Effects of different flow velocities on behavior and TRPA1 expression in the sea cucumber Apostichopus japonicas*
2020-07-31LINChenggangLIUXiaoluSUNLinaLIUShilinSUNJingchunZHANGLibinYANGHongsheng
LIN Chenggang , LIU Xiaolu , SUN Lina LIU Shilin SUN Jingchun ZHANG Libin YANG Hongsheng ,
1 CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China
3 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
4 The Innovation of Seed Design, Chinese Academy of Sciences, Wuhan 430071, China
5 College of Marine Life Sciences, Ocean University of China, Qingdao 266003, China
Abstract A water flow simulation device capable of adjusting flow velocity was designed in flow velocity range of 0–30 cm/s, with which an indoor experiment was conducted to simulate the movement and adhesive behaviors of different-sized Apostichopus japonicus under different flow velocities. Observation showed that, in slow flow (~5 cm/s), A. japonicus moved more distance than in still water, and hardly moved in the riptide (~30 cm/s); and the adhesive capacity of A. japonicus was related to the flow velocity and attachment time. A. japonicus were able to attach to the bottom after any attachment time in the slow flow, after 10 s in the medium flow (~15 cm/s), and after 60 s in the riptide (~30 cm/s). In addition, larger A. japonicus were stronger with adhesive ability than smaller ones. The transcriptome data showed that the expression of transient receptor potential cation channel subfamily A, member 1 (TRPA1) in the tube feet was increased signifi cantly in a flowing water, but those in the tentacles and tube feet were not signifi cantly changed. Fluorescence in-situ hybridization results showed that TRPA1 was expressed around the watervascular of tentacles, tube feet, body wall, and spines. Therefore, tube feet were important for sea cucumbers to keep themselves stable in relatively swift flow with adhesion ability.
Keyword: sea cucumber; Apostichopus japonicus; behavior; flow velocity; transient receptor potential cation channel subfamily A, member 1 (TRPA1)
1 INTRODUCTION
It is commonly known that water flow is an important physical factor affecting the structure and function of marine benthic communities. For example, low-velocity tides affect the community structure of deposit-feeding organisms, which include polychaetes and nematodes, and high-velocity tides affect some bivalves and sea cucumbers cultured on rafts (Mann and Lazier, 1992). Flow velocity affects the characteristics of some marine invertebrates, such as the body shape of anemones (Koehl, 1977), the growth rate of bivalves and polychaetes (Wildish and Saulnier, 1992; Hentschel and Larson, 2005), and the feeding rate/fi ltration rate of bivalves (Wildish and Miyares, 1990; Newell et al., 2001). Although certain groups, such as fi shes, bivalves, and polychaetes, have been adequately studied, few in-depth studies have been conducted on Japanese sea cucumber Apostichopus japonicus.
In the proliferation and culturing of sea cucumbers, the influence of water flow on their behavior must be studied fi rst when determining the place and time of seedling, that is, to determine whether the water flow of the aquaculture area meets their living needs and whether the flow conditions can ensure that their attachment to the aquaculture area is smooth at seedling, and do not move them to other areas during future proliferation as to avoid difficulties in their recapture. In addition, the influence of water flow on the growth process of sea cucumbers must be considered as well. Some scholars believe that the flow of water could impede the movement of fi sh and increase their consumption when in motion (Pavlov et al., 2000). Furthermore, such a flow-shearing stress could even cause damage to their body (Odeh, 2002). Does water flow affect the growth of sea cucumbers? At what flow velocity do sea cucumbers grow best? How long does it take for sea cucumbers to grow into the size necessary for commercial purposes under the flow conditions of the aquaculture area? These questions need to be considered in terms of their behavioral ecology as well as their proliferation and culturing. Finally, the influence of water flow on sea cucumbers must be considered when they are recaptured, namely, the appropriate flow conditions under which they swim out of rock cracks for feeding and movement should be understood to reduce difficulties in their recapture. A trapping device should be designed based on the influence that water flow has on their behavior to realize mechanized capturing. To answer the above questions, the influence that the flow of water has on the behavior and feeding of sea cucumbers needs to be studied. As one of the most important indicators of water flow, flow velocity should be studied fi rst. However, the research carried out in the fi eld in this regard is affected by uncontrollable natural factors, which require ships and labor, which could be at high costs, in poor stability, and with weak risk resistance, and difficulties in timely observing and recapturing such creatures. Moreover, multiple flow velocities are difficult to simulate continuously in indoor experiments, thus resulting in low reliability.
At present, there are no related reports on the flow sensor of sea cucumber. Based on the research methods of biomechanical vibration receptors (water sensing is mechanical vibration sensing) such as sea anemone (Mahoney et al., 2011), the transient receptor potential channel A1 (TRPA1) that mediates vibration sensory function on specifi c tissues and organs of sea cucumber can be measured by immunohistochemistry to determine if it has a water sensor function. Transient receptor potentials (TRPs) are a class of important cation channels located on the cell membrane. The TRPs family exists in yeast, algae, and various animals, including worms, insects, and mammals (Venkatachalam and Montell, 2007). TRPA is one of subfamily in TRP family, the “A” means “Ankyrin”, there was at least 14 ankyrin repeat in N-terminus (Sotomayor et al., 2005). TRPA1 was thought to be related to thermosensation and thermoregulation (Caterina, 2007), mechanosensitive transduction (Corey et al., 2004; Zhong et al., 2012), cold nociception (Kwan et al., 2006; Karashima et al., 2009), and auditory (García-Añoveros and Nagata, 2007) etc. In 2006, sea urchin Strongylocentrotus purpuratus, as the fi rst species in the echinoderms to perform whole genome sequencing, discovered the homologous gene of TRPA1, which was considered to be the orthologs of vertebrate mechanosensory genes (Sea Urchin Genome Sequencing Consortium, 2006). Zhao et al. (2019) found that TRPA1 played the role of covering to response for UV-B radiation. Ding et al. (2019) regarded TRPA1 as a key gene for thermal perception. In starfi sh, TRPA1 was considered as thermal activator (Saito et al., 2017). There was no evidence in echinoderms for mechanosensory mechanoreceptor. Sea anemone, also as invertebrate, was proved to use their hair bundle for mechanoreception and TRPA1 participates in signal transduction of it (Mahoney et al., 2011). However, there was no research on the function of TRPA1 in echinoderms about mechanosensitive transduction. In this experiment, a water flow simulation system was developed and designed to realize the effect of water flow at different speeds on the behavior of sea cucumber. The transcriptome data of the epithelial tissues (tentacles, tube feet, and body wall) in different flow condition was used to characterize the role of TRPA1 in mechanosensitive transduction. Fluorescence in-situ hybridization (FISH) was used to analyze TRPA1 expression pattern.
