Treatment Effect of Pig Manure-derived Biochar-based Metal Catalyst for Pig Breeding Wastewater
2020-08-01ZhijunZHANGYijueGAOJiangchengSHUJiaweiHU
Zhijun ZHANG, Yijue GAO, Jiangcheng SHU, Jiawei HU
1. Department of Architectural and Environmental Engineering, Huaide College, Changzhou University, Taizhou 214500, China; 2. School of Environmental & Safety Engineering, Changzhou 213164, China
Abstract [Objectives] To treat pig farm wastewater and solve the problem of pollution caused by it to surface water or groundwater. [Methods] Fe and Zn/biochar (AC) was prepared by dipping method using pig manure-derived biochar as carrier. The preparation conditions were investigated, and the screened metal-loaded biochar was characterized. Pig farm waste water was treated with metal-loaded biochar-H2O2 catalytic oxidation method. [Results] At the COD concentration of 2 904 mg/L, 0.02 g Zn/AC and 0.005 mL H2O2 showed the highest COD removal rate (qe) from pig breeding wastewater under conditions of reaction time of 8 h, pH value of 7 and temperature of 55 ℃, reaching 70.98%. [Conclusions] Fe or Zn-loaded biochar made from pig manure-derived activated carbon has a certain catalytic capacity for the actual oxidation treatment of pig farm wastewater. The activity of Zn/AC was higher, and its COD removal rate from pig farm wastewater was also higher.
Key words Pig manure, Metal-loaded biochar, Hydrogen peroxide, Catalytic oxidation, Pig farm wastewater
1 Introduction
Pig farm wastewater mainly comes from pig manure and flushing water at the breeding site. Its chemical oxygen demand (COD), ammonia nitrogen, SS and other indicators are relatively high. Direct discharge or inappropriate storage of pig farm wastewater will cause serious deterioration of surface water or groundwater quality. The leaching of livestock and poultry manure is very strong, and the content of water-soluble organic matter in feces and urine is high. If not handled properly, livestock and poultry manure will enter the groundwater layer through surface runoff and infiltration to contaminate groundwater.
Biochar is a type of carbonaceous material prepared by anoxic pyrolysis of animal and plant residues. It is an efficient adsorbent for pollutants such as heavy metals and pesticides for its high cation exchange capacity, high porosity, large specific surface area, and multiple functional groups on the surface. At present, China’s farmland wastes are mainly straw and animal feces, of which the annual output is huge but the effective utilization rate is low. In the case of long-term accumulation of feces in the open air, leachate pollutes groundwater with runoff, making the water body eutrophic. In addition, pathogens in the water body may cause water and soil pollution and make them deteriorate[1-3]. Pig manure has a finer texture and contains more organic matter and nitrogen, phosphorus, potassium. In this study, the preparation process of pig manure-derived activated carbon was optimized. On the basis of burning raw materials into biochar, a certain concentration of metal ions was loaded to it to improve its special performance and specific adsorption and catalytic performance in the treatment of wastewater.
Hydrogen peroxide has a strong oxidizing ability, and it can quickly separate atomic oxygen or generate hydroxyl radicals on the surface of activated carbon. This type of strong oxidants can quickly oxidize and decompose organic matter in wastewater, to achieve the purpose of reducing the COD concentration and chromaticity of wastewater. Biochar, as a solid catalyst, can be reused many times after proper treatment[4].
Therefore, in this experiment, iron-loaded biochar (Fe/AC) and zinc-loaded biochar (Zn/AC) were prepared by dipping method, and taking hydrogen peroxide (H2O2) as oxidant, catalytic oxidation of pig farm wastewater was carried out, thus realizing efficient degradation of high-concentration pig farm wastewater.
2 Materials and methods
2.1ReagentsandinstrumentsThe raw material of pig manure for preparing biochar and pig breeding wastewater were all from a pig farm in Wujin District, Changzhou City, which was naturally dried and ground for use. The reagents and equipment used in the experiment are shown in Table 1 and Table 2.

Table 1 Main reagents and drugs used
2.2Experimentalmethods
2.2.1Preparation of catalyst. A certain amount (5 g) of crushed pig manure was poured into 0.8 mol/L KOH solution. After stirring evenly, the mixture was placed in a constant-temperature shaker at 40 ℃ for 24 h. Then, the thick substance was poured into a Petri dish to dry to a constant mass. The dried solid was ground and placed in a vacuum tube furnace, in which the temperature was increase to 600 ℃ at 15 ℃/min and the temperature of 600 ℃ was kept for a while. After cooling the pyrolysis to room temperature, it was dipped in hydrochloric acid, rinsed with deionized water repeatedly, dried, ground and sieved to obtain pig manure-derived biochar.
