Clinical applicability of gastroscopy with narrow-band imaging for the diagnosis of Helicobacter pylori gastritis,precancerous gastric lesion,and neoplasia
2020-09-14
Jun-Hyung Cho,Seong Ran Jeon,Digestive Disease Center,Soonchunhyang University Hospital,Seoul 04401,South Korea
So-Young Jin,Department of Pathology,Soonchunhyang University Hospital,Seoul 04401,South Korea
Abstract
Premalignant gastric lesions such as atrophic gastritis and intestinal metaplasia frequently occur in subjects with long-term Helicobacter pylori (H.pylori) infection.The regular arrangement of collecting venules (RAC) is seen in the normal gastric corpus,whereas mucosal swelling and redness without RAC are observed in H.pylori-infected mucosa.Despite successful H.pylori eradication,the presence of atrophic gastritis and/or gastric intestinal metaplasia (GIM) is a risk factor for gastric cancer.With the development of advanced imaging technologies,recent studies have reported the usefulness of narrow-band imaging (NBI) for endoscopic diagnosis of atrophic gastritis and GIM.Using NBI endoscopy with magnification (M-NBI),atrophic gastritis is presented as irregular coiled microvessels and loss of gastric pits.Typical M-NBI endoscopic findings of GIM are a light blue crest and a white opaque substance.Based on the microvascular patterns,fine network,core vascular,and unclear patterns are useful for predicting gastric dysplasia in polypoid lesions.For diagnosis of early gastric cancer (EGC),a systematic classification using M-NBI endoscopy has been proposed on the basis of the presence of a demarcation line and an irregular microvascular/microsurface pattern.Furthermore,M-NBI endoscopy has been found to be more accurate for determining the horizontal margin of EGC compared to conventional endoscopy.In this review,we present up-to-date results on the clinical usefulness of gastroscopy with NBI for the diagnosis of H.pylori gastritis,precancerous gastric lesion,and neoplasia.
Key words:Gastroscopy;Narrow-band imaging;Magnification;Helicobacter pylori;Atrophic gastritis;Intestinal metaplasia;Dysplasia;Cancer
INTRODUCTION
Gastric cancer is the third most common cause of cancer-related mortality worldwide[1].Long-termHelicobacter pylori(H.pylori) infection causes premalignant gastric conditions,such as atrophic gastritis and intestinal metaplasia[2].In particular,gastric intestinal metaplasia (GIM) is a risk factor for gastric cancer development[3].Although GIM is reportedly improved afterH.pylorieradication,complete elimination of the gastric cancer risk cannot be guaranteed[4].Thus,surveillance endoscopy is recommended in subjects with precancerous lesions at the time of eradication[5].The diagnostic accuracy for precancerous lesions and gastric neoplasia of image-enhanced endoscopy has been increased by the advent of narrow-band imaging (NBI)endoscopy[6,7].Before endoscopic submucosal dissection,NBI endoscopy can determine the margin of gastric dysplasia and cancer to promote complete removal[8].Although pathologic diagnosis is the gold standard,accurate endoscopic prediction is important to minimize the number of biopsies and prevent post-biopsy bleeding.Herein,we present up-to-date results on the clinical usefulness of NBI endoscopy for the diagnosis ofH.pylorigastritis,precancerous gastric lesion,and neoplasia.This review consists of the following:(1)H.pylorigastritis;(2) Atrophic gastritis and GIM;(3)Gastric dysplasia;and (4) Early gastric cancer (EGC).
PRINCIPLE OF NARROW-BAND IMAGING WITH MAGNIFICATION
In 2005,technological advances resulted in the advent of NBI.NBI is an innovative optical method that modifies the wavelengths and bandwidths of the light into narrow bands of 415 ± 30 nm and 540 ± 30 nm[9].In this endoscopy system,which uses a red(R),green (G),and blue (B) sequential imaging system,the gastrointestinal mucosa is illuminated sequentially with R,G,and B light through a rotating RGB filter wheel[10].When the endoscopist presses the button on the handle,a narrow-band filter is inserted between the lamp and the RGB filter.Red light (long wavelength) diffuses widely and deeply,whereas blue light (short wavelength) diffuses within a smaller range and less deeply.Because short-wavelength light strongly reflects from the epithelial surface,it is suitable for visualizing its morphology.Therefore,NBI improves upon the detailed visualization possible by magnifying endoscopy(Figure 1).