2 MATERIAL AND METHOD
2.1 Test sample
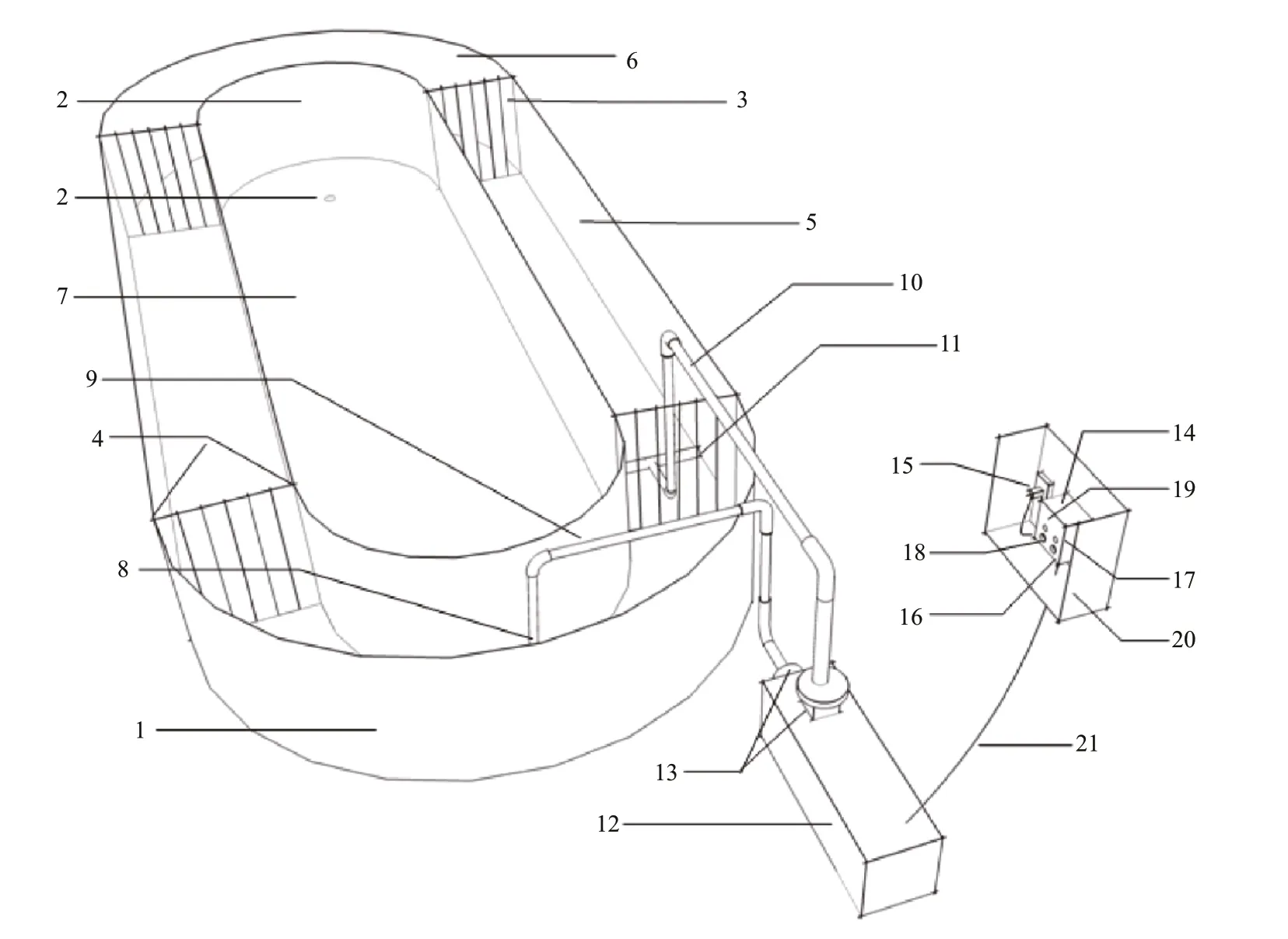
Fig.1 The experimental apparatus for flow simulation with adjustable flow velocity
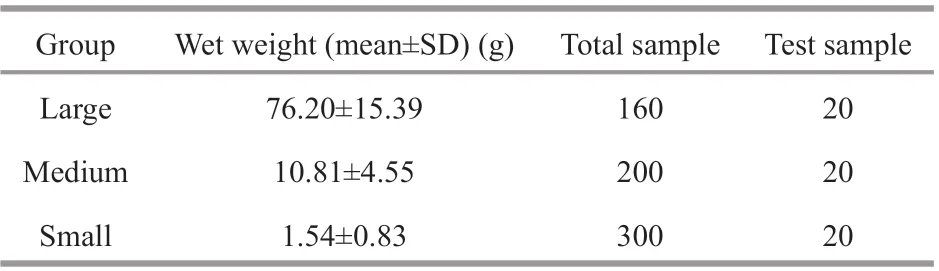
Table 1 Overview of sampled sea cucumbers
Sea cucumbers used in this study were sampled from the culture area and nursery workshop of Shandong Blue Ocean Science and Technology Co. Ltd., and divided into three groups (small, medium, and large) in wet weight. These groups represented typical periods during their shallow seabed-sown cultivation, namely nursery (small), seedling (medium), and grow-out (large). In addition, the sea cucumbers were temporarily reared in an experimental pond for seven days. When they had adapted to the experimental environment and engaged in normal activities, the same number of damage-free and healthy sea cucumbers was randomly selected from the groups of various sizes to test (Table 1).