Table 2 Main instruments and equipment used
A certain amount (0.5 g) of pre-treated pig manure-derived biochar was dipped in 100 mL of metal ion solution of a certain concentration, shaken at 40 ℃ for 24 h, suction-filtered, dried in an oven at 105 ℃ to constant mass, calcined in a vacuum tube furnace for a certain period of time to produce metal-loaded biochar.
2.2.2Adsorption experiment of pig farm wastewater. A certain volume (50 mL) of pig farm wastewater (pH 7, COD concentration 2 904 mg/L) was transferred to a 100-mL conical flask with a stopper and added with a certain amount of biochar. After shaking at 140 r/min in a constant-temperature shaker for a certain period of time, the COD concentration was determined. The influence of biochar addition amount, pH value and adsorption time on the adsorption effect was investigated.
2.2.3Characterization of biochar. The compound activated carbon prepared by optimized process was characterized. The changes in the surface morphology of biochar before and after modification were observed using SEM, and the changes in the phase structure of biochar before and after metal loading were analyzed using XRD.
3 Results and discussion
3.1Effectsofdifferentmetalionconcentrationsofmetal-loadedbiocharonremovalofCODfrompigbreedingwastewaterA certain amount (0.5 g) of pre-treated pig manure-derived biochar was dipped in 100 mL of metal ion solution of a certain concentration, shaken at 40 ℃ for 24 h, suction-filtered, dried in an oven at 105 ℃ for 6 h and calcined in a vacuum tube furnace for 2 h. The effects of different metal ion concentrations in the metal-loaded activated carbon on removal of COD from wastewater were investigated. As shown in Fig.1, the COD removal rate increases first and then decreases. When the zinc ion concentration was 0.5 mol/L and the iron ion concentration was 1 mol/L, the COD removal rate (qe) reached the maximum value. At the beginning of the dipping, with the increase in the amount of zinc chloride and ferric nitrate, there were more zinc ions and iron ions loaded on the inner surface of activated carbon. When the loading amount was lower than the maximum value of the distribution of monolayer on the surface of activated carbon, the metal ions are distributed in a dispersed form on the surface of pig manure-derived activated carbon and therefore show a high catalytic activity. When the amount of zinc chloride and ferric nitrate exceeded the maximum distribution of monolayer, the COD removal rate reduced. The reason was that the loading was higher than the maximum distribution of monolayer on the surface of activated carbon, and part of the metal ions precipitated in the form of large crystalline particles, resulting in decreased catalyst activity[5].