Magnifying endoscopy enables examination of the microanatomy of the gastric mucosa[11].When a soft black cap is fixed to the tip of the endoscope,it is possible to maintain a distance of approximately 2 mm,at which up to 100-fold magnification is feasible.This system produces sharp images of microvascular architecture and microsurface structure.In the upper gastrointestinal tract,the line of sight may be disrupted by respiration movement and great vessel pulsation.To climb the learning curve of magnifying endoscopy,a proper training system under an experienced supervisor is required.

Figure1 Narrow-band imaging system.
H.PYLORI GASTRITIS
Initially,the main tests forH.pyloriinfection were the rapid urease test and pathologic examination,both of which require a biopsy specimen[12].The development of endoscopy techniques enabled detection in real time,earlier than possible using these biopsy-based tests.In 2005,a regular arrangement of collecting venules (RAC) was suggested as the normal pattern in gastric mucosa withoutH.pyloriinfection[13].When the gastric corpus was examined by magnifying endoscopy,H.pylori-infected mucosa showed dilation of gastric pits and disappearance of RAC[14].For predicting histological chronic gastritis,Taharaet al[15]observed the gastric corpus using magnifying NBI (M-NBI) endoscopy,which enabled visualization of the micromucosal pattern.The normal pattern was defined as a honeycomb-like subepithelial capillary network (SECN) and the presence of RAC (Figure 2A).The abnormal pattern ofH.pylori-induced gastritis is typically polygonal swollen mucosa with enlarged crypt openings (Figure 2B).The sensitivities and specificities of magnifying endoscopy forH.pyloriinfection are 93.8% to 100% and 82.2% to 96.2%,respectively[13-15].
Recently,endoscopists have attempted to diagnoseH.pyloriinfection by white-light imaging (WLI) or non-magnifying endoscopy with other image-enhanced techniques[16,17].The typical endoscopic findings ofH.pylori-induced gastritis are mucosal swelling and redness with disappearance of RAC at the gastric corpus[18].Although M-NBI has good diagnostic accuracy,non-magnifying endoscopy is also useful for detectingH.pylorigastritis by close observation of the greater curvature side of the corpus[19].Furthermore,the rapid urease test from the corpus mucosa had a faster positive reaction,compared to the antrum[20].However,an endoscopic classification based on WLI has not yet been established.Furthermore,a comparison study between M-NBI and WLI endoscopy forH.pyloridiagnosis is needed.

Figure2 Magnifying narrow-band imaging endoscopy images of Helicobacter pylori-positive corpus mucosa and atrophic gastritis.
ATROPHIC GASTRITIS
In theH.pylori-infected stomach,chronic active inflammation becomes persistent,leading to mucosal atrophy with destruction of gastric glands.In Japan,atrophic gastritis is often evaluated according to the endoscopic Kimura-Takemoto classification[21].This classification has showed the good agreement with the histological assessment of atrophic gastritis[22,23].Because of high risk for gastric cancer development,surveillance endoscopy is required in subjects with severe atrophic gastritis[24].Compared to conventional WLI endoscopy,M-NBI enables a reliable diagnosis for the degree of atrophic gastritis.
Before the NBI system was introduced,Yagiet al[13]classified magnified endoscopic findings of theH.pylori-infected corpus mucosa into three types (Z-1 to Z-3).Of these,type Z-3 mucosal pattern corresponded to histological features of marked atrophy of the gastric glands.Recent studies have reported the typical endoscopic findings using M-NBI in diagnosing atrophic gastritis[25,26].Ridged surface structures encasing dilated coiled subepithelial capillaries indicates the presence of atrophic gastritis (Figure 2C).With progression to severe mucosal atrophy,irregular coiled microvessels and loss of gastric pits are observed by M-NBI endoscopy (Figure 2D).The sensitivities and specificities of M-NBI endoscopy for atrophic gastritis are 50.0% to 90.0% and 96.0% to 96.3%,respectively[14,15].
GASTRIC INTESTINAL METAPLASIA
All subjects with intestinal metaplasia are at risk for gastric cancer development.Whether the gastric cancer risk is low or high depends on the extent and severity of intestinal metaplasia[27].Since the advent of endoscopy,pathologic examination by forceps biopsy has been the gold standard for diagnosis of GIM.In the updated Sydney System,multiple mucosal biopsies from the gastric corpus and antrum are required to obtain the specimens[28].The diagnostic criteria of the operative link for gastritis assessment (OLGA) and operative link for gastric intestinal metaplasia assessment (OLGIM) enable more reliable risk stratification of gastric cancer and identification of patients at high risk who need endoscopic surveillance[29,30].However,OLGA and OLGIM staging are based on pathologic analyses of gastric biopsies from five sites.In clinical practice,elderly patients taking antiplatelets or anticoagulants are at risk for post-biopsy bleeding.Furthermore,biopsy-based methods can considerably increase the medical costs and procedure time.
