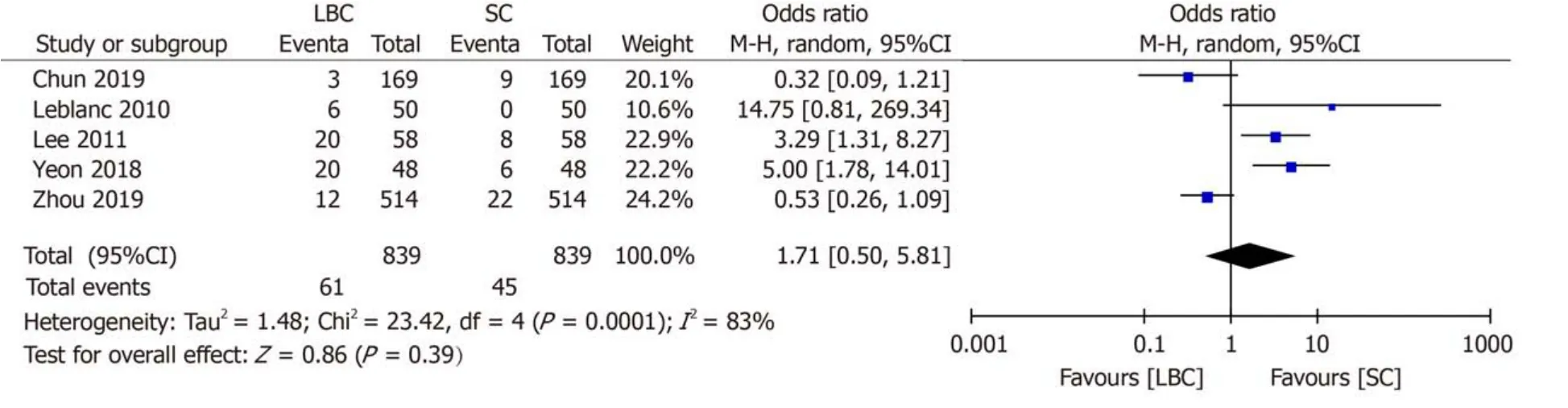
Figure3 Forrest plot of inadequate smears (dichotomous).
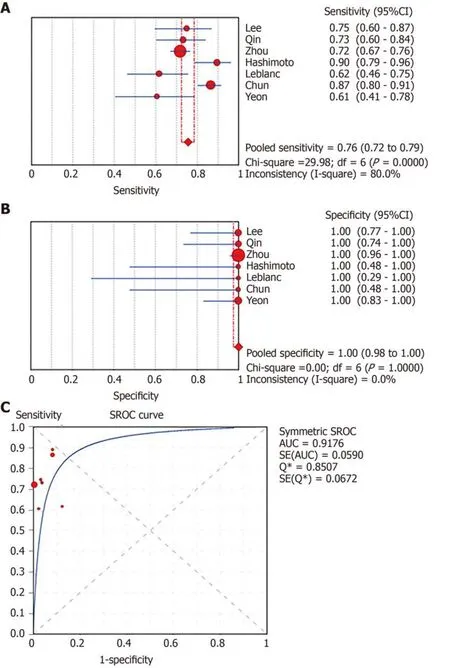
Figure4 Forest plots of pooled sensitivity and specificity and summary receiver operating characteristic (SROC) curve of liquid-based cytology.
There are several limitations to this meta-analysis.First,the high degree of statistical heterogeneity with highI2value could not be avoided.The cytology diagnostic category,LBC processing type,and the number of pancreatic samples possibly affect the heterogeneity of the included studies.Second,there is no classification of pancreatic lesions in most of the included studies.The diagnostic sensitivity of cytological methods for pancreatic solid lesions is different from that of cystic lesions,which may affect the results.Although a high degree of statistical heterogeneity is expected and known to occur in pathology publications,it does indicate a potential need for studies that compare cytopreparatory techniques that have a higher level of standardization than that is currently reported[23].