2.2 Flow generator
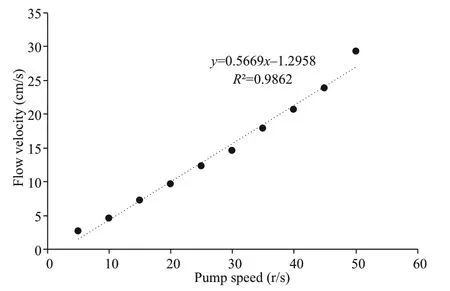
Fig.2 Relationship between flow velocity and pump speed
The flow generator used in this study is an experimental apparatus that we designed for flow simulation in adjustable flow velocity (Fig.1). The apparatus is comprised of a flow generator and a flow regulator. The device has a water tank (1) that has multiple diffuser grids (3). The inner part of the water tank (1) is divided into multiple water channels by multiple diffuser grids (3). The flow generator has a self-priming pump (12), a water inlet device (8), and a water outlet device (11). The inlet device (8) and the outlet device (11) are connected to the inlet and outlet of the self-priming pump (12) through an inlet pipe (9) and an outlet pipe (10). The inlet unit (8) and the outlet unit (11) are installed in different water channels of the tank (1). Water is pumped into the self-priming pump (12) by the inlet unit (8) and pumped out by the outlet unit (11) to form a water flow. The self-priming pump (12) is electrically connected to the flow regulator, which controls the frequency of the selfpriming pump (12) to regulate flow velocity.
The water flow in the apparatus was measured by a point current meter, and it was found that there was a positive correlation between pump speed and flow velocity (Fig.2).
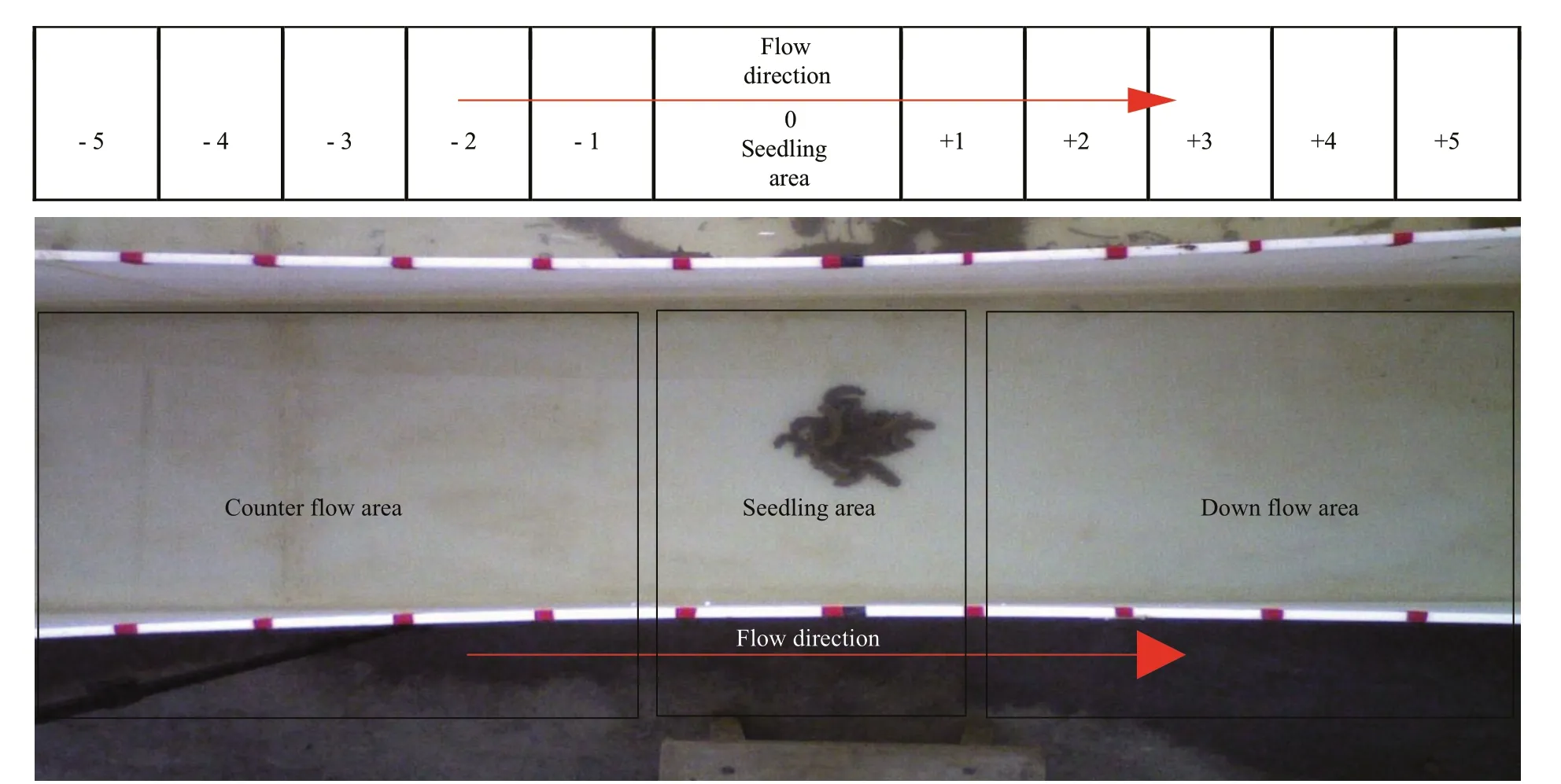
Fig.3 Schematic diagram (top) and real image (bottom) of experimental area
To ensure the stability of water flow in the experiment, experiments on water flow and attachment ability were carried out in the straight channel (5). The water channel (5) was marked by watertight tape from the center to two sides every 10 cm to form 11 areas. The central position indicates the area where the sea cucumbers were placed. Each area had its own representative value. The central area was represented by a zero (0). The value of the down-flow direction in the central area was positive. The greater the distance from the central area, the greater the value. The value of the counter-flow direction was negative. The greater the distance from the central area, the smaller the value (Fig.3).
2.3 Monitoring device
The movement and attachment behaviors of the sea cucumbers were recorded by a Brinno Time Lapse Camera. Two Brinno cameras were fi xed on a triangular bracket. One was used for vertical shooting and the other one for horizontal shooting (Fig.4). When the experiment was started, images were taken every 20 s.