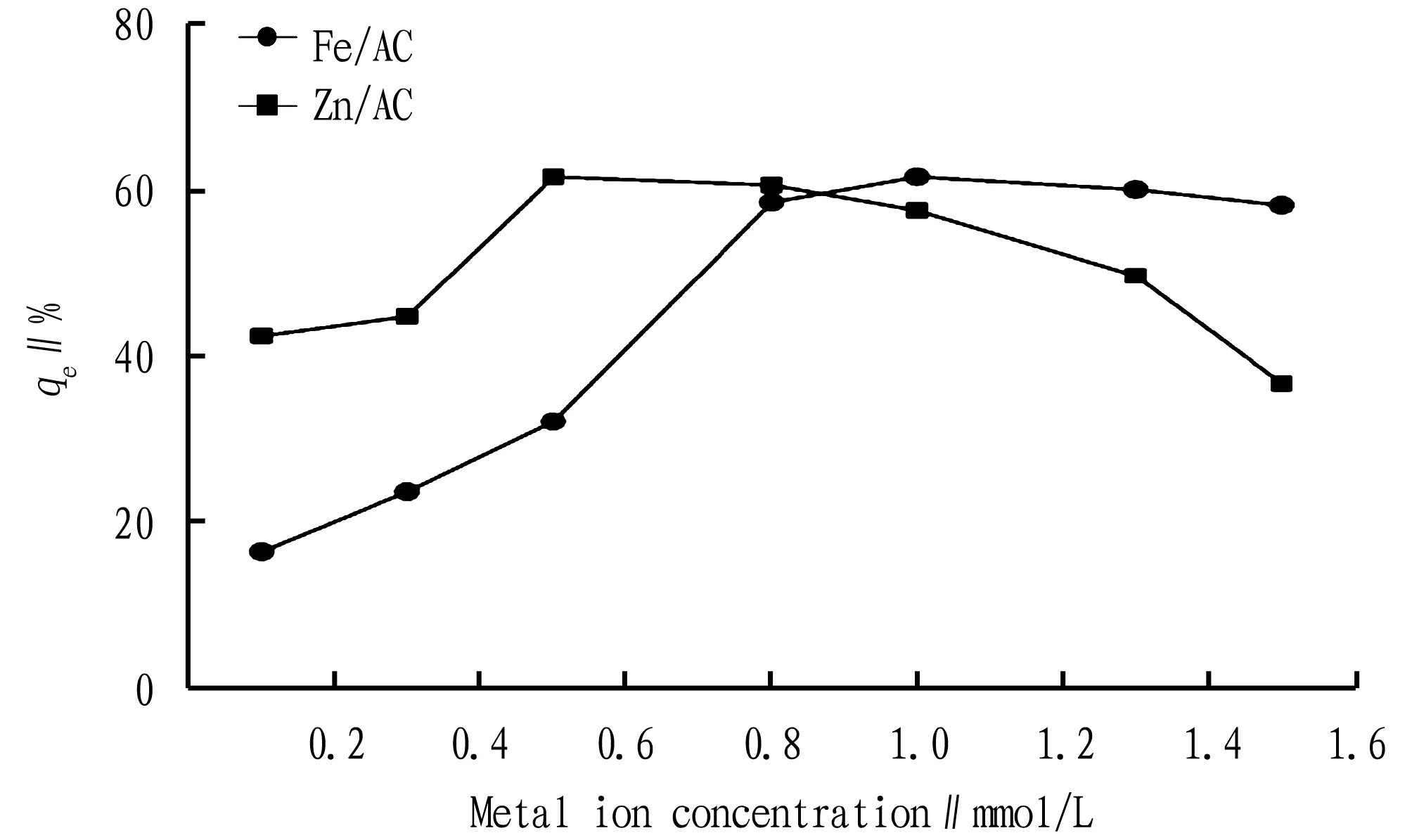
Fig.1 Effects of different metal ion concentrations on COD removal rate
3.2Effectsofdifferentactivationtemperaturesofmetal-loadedbiocharonremovalofCODfrompigbreedingwastewaterA certain amount (0.5 g) of pre-treated pig manure-derived biochar was dipped in 100 mL of metal ion solution (0.5 mol/L), shaken at 40 ℃ for 24 h, suction-filtered, dried in an oven at 105 ℃ for 6 h, and calcined in a vacuum tube furnace for 2 h. The effects of different activation temperatures of metal-loaded activated carbon on removal of COD from pig farm wastewater. As shown in Fig.2, the removal rate of COD increased first and then decreased with the increase of the calcination temperature of Zn/AC and Fe/AC. As the temperature continued to increase, to a greater extent, the active components accelerated their migration speed and also improved their dispersion, thereby significantly improving the performance of the catalyst and the COD removal rate of wastewater. When the calcination temperature reached 400 ℃ and 500 ℃, respectively, the COD removal rate both reached the maximum. When the calcination temperature exceeded the temperature of the maximum adsorption capacity, the pore structure of the biochar encountered burning and was destroyed, thereby reducing the catalytic ability of the catalyst and the removal rate of COD[6].
In summary, regardless of metal ion concentration and activation temperature, Zn-loaded pig manure-derived biochar is a good catalyst. At the zinc ion concentration of 0.5 mol/L and calcination temperature of 500 ℃, Zn/AC showed the best adsorption effect on pig farm wastewater[7]. Therefore, the Zn/AC prepared under the conditions above was selected to continue the following experiment.

Fig.2 Effects of different calcination temperatures on COD removal rate
3.3Characterizationofbiochar
3.3.1XRD analysis. As shown in Fig.3, the metal-unloaded pig manure-derived biochar showed broad and strong carbon diffraction peaks at 20°-30° and 30°-35°, indicating that a large number of graphite crystallites existed in the pig manure-derived biochar. After modified by ZnCl2, the diffraction peaks showed different degrees of reduction, while 2θshowed sharper ZnO diffraction peak at 30°-40°, 47.46°, 56.52°, 62.78° and 67.88°[8](Fig.4). It indicates that the main component of zinc-loaded pig manure-derived biochar is ZnO. The sharp diffraction peak indicates that it has high crystallinity[9].