Based on WLI,the presence of light gray granular patches is the only endoscopic finding of GIM[31].However,endoscopic diagnosis using only WLI is limited by its low sensitivity.Several investigators have proposed NBI endoscopic criteria for diagnosis of GIM (Table 1).In 2006,Uedoet al[32]suggested a light blue crest (LBC) as a new diagnostic criterion for GIM.In M-NBI endoscopy,LBC was observed as a fine,blue line on the crests of the epithelial surface (Figure 3).The presence of LBC was correlated with histological GIM (sensitivity 89% and specificity 93%).Savarinoet al[33]reported that LBC was a good indicator of GIM (sensitivity 80%,specificity 96%,and accuracy 93%).For diagnosing GIM,Anet al[34]suggested the marginal turbid band(MTB),which was defined as an enclosed,white turbid band on the epithelial surface.The presence of both MTB and LBC is more frequent in moderate to severe GIM.The presence of MTB without LBC is considered indicative of early-stage GIM.LBC may be present in subjects who progress to severe GIM.In Japan,Kanemitsuet al[35]reported that a white opaque substance (WOS) was a marker for M-NBI diagnosis of GIM.The sensitivity of LBC for histological GIM was 62.5%.When the presence of WOS was added to the criteria,the sensitivity increased to 87.5%.The specificity and accuracy of WOS and/or LBC were 93.8% and 90.0%,respectively.Sakaet al[36]performed M-NBI endoscopic staging of gastritis using the diagnostic criteria of a tubular/granular mucosal pattern plus LBC or WOS.The accuracy for assessing OLGA and OLGIM was 69.1% for the antrum and 72.7% for the corpus.The degree of gastritis was classified as low or high risk by summing the scores of the corpus and antrum.The concordance of M-NBI endoscopy with histologic severity for differentiating the low- and high-risk groups was 89.1%.
In 2012,Pimentel-Nuneset al[37]performed a validation study for endoscopic classification of premalignant gastric lesions and dysplasia.Using NBI endoscopy without magnification,they defined a regular tubulovillous or ridge glandular pattern as intestinal metaplasia.This mucosal pattern showed 90% sensitivity,81% specificity,and 84% accuracy for diagnosing GIM.They used a web-based video system to address the learning curve of this classification by endoscopists[38].The sensitivity and specificity for GIM reached 73% and 81% by the trainees,respectively.An NBI classification seemed to be easily learned for the identification of precancerous gastric lesions.A multicenter prospective study evaluated endoscopic grading of GIM using the same NBI classification[39].Five different areas (each of the lesser and greater curvature of the corpus and antrum,and the gastric angle) were closely observed by NBI endoscopy.The endoscopic grades of GIM were evaluated on a three-point scale[no = 0,focal (≤ 30%) = 1,and extensive (>30%) = 2] according to the extent of metaplastic mucosa.Compared to WLI,NBI had a higher diagnostic accuracy for GIM(83%vs94%).NBI increased the sensitivity for GIM from 53% to 87%.The endoscopic grading was concordant with an extensive degree of histological GIM (OLGIM III/IV).If the cutoff value was >4 (total score = 5–10),the sensitivity and specificity for OLGIM III/IV were 94.2% and 95.2%,respectively.The area under the receiver operating characteristic curve was 0.98.Espositoet al[40]suggested that NBI endoscopy for diagnosing GIM is useful for evaluating the risk for OLGIM without performing mucosal biopsy.By contrast,another study reported that the diagnostic yield for GIM using NBI endoscopy was 53% to 65%[41].NBI-targeted biopsy is still recommended for detection of GIM.A further largescale study is required to standardize the diagnosis of GIM using NBI endoscopy.