In summary,this meta-analysis has clearly shown that LBC has a superior sensitivity to SC in the diagnosis of benign and malignant pancreatic lesions,and the advantages may be further revealed with the progress of EUS-FNA technology.The diagnostic performance of LBC combined with SC is significantly better than that of LBC or SC,alone,which suggests that we should promote the combined application of the two techniques for pancreatic lesions in clinical practice.
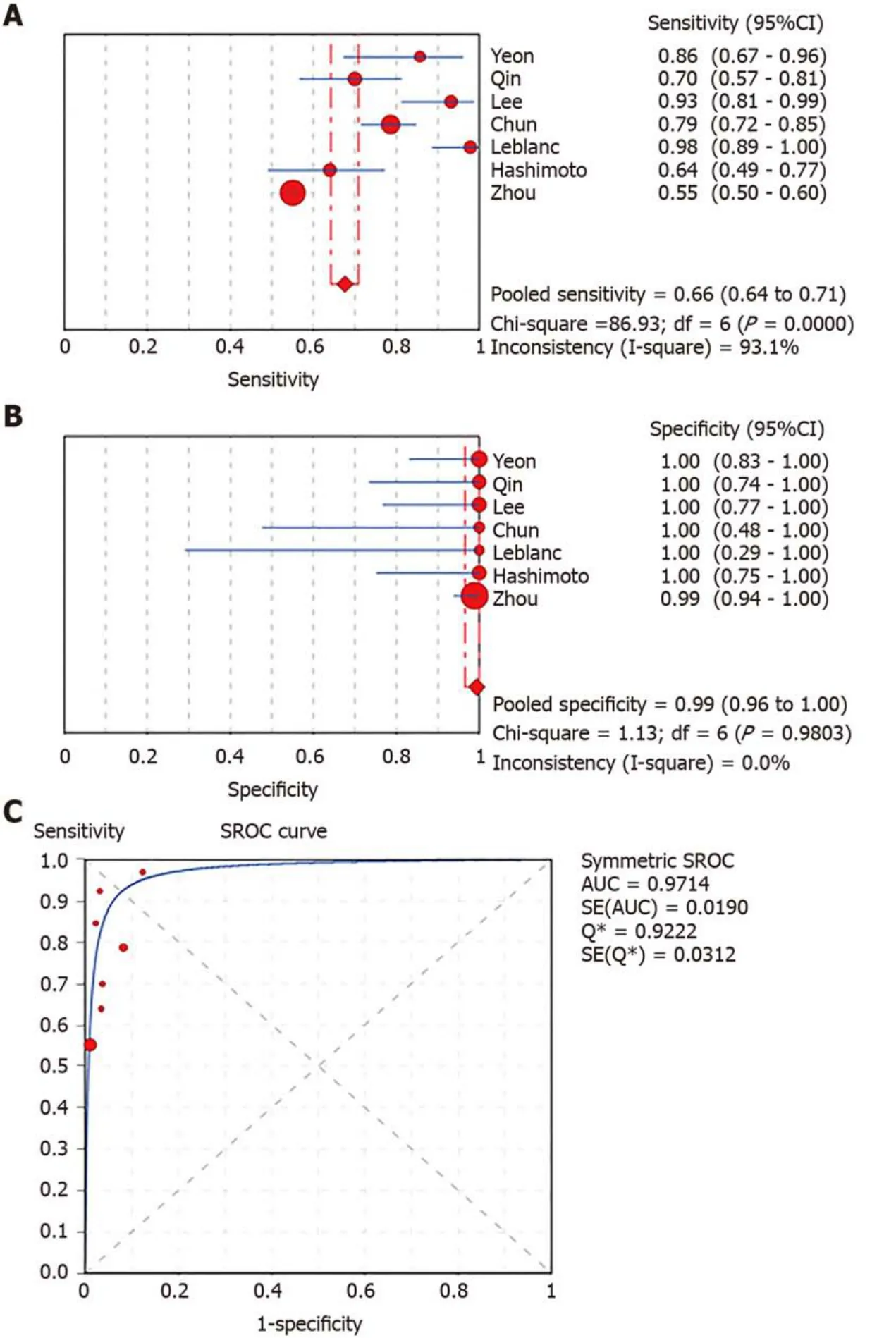
Figure5 Forest plots of pooled sensitivity and specificity and summary receiver operating characteristic (SROC) curve of smear cytology.