Fig.3 XRD spectrum of pig manure-derived biochar
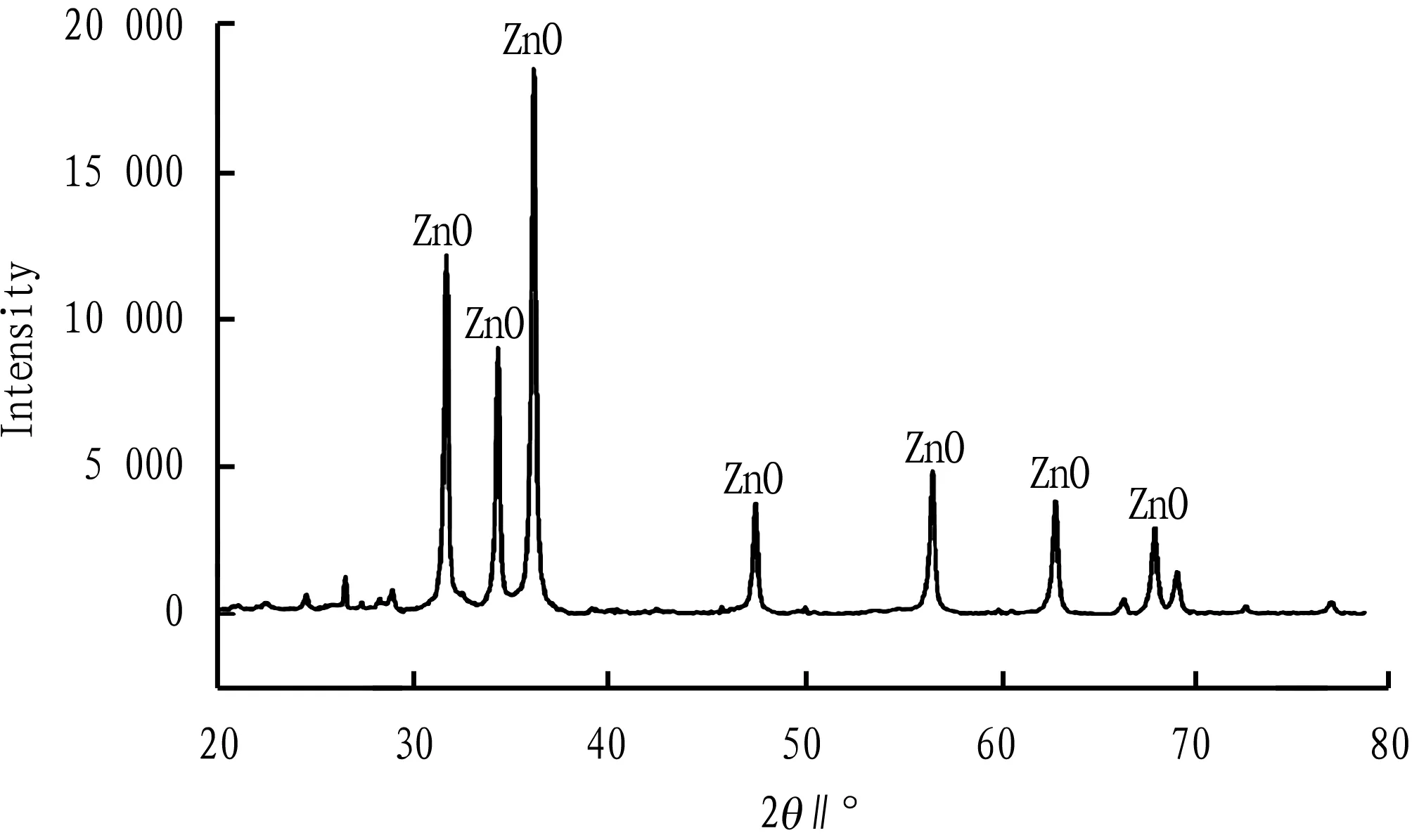
Fig.4 XRD spectrum of zinc-loaded pig manure-derived biochar
3.3.2SEM analysis. As shown in Fig.5a and Fig.5b, there are many voids on the surface of metal-unloaded pig manure-derived biochar. In contrast, the pig manure-derived biochar modified by ZnCl2has more pores and uneven surface, so that its specific surface area is greatly increased[10]. More ZnO molecules adhered to the surface and pores of the pig manure-derived biochar, which was conducive to the improvement of adsorption capacity.
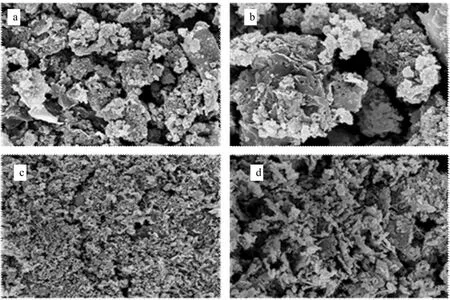
Note: a. Pig manure-derived biochar (×1 000); b. Pig manure-derived biochar (×5 000); c. Zn-loaded pig manure-derived biochar (×1 000); d. Zn-loaded pig manure-derived biochar (×5 000).