2.4 Movement measurement

Fig.4 The experimental setup
The sea cucumbers were temporarily reared for a week prior to the experiment. During the experiments, water temperature was 13.6–17.4°C, salinity was 30.5±0.5, and the concentration of dissolved oxygen was greater than 6 mg/L. Water in the tank where the sea cucumbers were temporarily reared was renewed once a day at rate of 2/3. The pond was cleared every two days. Baits were cast at 09∶00 every day by 2%–5% of the sea cucumbers’ body weight.
The water flow of four velocities was generated by adjusting the pump speed and the velocities were measured by the point current meter: high velocity of 29.3±3.7 cm/s, medium velocity of 14.7±0.3 cm/s, low velocity of 4.6±0.5 cm/s, and still water of 0 cm/s. The movement and attachment abilities of the sea cucumbers of different sizes at different flow velocities were measured and compared.
The movement of the sea cucumbers of three sizes was measured; 15 groups of each size were measured. During the measurement, 20 samples were randomly selected from the sea cucumbers to be tested. The samples were placed in the seedling area of the water channel and picture taken by the Brinno camera. A minute later, the pump was started and adjusted to the corresponding speed. The movement of the sea cucumbers was continuously recorded. An hour later, the samples were collected and the next group was performed with the experiments.
The attachment ability of the sea cucumbers of three sizes was measured in fi ve lengths of standing time (3 s, 10 s, 30 s, 60 s, and 120 s), where 15 groups of sea cucumbers of each size were measured each group. Before the experiments, the pump was started and adjusted to the corresponding speed. When the flow of water was stabilized, 20 samples were randomly selected from the sea cucumbers to be tested and placed in the seedling area. At this time, the sea cucumbers were protected by a Plexiglas cover in which the water was still. After standing for the corresponding time, the protective cover was removed from the sea cucumbers, which were instantly exposed to the water flow. The attachment of the sea cucumbers was recorded. A minute later, the samples were collected and the next group was tested.
2.5 TRPA1 expression in transcriptome data
We had performed an RNA-Seq produced from the epithelial tissues (tentacles: C, tube feet: G, and body wall: T) in different flow conditions (still water: 0 cm/s and flowing water: 35 cm/s). The transcriptome data were used to detect the gene expression changes in the same organization in high-speed flowing water and still water comparison group (C35 vs. C0; G35 vs. G0; T35 vs. T0). Then we selected the expression of TRPA1 in each group. The mean of normalized TRPA1 readcount was used to chart by GraphPad Prism 5 (GraphPad Software, San Diego, CA). The gene which possessed log2(Fold Change)>1 and P< 0.05 was considered as differentially expressed genes (DEGs).
2.6 Fluorescence in-situ hybridization (FISH)
Fluorescence in-situ hybridizations (FISH) were performed as described by Arendt et al. (2004). For FISH, the staining protocol outlined by Cole et al. (2009) was followed and signal was developed with FAM-fluorophore-conjugated tyramide (PerkinElmer). Both antisense- and sense- digoxigenin-labeled RNA probes were obtained using a DIG-RNA labeling kit (Roche), following the manufacturer’s instructions by using 1 μg of linearized plasmids. Anti-TRPA1 RNA probes (5′-DIG-CAGATGGAGTGGGGTGACCTTAGCTTTGTCTGDIG-3′) were purifi ed using the Mini Quick Spin RNA Columns (Roche). After staining, samples were collected the images under the Nikon ECLIPSE NI positive fluorescence microscope (Tokyo, Japan).
2.7 Data and statistical analysis
The sea cucumbers at different flow velocities were measured according mainly to their movement trend and movement ability. The movement trend is expressed in the value of the total distribution (VTD). A negative VTD indicates that the sea cucumbers performed counter-flow movement. A positive VTD indicates that the sea cucumbers performed downflow movement. The equation is as follows:
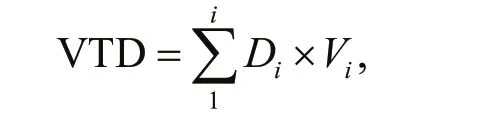
where the sum of the product of the quantity of sea cucumbers is Diand its representative value is Viin the area i for any given time.
The movement ability of the sea cucumbers is expressed by the absolute value of the total distribution (AVTD). The greater the value, the stronger their movement ability. The equation is as follows:
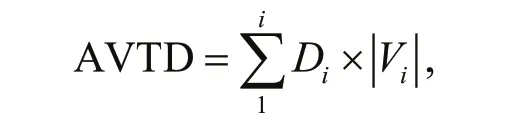
where the sum of the product of the quantity of sea cucumbers is Diand its absolute representative value is | Vi| in the area i for any given time.
The attachment ability of the sea cucumbers is expressed by an adsorption rate (AR). The greater the value, the stronger the attachment ability of the sea cucumbers. The equation is as follows:
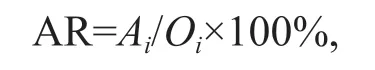
where the quantity of sea cucumbers attached in the experimental area Aiis in proportion to the quantity of placed sea cucumbers Oifor any given time.
All analyses were performed using SPSS 22.0 statistical software. Prior to statistical analysis, data were examined for normality of distribution and homogeneity of variance using Shapiro-Wilk and Levene’s tests, respectively. Data that conformed to normal distribution and homogeneity of variance were tested using a one-way analysis of variance followed by Tukey’s multiple comparison analysis. Data that conformed to normal distribution, but did not conform to homogeneity of variance were tested using Games-Howell test. Data that did not conform to normal distribution and homogeneity of variance were analyzed using Kruskal-Wails test. The results with a P-value of 0.05 were considered statistically signifi cant.
3 RESULT
3.1 Movement trend of sea cucumbers of different sizes under different flow conditions
Under flow conditions of four velocities, the sea cucumbers showed different movement trends. Under hydrostill conditions, the VTD of large sea cucumbers was negative, and the VTDs of medium and small sea cucumbers were positive; however, there were large standard errors, and no signifi cant trends were evident. At low and medium velocities, the VTDs of all sea cucumbers were positive, showing a signifi cant down-flow trend. At high velocity, the VTDs of all sea cucumbers were also positive; however, those of small and medium sea cucumbers tended to be zero, with no signifi cant movement trends evident; meanwhile, large sea cucumbers showed a signifi cant down-flow trend (Fig.5).