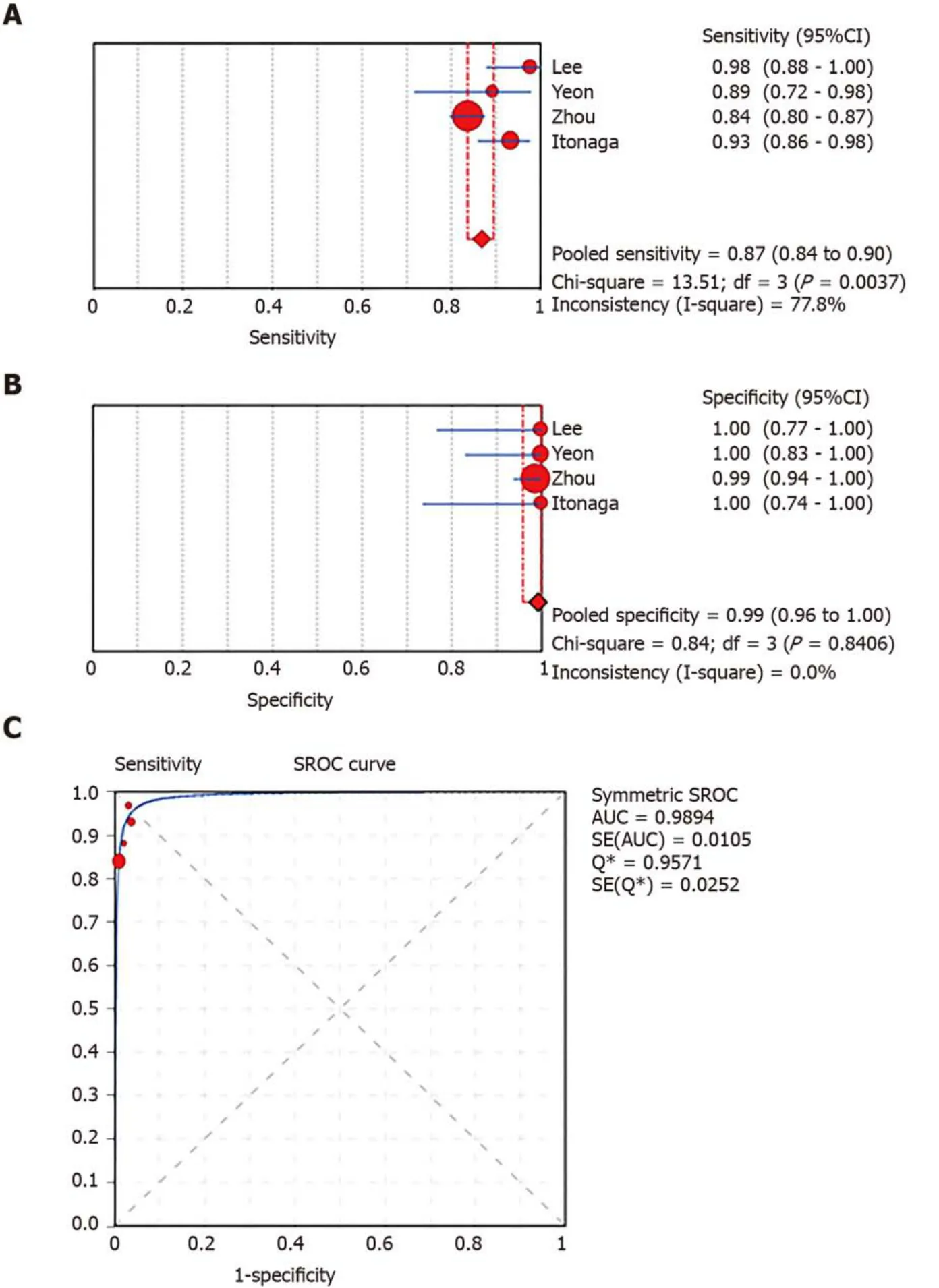
Figure6 Forest plots of pooled sensitivity and specificity and summary receiver operating characteristic (SROC) curve of combined liquid-based cytology and smear cytology.