Fig.5 SEM photographs of pig manure-derived biochar and zinc-loaded pig manure-derived biochar
3.4InfluenceofdifferentfactorsontheCODremovaleffectofpigbreedingwastewater
3.4.1Amount of biochar added. The dynamics of COD removal rate with the increased dosage of AC and Zn/Ac are shown in Fig.6. As the addition amount of AC increased, the removal rate of COD showed an upward trend overall. At the dosage of 0.05 g, the COD removal rate reached the maximum (43.59%). For Zn/AC, the COD removal rate reached the peak at the dosage of 0.02 g; and as the addition amount was further increased, the COD removal rate declined gradually. This was mainly due to the fact that biochar had gradually desorbed after reaching adsorption saturation[11]. The COD removal rate of Zn/AC was the highest at the dosage of 0.02 g. Under the same conditions, Zn/AC had the more obvious COD removal effect on pig farm wastewater, and the highest removal rate reached 61.64%.

Fig.6 Effects of addition amount of biochar on COD removal rate
3.4.2Hydrogen peroxide added. Different dosages of H2O2, (AC+H2O2) and (Zn/AC+H2O2) were used to oxidize pig farm wastewater, and the dynamics of COD removal rate are shown in Fig.7.
The maximum COD removal rate of H2O2was 34.44%, a relatively low value, indicating that H2O2has little oxidizing and decomposing effect on wastewater in the absence of catalyst. The maximum COD removal rates of (AC+H2O2) and (Zn/AC+H2O2) were 58.54% and 68.38%, respectively. In the reaction systems, Ac and Zn/Ac both increased the decomposition rate of H2O2. Between them, the decomposition rate of H2O2was higher when Zn/AC was added. In the process of catalyzing H2O2’s oxidation for organic matter, biochar can initiate a series of chain decomposition reactions like OH-in alkaline solution. According to the mechanism of catalytic oxidation reaction, the catalyst in the reaction system can improve the efficiency of the decomposition of H2O2to generate hydroxyl radicals, thereby increasing the concentration of hydroxyl radicals in the solution and increasing the removal rate of organic matter[12]. However, as the amount of H2O2added increased, the COD removal rate decreased slowly. Zn/AC catalyst has more obvious degradation effect on wastewater. The best dosage of H2O2is 0.005 mL.
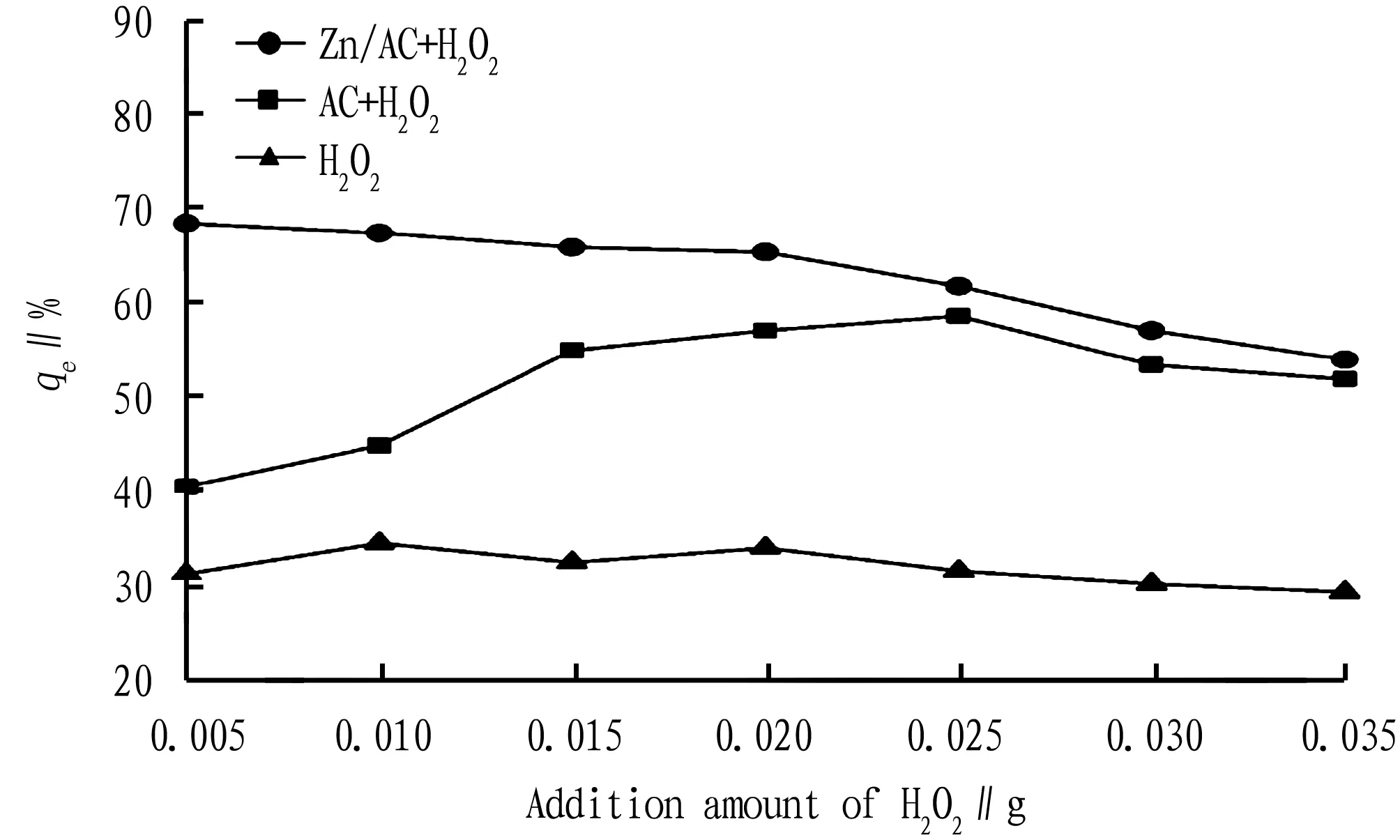
Fig.7 Effects of addition amount of H2O2 on COD removal rate
3.4.3pH value. Under different pH conditions, AC and Zn/AC were used to catalyze H2O2’s oxidation for pig farm wastewater, and the dynamics of COD removal rate are shown in Fig.8.