GASTRIC DYSPLASIA
Gastric dysplasia is the precursor lesion of gastric adenocarcinoma,particularly of the intestinal type[42].The World Health Organization (WHO) defines dysplasia in the gastrointestinal tract as the presence of histologically unequivocal neoplastic epithelium without evidence of tissue invasion[43].According to the revised Vienne classification,low and high grade dysplasia (category 3 and 4.1) is classified as noninvasive gastric neoplasia[44].Endoscopic resection is recommended for the management of gastric dysplasia due to the possibility of malignant transformation[45].Indefinite pathology for neoplasia on forceps biopsy specimens (category 2 of the revised Vienne classification) is often observed in clinical practice.Althoughendoscopic biopsy is essential before planning an endoscopic resection,there are reportedly histological discrepancies between forceps biopsy and post-resection specimen[46].In a study by Leeet al[47],up to 64.5% of gastric lesions with indefinite pathology were upgraded to dysplasia and cancer after endoscopic submucosal dissection (ESD).Endoscopic biopsy may not be representative of the entire lesion due to its superficiality and sampling errors[48].Repeated biopsy can make the subsequent endoscopic treatment to be difficult due to submucosal fibrosis.Therefore,advanced endoscopic imaging such as M-NBI is required for the management of gastric dysplasia.

Table1 Endoscopic criteria using narrow-band imaging for diagnosis of gastric intestinal metaplasia

Figure3 Narrow-band imaging endoscopy images of intestinal metaplastic mucosa.
Using M-NBI endoscopy,Omoriet al[49]suggested the characteristics of fine mucosal structures and microvascular pattern for diagnosing the gastric polypoid lesions.Most reliable microvascular patterns were honeycomb for fundic gland polyp (sensitivity 94.7%,specificity 97.4%) and dense vascular patterns for hyperplastic polyp(sensitivity 93.6%,specificity 91.6%).For predicting gastric neoplasia,fine network,core vascular,and unclear patterns showed the high specificity of 97%,100%,and 100%,respectively (Figure 4).Hwanget al[50]reported the association between the MNBI findings and upgraded histology in biopsy-proven low grade dysplasia.Positive M-NBI findings were defined as the irregularity of microvascular and/or microsurface patterns within the lesion (Figure 5).In cases with positive M-NBI findings,76.6% (n=59/77) was diagnosed as high grade dysplasia and cancer in post-resection pathology.If either an irregular microvascular or microsurface pattern is present,the gastric lesion can be diagnosed as high grade dysplasia or EGC[51].In addition,M-NBI endoscopy is useful for determining the horizontal margin of gastric dysplasia before ESD (Figure 6).
EARLY GASTRIC CANCER
Differential diagnosis between focal gastritis and small depressed cancer
During endoscopy,EGCs are recognized based on a color or morphological change.Particularly in small (≤ 10 mm) gastric cancer,pathologic examination may be misdiagnosed due to targeted biopsy failure.If the opportunity for treatment by endoscopic resection is missed,false negativity based on the pathologic result alone is of great concern.Because conventional WLI endoscopy cannot be used to examine the gastric micromucosal pattern in detail,its utility for real-time endoscopic diagnosis is limited.To overcome this,the diagnostic efficacy of magnifying endoscopy has been investigated (Table 2).
In 2007,Yaoet al[52]reported that cancerous lesions can be diagnosed by close observation using magnifying endoscopy.They defined the characteristics of EGC as a demarcation line (DL) between the lesion and the background mucosa,and an irregular microvascular pattern within the lesion.When these criteria were used,the sensitivity,specificity,and accuracy for distinguishing cancerous from benign lesions were 92.9%,99.3%,and 98.7%,respectively.Ezoeet al[53]performed a prospective comparative study of magnifying NBI and magnifying WLI for diagnosing cancer in small depressed lesions.The NBI mode enabled the gastric micromucosal patterns around the lesion to be more clearly visualized,increasing the diagnostic performance(sensitivity of 70.0%vs33.3%,specificity of 88.8%vs66.6%,and accuracy of 78.9%vs43.8%).Yamadaet al[54]compared WLI alone and M-NBI after WLI for the diagnosis of small,depressed EGC.When WLI endoscopy alone was performed according to criteria including irregular margin and spiny depressed area,the sensitivity was 40%,specificity was 68%,and accuracy was 65%.Remarkably,M-NBI after WLI showed improved sensitivity of 95%,specificity of 97%,and accuracy of 97%.