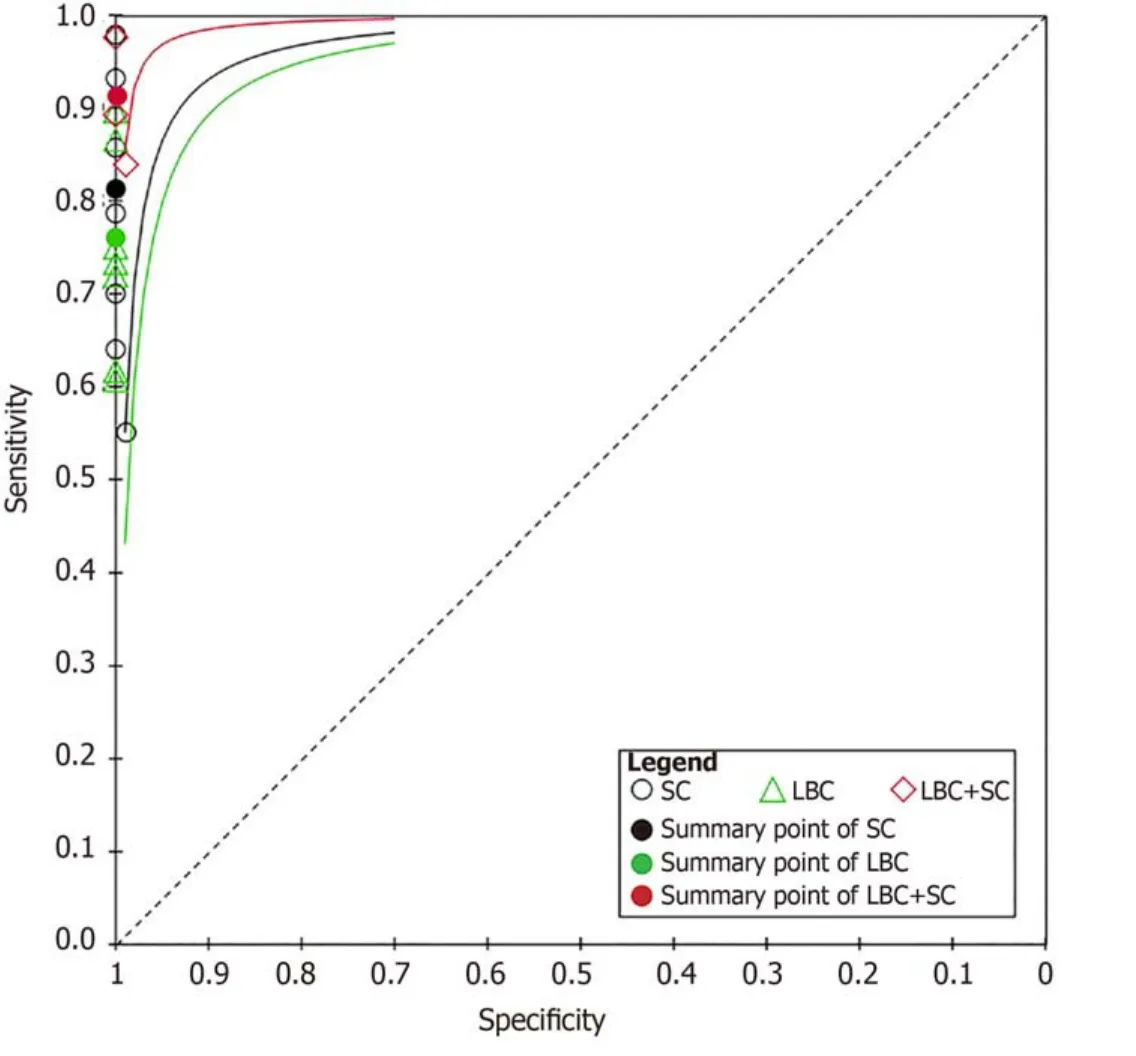
Figure7 Corresponding summary receiver operating characteristic curves of the studies using smear cytology,liquid-based cytology,and combined liquid-based cytology and smear cytology.