It can be seen from Fig.8 that as the pH value gradually increases, theqeof Zn/AC increased gradually from 33.68%, and the highest COD removal rate was 68.39% (pH=7); and theqeof AC also gradually rose from 24.79% at pH=2 to 61.67% at pH=7. But when the pH value continued to increase,qegradually decreased.
This was because that the adsorption sites of activated carbon combine with H+when the pH value was low, resulting in a very low COD removal rate; as the pH increased, since the adsorption sites bound to H+were released to bind to the pollutants, the removal rate of COD increased rapidly; and when the pH value was further increased, as the reaction between free OH-in the solution and ·OH decomposed by hydrogen peroxide accelerated the decomposition of hydrogen peroxide, the oxidation effect was reduced, and so,qeshowed a downward trend[13]. In summary, for the removal of COD from pig farm wastewater by Zn/AC and AC, the optimal pH of the reaction is 7.
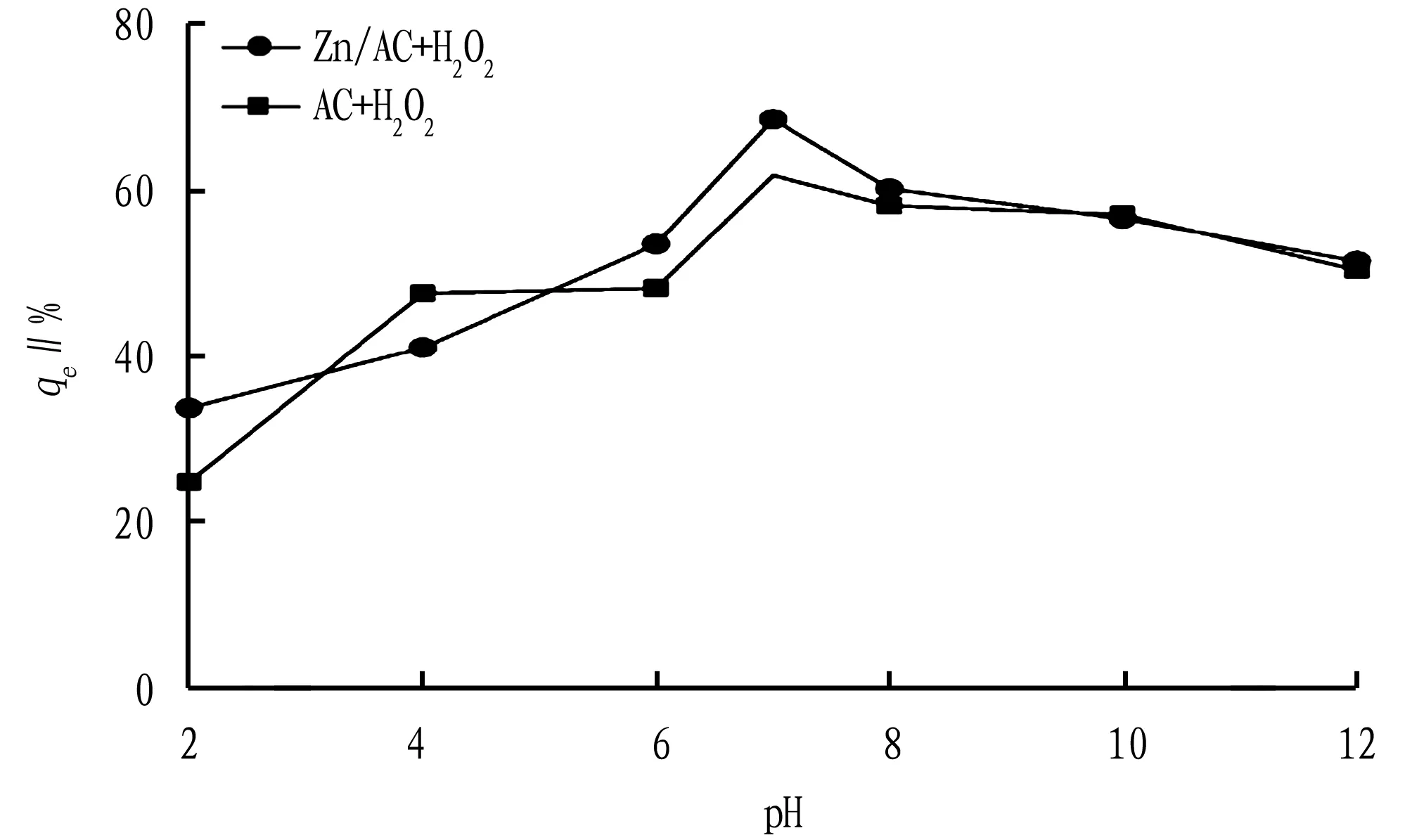
Fig.8 Effect of pH value on COD removal rate