Systematic classification using M-NBI endoscopy based on microvascular and microsurface pattern (the VS classification) has been proposed[55].When a suspicious mucosal lesion with color or morphological change is detected,the first step is to identify the presence of DL,which separates the lesion from the background mucosa.A lesion without DL is unlikely to be cancer.If a DL is seen,the diagnosis of gastric cancer can be determined by the presence of an irregular microvascular and/or microsurface pattern within the DL (Figure 7).In a prospective multicenter study,the sensitivity,specificity,and accuracy of M-NBI for diagnosis of EGC were 85.7%,99.4%,and 98.1%,respectively[56].However,there were false-negative cases of signet ring cell carcinoma despite the diagnosis being performed by well-trained endoscopists.Palecolored lesions suspicious of undifferentiated-type carcinoma are indications not for M-NBI endoscopy but rather for pathologic study by targeted biopsy.Fugiwaraet al[57]evaluated M-NBI diagnosis of minute gastric cancer (≤ 5 mm) compared to chromoendoscopy using indigo carmine.The sensitivity and diagnostic accuracy were significantly higher for M-NBI endoscopy than chromoendoscopy (78.0%vs43.7% and 88.3%vs69.9%,respectively).
Katoet al[58]suggested a diagnostic triad for gastric cancer by M-NBI endoscopy:Disappearance of fine mucosal structure,microvascular dilation,and heterogeneous shape.The sensitivity and specificity were significantly higher than those ofconventional WLI endoscopy (92.9%vs42.9% and 94.7%vs61.0%,respectively).Kanesakaet al[59]categorized the microvascular patterns of small depressed lesions as microvascular dilation,microvascular tortuosity,difference in caliber,and variation in shape.Among these microvascular findings by M-NBI endoscopy,variation in shape was the most significant feature,with a diagnostic accuracy of 92%.

Table2 Diagnostic performance of narrow-band imaging with magnification for early gastric cancer
Determination of the horizontal extent of early gastric cancer before endoscopic submucosal dissection
ESD is curative in selected patients with EGC[60].For a successful outcome of ESD,the tumor margin should be clearly examined.In M-NBI endoscopy,EGC margin delineation can be achieved by close-up observation of DL (Table 3).In 2010,Kiyotokiet al[61]evaluated the usefulness of M-NBI endoscopy for determining the gastric tumor margin compared to conventional chromoendoscopy using indigo carmine.Before ESD,marking dots were made at the tumor margin using M-NBI or chromoendoscopy.If the distance was less than 1 mm between the endoscopic marking dot and pathologically confirmed margin,the diagnosis was defined to be accurate.The diagnostic accuracy was significantly higher for M-NBI endoscopy than chromoendoscopy (97.4%vs77.8%,P =0.009).In another comparison study,there was a significant difference in delineating the margin of EGC between M-NBI endoscopy and chromoendoscopy (89.4%vs75.9%,P =0.007)[62].Similarly,several studies have demonstrated that M-NBI endoscopy improves the determination of horizontal extent before ESD in patients with an unclear margin of EGC[63,64].Horiiet al[65]showed that diagnostic accuracy using M-NBI was 96.7% when the successful demarcation of EGC was evaluated on the basis of the biopsy-negative rate outside the tumor.Complete resection of EGC with a tumor-negative horizontal margin was achieved in 97.9% (n=323/330).However,Nagahamaet al[66]reported that the diagnostic accuracy for EGC margin delineation of M-NBI endoscopy was not superior to that of chromoendoscopy using indigo carmine.Indeed,M-NBI endoscopy does not need a dye solution and so is less time consuming.

Table3 Determination of the horizontal extent of early gastric cancer by magnifying narrow-band imaging

Figure4 Magnifying narrow-band imaging findings of microvascular patterns for diagnosing the gastric polypoid lesions.
CONCLUSION
In an era of high imaging quality in medical devices,magnifying endoscopy and NBI have enabledH.pyloridiagnosis,endoscopic grading of GIM,and detailed characterization of small gastric cancer.Previously,the pathological report was the absolute authority for diagnosis of gastrointestinal tract diseases.Henceforth,imageenhanced endoscopy can make a big step to optical biopsy,which is a real-time diagnosis during gastrointestinal endoscopy.Furthermore,confocal laser endomicroscopy and endocytoscopy may become available in the future[67].If endoscopists are well-trained in advanced endoscopic imaging,they may evolve into endo-pathologists[68].

Figure5 White-light endoscopy and magnifying narrow-band imaging images of high grade dysplasia.

Figure6 Narrow-band imaging endoscopy for determining the horizontal margin of gastric dysplasia before endoscopic submucosal dissection.