3.2 Movement ability of sea cucumbers of different sizes under different flow conditions
3.2.1 Comparison of movement ability of sea cucumbers of different sizes under the same flow conditions
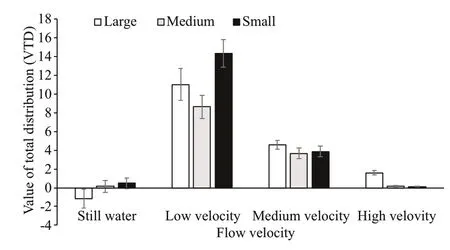
Fig.5 VTDs of sea cucumbers of different sizes under different flow conditions (mean±SEM)
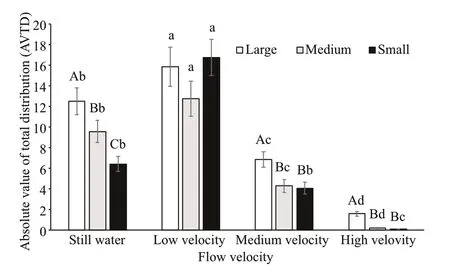
Fig.6 Comparison of AVTDs of sea cucumbers of different sizes under different flow conditions (mean±SEM)
Under the flow conditions of four velocities, the sea cucumbers of different sizes showed different movement abilities. Under hydrostill conditions, the AVTD of large sea cucumbers was highest, signifi cantly greater than that of medium sea cucumbers, and the AVTD of medium sea cucumbers was signifi cantly greater than that of small sea cucumbers ( P <0.05). At low velocity, there was no signifi cant difference in the AVTDs of all sea cucumbers. At medium and high velocities, the AVTDs of large sea cucumbers were still the highest and signifi cantly greater than those of small and medium sea cucumbers; however, there was no signifi cant difference between small and medium sea cucumbers. At high velocity, the AVTDs of small and medium sea cucumbers were close to zero (Fig.6).
The AVTDs of the sea cucumbers of three sizes under four flow velocities showed a uniform change. At low velocity, the AVTDs were highest and signifi cantly greater than those at the other flow velocities. At high velocity, the AVTDs were signifi cantly lower than those at the other flow velocities ( P <0.05). The AVTDs of medium and large sea cucumbers under hydrostill conditions were signifi cantly greater than those at medium velocity ( P <0.05), but there was no signifi cant difference in the AVTDs of small sea cucumbers under hydrostill condition and at medium flow velocity (Fig.6).
3.2.2 Comparison of movement ability of same-sized sea cucumbers under different flow conditions
The AVTDs of large sea cucumbers at different flow velocities increased with time and were signifi cantly different between different flow velocities. Although such AVTD was highest at a low flow velocity and not signifi cantly different from that under hydrostill conditions, it was signifi cantly greater than that at a medium flow velocity. The AVTD was lowest at a high flow velocity and signifi cantly lower than those at the other flow velocities ( P< 0.05) (Fig.7).
The AVTDs of medium sea cucumbers at different flow velocities increased with time and were signifi cantly different between different flow velocities. The AVTD was highest at a low flow velocity and signifi cantly greater than that under hydrostill conditions. The AVTD under hydrostill conditions was signifi cantly greater than that at a medium flow velocity. The AVTD at a high flow velocity was close to zero and signifi cantly lower than those at other flow velocities ( P< 0.05) (Fig.8).
The AVTDs of small sea cucumbers at different flow velocities also increased with time and were signifi cantly different between different flow velocities. The AVTD at a low flow velocity was the highest and signifi cantly greater than that under hydrostill conditions and at a medium flow velocity. Although such AVTD under hydrostill conditions was not signifi cantly different from that at a medium flow velocity, both were signifi cantly greater than that at a high flow velocity ( P< 0.05). The AVTD at a high flow velocity was close to zero (Fig.9).
3.3 Attachment ability of sea cucumbers of three sizes at different flow velocities
3.3.1 Comparison of attachment ability of sea cucumbers of different sizes at the same flow velocity
At a high flow velocity (29.3±3.7 cm/s), the attachment of the sea cucumbers of different sizes showed a uniform change. With the increase of standing time, the attachment ability increased gradually. For 3 s of standing time, the attachment ability of large sea cucumbers was signifi cantly stronger than that of small and medium sea cucumbers ( P <0.05). For 60 s of standing time, the attachment ability of small sea cucumbers was the strongest, being signifi cantly stronger than that of medium sea cucumbers ( P <0.05). For 120 s of standing time, the sea cucumbers of all sizes were completely attached (Fig.10).