3.4.4Time. At different times, the COD removal rate of pig farm wastewater catalyzed by AC and Zn/AC is shown in Fig.9. With the increase of time, the removal rate of COD first increased and then decreased. The metal-unloaded pig manure-derived biochar also showed a certain catalytic capacity for the catalytic oxidation of pig farm wastewater, and it had strong adsorption capacity for organic components in wastewater. When the reaction time was less than 4 h,qegradually increased with time and reached 61.67% at 4 h; and when the reaction time exceeded 4 h,qedecreased with time.
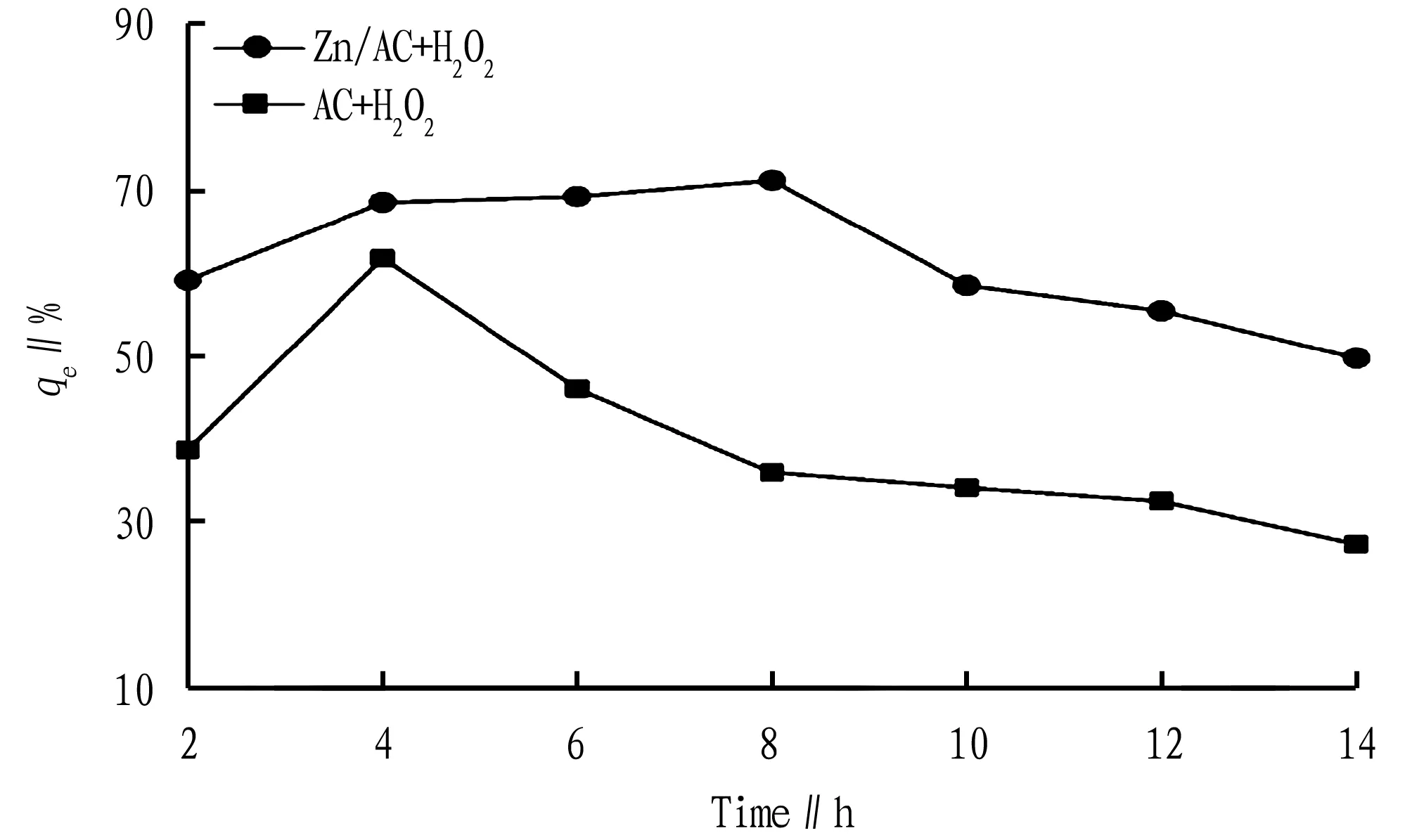
Fig.9 Effect of time on COD removal rate
For Zn/AC, the maximum is reached at 8 h, and then with the extension of the time, the removal rate of COD gradually decreased. Therefore, the best removal time is 8 h. This was mainly due to the fact that at the beginning, there were a large number of active sites in the biochar, so it showed a larger adsorption rate, and as the active sites were gradually occupied, the rate of adsorption began to gradually decrease and eventually reached the saturation equilibrium.