ARTICLE HIGHLIGHTS
Research background
Smear cytology (SC) using endoscopic ultrasound-guided fine needle aspiration (EUSFNA) is the established and traditional choice for diagnosing pancreatic lesions.Liquid-based cytology (LBC) is a novel alternative cytological method,however,the comparative diagnostic efficacy of LBC remains inconclusive.
Research motivation
Although previous studies have reported that use of LBC for pancreatic EUS-FNA specimens is acceptable,to what extent we can trust the results of LBC and whether it is feasible to use LBC alone or whether LBC should be used in combination with SC are unclear aspects.Further,cumulative evidence in the form of systematic review and meta-analysis of the studies is unavailable.
Research objectives
To perform a systematic review and meta-analysis on comparative diagnostic efficacy of LBC and SC for pancreatic specimens obtained by EUS-FNA.
Research methods
A systematic literature search was performed using PubMed,EMBASE,the Cochrane Library,and Web of Science.The pooled sensitivity and specificity and the area under the summary receiver operating characteristic curve (AUC) were calculated,and the AUC was compared by Tukey's multiple comparisons test.
Research results
A total of 1656 patients in eight studies were included.The pooled sensitivity and specificity and the AUC for LBC were 0.76 (95%CI:0.72-0.79),1.00 (95%CI:0.98-1.00),and 0.9174,respectively,for diagnosing pancreatic lesions.The pooled estimates for SC were as follows:Sensitivity,0.68 (95%CI:0.64-0.71);specificity,0.99 (95%CI:0.96-100.00);and AUC,0.9714.Similarly,the corresponding values for LBC combined with SC were 0.87 (95%CI:0.84-0.90),0.99 (95%CI:0.96-1.00),and 0.9894.The results revealed a higher sensitivity of LBC than SC in the diagnosis of benign and malignant pancreatic lesions.Additionally,the diagnostic performance of LBC combined with SC was higher than that of LBC or SC,alone (P<0.05).
Research conclusions
LBC may have a superior sensitivity to SC in the diagnosis of benign and malignant pancreatic lesions.The diagnostic performance of LBC combined with SC is significantly better than that of LBC or SC,alone.
Research perspectives
Our study found superior outcomes of LBC combined with SC performed in pancreatic lesions.These findings suggest that we should promote the combined use of these two techniques to guide clinical practice.Additionally,with the continuous progress of EUS-FNA technology,the advantages of LBC may be further revealed.Moreover,future research studies should assess the differences between solid and cystic pancreatic lesions to confirm our results.
杂志排行
World Journal of Clinical Cases的其它文章
- Recommendations for perinatal and neonatal surgical management during the COVID-19 pandemic
- Clinical applicability of gastroscopy with narrow-band imaging for the diagnosis of Helicobacter pylori gastritis,precancerous gastric lesion,and neoplasia
- Identification of APEX2 as an oncogene in liver cancer
- Restenosis after recanalization for Budd-Chiari syndrome:Management and long-term results of 60 patients
- Comparison of microendoscopic discectomy and open discectomy for single-segment lumbar disc herniation
- Clinical characteristics of patients with COVID-19 presenting with gastrointestinal symptoms as initial symptoms:Retrospective case series