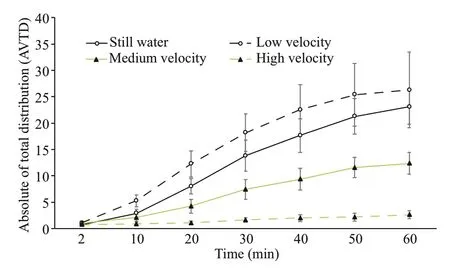
Fig.7 Comparison of AVTDs of large sea cucumbers at different flow velocities for different periods of time (mean±SEM)
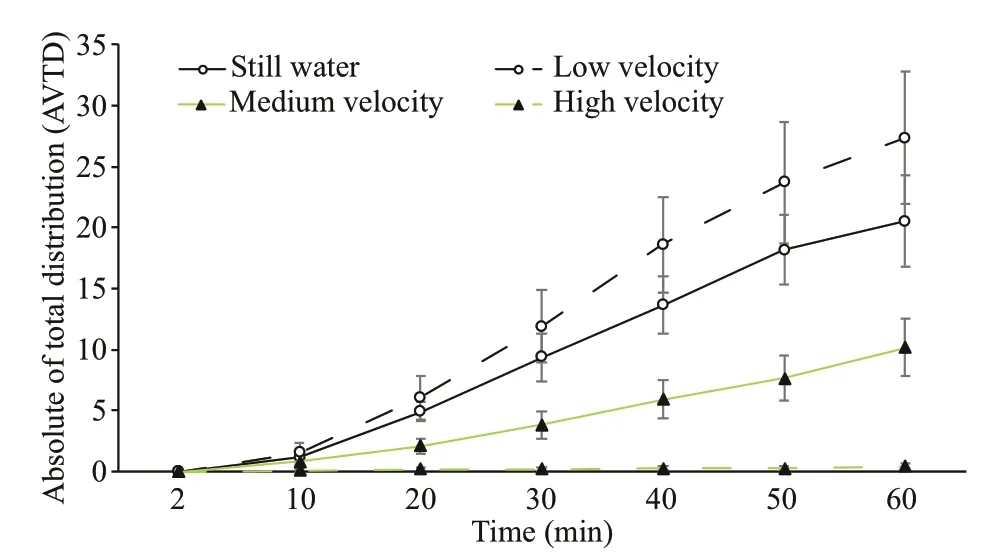
Fig.8 Comparison of AVTDs of medium sea cucumbers at different flow velocities for different periods of time (mean±SEM)
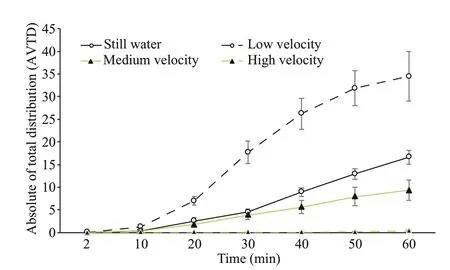
Fig.9 Comparison of AVTDs of small sea cucumbers at different flow velocities for different periods of time (mean±SEM)
At a medium flow velocity (14.7±0.3 cm/s), the attachment of the sea cucumbers of different sizes was the same as that at a high flow velocity. With the increase of standing time, the attachment ability increased gradually. For 3 s of standing time, the attachment ability of large sea cucumbers was signifi cantly stronger than that of medium sea cucumbers, and the attachment ability of medium sea cucumbers was signifi cantly stronger than that of small sea cucumbers ( P <0.05). For 10 s of standing time, the attachment ability of large sea cucumbers was signifi cantly stronger than that of small sea cucumbers ( P< 0.05). For 30 s of standing time, the ARs of sea cucumbers of all sizes tended to be 100% with no signifi cant difference between all the sizes (Fig.11).
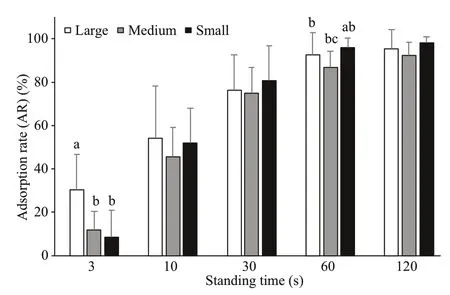
Fig.10 Comparison of ARs of sea cucumbers of different sizes at a high flow velocity (mean±SEM)
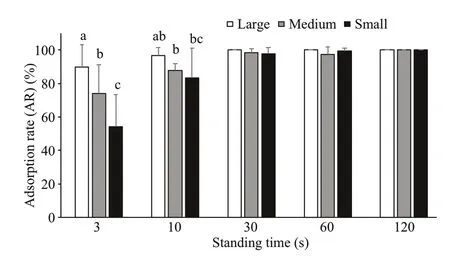
Fig.11 Comparison of ARs of sea cucumbers of different sizes at a medium flow velocity (mean±SEM)
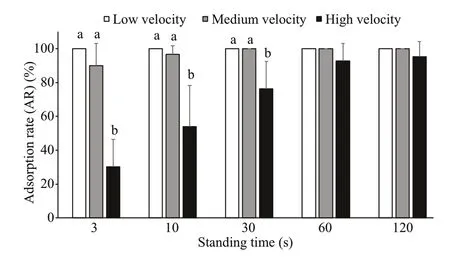
Fig.12 Comparison of ARs of large sea cucumbers at different flow velocities (mean±SEM)
At a low flow velocity (4.6±0.5 cm/s), the sea cucumbers of all sizes could be completely attached under fi ve standing conditions with no signifi cant difference.
3.3.2 Comparison of attachment ability of same-sized sea cucumbers at different flow velocities
The ARs of large sea cucumbers at different flow velocities were different. Overall, the ARs at low and medium velocities were signifi cantly greater than those at high velocity ( P <0.05). For 3 s, 10 s, and 30 s of standing time, the ARs were the same overall, that is, the ARs at a low flow velocity was not signifi cantly different from those at a medium flow velocity, and both were greater than those at a high flow velocity. For 60 s and 120 s of standing time, there was no signifi cant difference in the ARs at the three flow velocities (Fig.12).
The ARs of medium sea cucumbers at different flow velocities were different. Overall, the ARs at low velocity were signifi cantly greater than those at medium velocity and the ARs at medium velocity were signifi cantly greater than those at high velocity ( P <0.05). For 3 s and 10 s of standing time, the ARs were in overall the same overall, that is, the ARs at a low flow velocity were highest and were signifi cantly greater than those at a medium flow velocity. In addition, the ARs at a medium flow velocity were signifi cantly greater than those at a high flow velocity ( P <0.05). For 30 s, 60 s, and 120 s of standing time, the ARs at a low flow velocity were not signifi cantly different from those at a medium flow velocity, and both were greater than those at a high flow velocity ( P< 0.05) (Fig.13).
The ARs of small sea cucumbers at different flow velocities were different. Overall, the ARs at low velocity were signifi cantly greater than those at medium velocity and the ARs at medium velocity were signifi cantly greater than those at high velocity ( P <0.05). For 3 s and 10 s of standing time, the ARs were basically the same overall, that is, the ARs at a low flow velocity were highest and signifi cantly greater than those at a medium flow velocity. In addition, the ARs at a medium flow velocity were signifi cantly greater than those at a high flow velocity ( P <0.05). For 30 s of standing time, the ARs at a low flow velocity were not signifi cantly different from those at a medium flow velocity, and both were greater than those at a high flow velocity ( P< 0.05). For 60 s and 120 s of standing time, there was no signifi cant difference in the ARs at the three flow velocities (Fig.14).