3.5ReuseofcatalystIn order to examine the effect of repeated use of Zn/AC, the biochar after each experiment was taken out, dried in an oven at 105 ℃ to constant mass, and subjected to oxidation experiment of pig farm wastewater under the conditions described above. As shown in Fig.10, after the biochar was reused 1 and 2 times, the COD removal rate could still reach 84.65% and 80.22%, respectively of the initial value, suggesting that zinc-loaded biochar is reusable. After the biochar was reused for 8 times, the COD removal rate of pig farm wastewater was 4.58%, indicating that the biochar had already basically lost its activity. The breakage in the use and the dissolution of the active component ZnO or the coverage of the active center with adsorbate may cause a reduction in the catalytic effect of biochar.
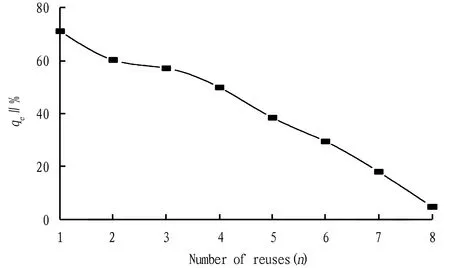
Fig.10 Effect of use times of catalyst on COD removal rate
4 Conclusions
Biochar made from pig manure-derived activated carbon loaded with Fe and Zn has a certain catalytic capacity for the actual oxidation treatment of pig farm wastewater. The activity of Zn/AC is relatively high, and its COD removal rate for pig farm wastewater is also the highest.
The optimized process for the preparation of Zn-loaded pig manure-derived biochar is as follows: dipping concentration 0.5 mol/L, dipping time 24 h, activation temperature 500 ℃ and activation time 120 min. After the wastewater with COD concentration of 2 904 mg/L was treated with 0.02 g of Zn/AC and 0.005 mL of H2O2under the following conditions, reaction time of 8 h, pH of 7 and temperature of 55 ℃, the COD removal rate is as high as 70.98%. Compared to Zn/AC, AC has poor adsorption effect on pig farm wastewater. Under the best conditions, the maximum removal rate for pig farm wastewater is only 61.67%. Biochar has strong adsorption capacity and certain catalytic ability. This may be related to the following aspects. (i) The surface of biochar contains a large number of acidic or basic groups such as hydroxyl and phenolic hydroxyl groups. (ii) The substance on the surface of biochar may crystallize, which provides effective active centers for the catalytic activity of metals and metal oxides. (iii) Biochar has excellent physical and chemical properties such as large specific surface area and strong lipophilicity that endow it with strong adsorption capacity for H2O2, thereby providing a larger reaction area for the reaction and increasing the reaction rate[14]. Repeated use experiments show that Zn/AC is reusable, and the biochar can still achieve a good removal effect when it is reused 1-2 times. The catalyst’s catalytic activity gradually decreases during repeated use.
杂志排行
Asian Agricultural Research的其它文章
- Empirical Study on Relationship between Income Structure and Consumption of Rural Residents in Jiangsu
- Report on Screening Test of Dazhou Superior Ramie Germplasm Resources
- Estimation of Economic and Ecological Value of Raising Sheep in Pastoral Area
- Construction of Platform-based Business Ecosystem
- Analysis of Industrial Transformation of Resource-based Towns in Qingyang City: Taking Qingcheng County as an Example
- Practice and Exploration of Scientific Recycling of Agricultural Plastic Film in Nanchong City