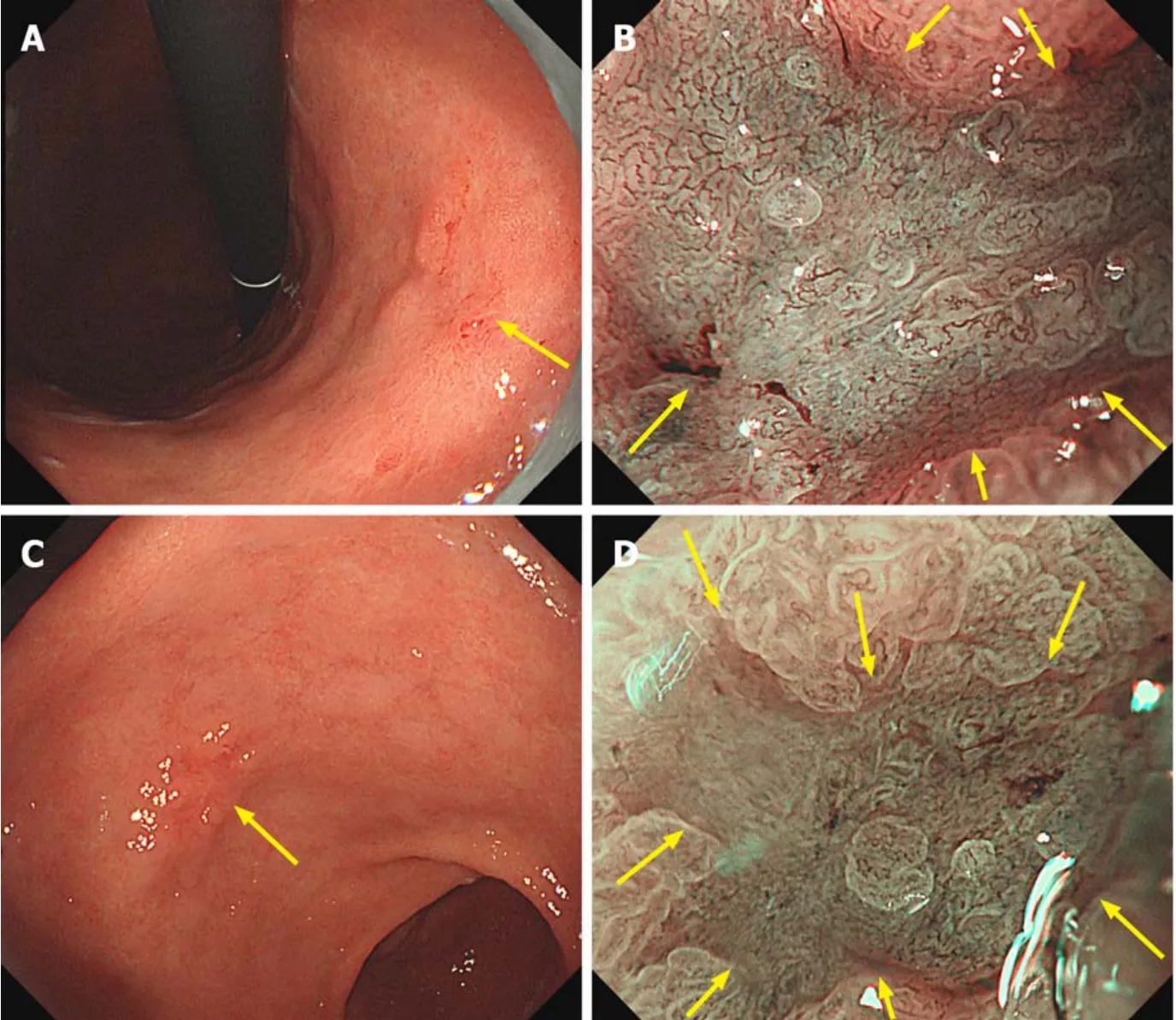
Figure7 Magnifying narrow-band imaging endoscopic findings of early gastric cancers.
杂志排行
World Journal of Clinical Cases的其它文章
- Recommendations for perinatal and neonatal surgical management during the COVID-19 pandemic
- Identification of APEX2 as an oncogene in liver cancer
- Restenosis after recanalization for Budd-Chiari syndrome:Management and long-term results of 60 patients
- Comparison of microendoscopic discectomy and open discectomy for single-segment lumbar disc herniation
- Clinical characteristics of patients with COVID-19 presenting with gastrointestinal symptoms as initial symptoms:Retrospective case series
- Effects of policies and containment measures on control of COVID-19 epidemic in Chongqing