3.4 TRPA1 expression analysis
RNA-Seq can indicate the gene changes influenced by different conditions at a specifi c time. We selected the control and maximum flow velocity in behavioral experiment as different environmental conditions and collected epithelial tissues (tentacles, tube feet, and spines) of sea cucumber to generate transcriptome data. The expressions of TRPA1 were shown in Fig.15. It showed that there was no differentially expressed change of TRPA1 even though it had upregulated trend in tentacles (log2FC=0.473 00). In body wall, the expression of TRPA1 was differentially expressed but not signifi cant (log2FC=1.300 1, P=0.129 94). However, there were differentially expressed changes of TRPA1 in tube feet (log2FC=3.082 3, P=0.000 97) with a signifi cant upregulation in 35 cm/s flow velocity group. FISH analysis showed that TRPA1 was expressed around the water-vascular of tentacles, tube feet, body wall, and spines (Fig.16).
4 DISCUSSION
Water flow is an important ecological factor for aquatic organisms living in the ocean, undoubtedly affecting their behavior. Studies pertaining to the influence of water flow on the behavior of sea cucumbers can serve as an important reference for their growth, feeding, breeding, seedling, and capture. In terms of the role of water flow, some believe that the flow of water would impede the movement of fi sh. Many documents proved that water flow could increase the consumption of fi sh in such movement (Pavlov et al., 2000). Moreover, flow shearing stress would even cause damage to fi sh located around a turbine of a hydroelectric dam (Odeh, 2002). Others believe that water flow can maintain the swimming behavior of fi sh. Many fi eld and indoor experiments showed that fi sh could reduce its consumption in the movement by generating propulsive flow with the help of water flow or other fi sh (Montgomery et al., 2003). Domestic scholars have studied the trends of many types of fi sh in the water flow. For example, although Kanglang fi sh ( Anabarilius graham) do not show a specifi c direction and flow trend when swimming in still water, they show a signifi cant flow trend when the flow velocity is 0.1 m/s. When the flow velocity increases to 0.25 m/s, the rate of their flow trend even reaches 100% (Zhong et al., 2013). Liang and He (1998), Song et al. (2008), and Li et al. (2010) studied the behavior of carp species, including common carp ( Cyprinus carpio), tinfoil barb ( Barbodes schwanenfeldii), and grass carp ( Ctenopharyngodon idella) in the water, and found that they show a signifi cant flow trend. Zhang and Chen (2005) and Li et al. (2011) found that the flow trend of hybrid sturgeon, namely male kaluga ( Huso dauricus Georgi, 1775) crossed with female Amur sturgeon ( Acipenser schrenckii von Brandt, 1869), and black-banded rockfi sh ( Sebastes fuscescens) increases with the increase of flow velocity within a certain range. The above studies show that some fi sh will show directional movement under the flow of water.
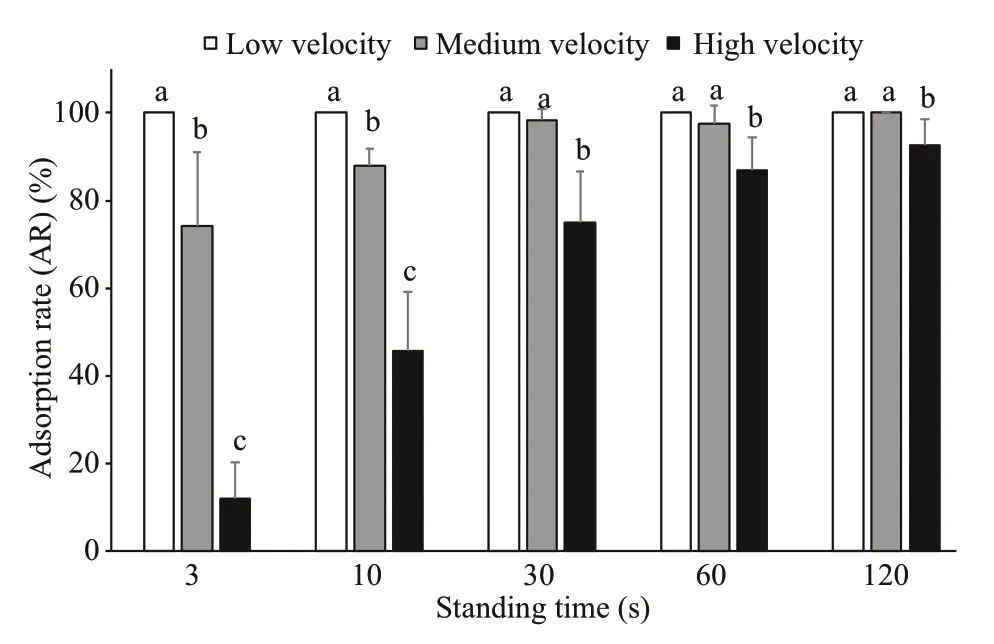
Fig.13 Comparison of ARs of medium sea cucumbers at different flow velocities (mean±SEM)
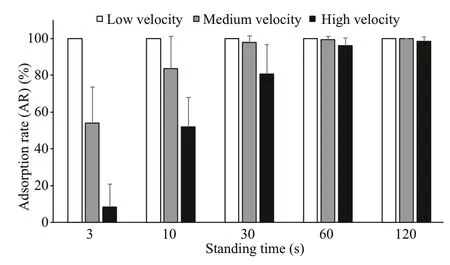
Fig.14 Comparison of ARs of small sea cucumbers at different flow velocities (mean±SD)
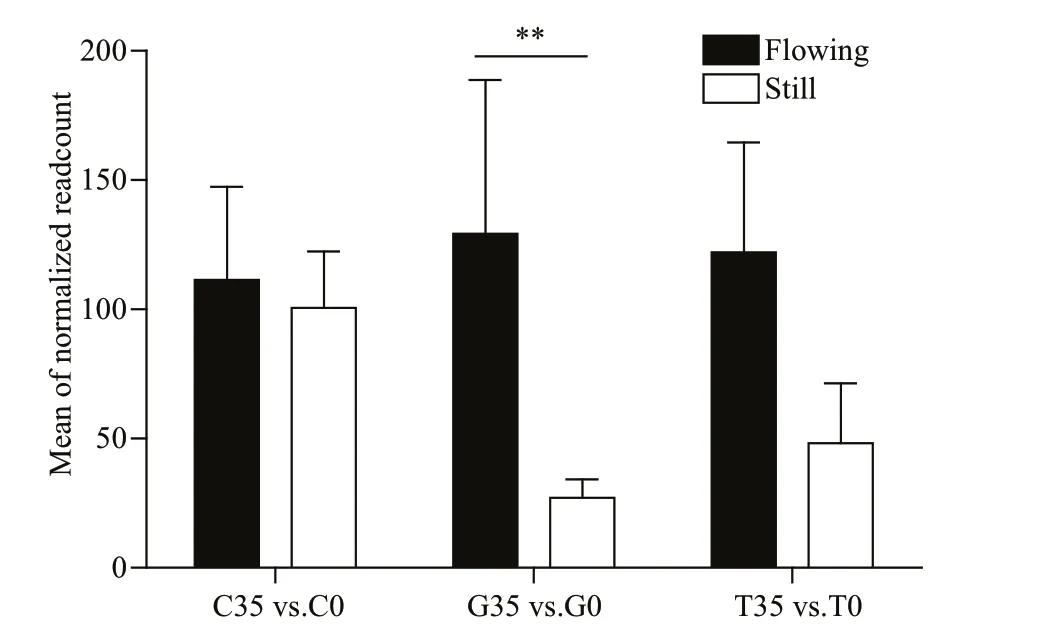
Fig.15 TRPA1 expressions in the tentacles (C), tube feet (G), and body wall (T) comparisons in still and flowing water conditions (mean±SD)
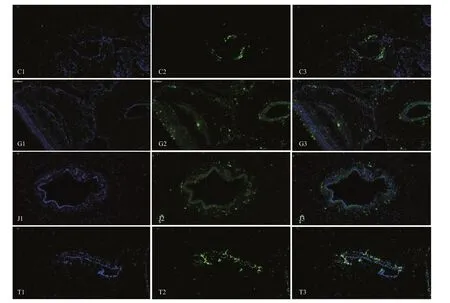
Fig.16 Fluorescence in-situ hybridization analysis for TRPA1 in the tentacles (C), tube feet (G), spines (J), and body wall (T)
Different from the above conclusions, this study proposes that under the flow of water, sea cucumbers prefer down-flow movement and have a maximum movement ability under weak flow conditions (~5 cm/s). This indicates that a weak flow can promote their behavior, which may be due to a simple propulsion from physics. However, at a high water velocity (~15 cm/s), these creatures slow down, or even stop moving, which may be due to the attachment of their tube feet. Under high flow conditions, their tube feet are difficult to attach on the substrate, thus making them unable to move. This inference has been proved by the experiments on their attachment. In addition, the attachment ability of sea cucumbers is related to flow velocity and attachment time. Under weak flow conditions (~5 cm/s), sea cucumbers could attach as they normally would at any time, indicating that the water flow of such velocity had no influence on their attachment with their tube feet. When the flow velocity increased to about 15 cm/s, the attachment required more than 10 s. When the flow velocity reached 30 cm/s, the attachment required more than 60 s, depending on their size. Large sea cucumbers had the best attachment ability, indicating that the attachment ability of their tube feet was higher than that of small and medium sea cucumbers. In addition, we did not observe the signifi cant morphological changes in the body wall of sea cucumbers. On the high velocity group, the body wall of some sea cucumbers showed a degree of contraction which were usually motionless for a long time in the experiments, but in general, it is not a common phenomenon.
The TRP channel has six transmembrane domains (TM1–TM6) with a channel region formed between TM5 and TM6 (Voets et al., 2004). Both the N- and C-terminus are located within the cell. The regulatory elements that control the opening of the channel may be located at TM1–TM4 and the intracellular N- and C-terminus. The TRP channel may form a homo- or hetero-mer functional domain and play an important role in signal transduction (Hoenderop et al., 2003). Liu and Montell (2015) put forward a completely new point of view, and they believed that the vast majority of the TRP channel family belonged to mechanosensitive channels (MSC). TRP channels can be directly activated by mechanical stimulation, or can be indirectly stimulated by signal cascades. The transcriptome data showed that the expression of TRPA1 in the tube feet was signifi cantly increased in the flowing water, but the tentacles and tube feet were not signifi cantly changed. The results of fluorescence in-situ hybridization showed that TRPA1 was expressed around the water-vascular of tentacles, tube feet, body wall, and spines. The water-vascular system can adjust the pressure change and thereby regulate the individual's movement, we had observed the difference in the tube feet adsorption rate in behavior experiments, and the transcriptome data supported that the TRPA1 expression was up-regulated in high velocity compared with still water, FISH results characterized the relationship between the TRPA1 expression pattern. All the above provided the preliminary evidences for exploring how the sea cucumbers response to the daedal seawater current.
The movement behavior and feeding behavior of sea cucumbers complement each other. The increase of movement ability expands their feeding range while the feeding behavior provides energy required by their movement behavior. Some scholars have studied the influence of water flow on the behavior of echinoderms. Kawamata (1998) studied the influence of water flow on the distribution and feeding of sea urchins, proposing that wave motion may both prevent the feeding activity of sea urchins and affect the distribution of seaweed. When waves become gentle, sea urchins will move to a new seaweed distribution area for feeding. Sea lilies can change their postures in response to the direction and velocity of water flow (Macurda and Meyer, 1974). Baumiller and Labarbera (1993) demonstrated that sea lilies could adapt to flow direction and velocity by changing their own postures within several minutes. The change in the sea lilies’ posture is to ensure that the flow direction and velocity of the water around them can be stable during feeding, thus ensuring efficient fi lter feeding (Kitazawa and Oji, 2014). Although the influence of water flow on the feeding behavior of sea cucumbers has not been studied, the experiments regarding the influence of water flow on the movement and attachment behavior can serve as a reference for future studies. Long-term research that examines the influence of flow velocity on the growth of sea cucumbers can also serve as a reference for evaluating their growth in mariculture.
5 CONCLUSION
Sea cucumbers prefer to move downstream and move actively at low velocity. However, when the water velocity is high, their moved slowly, which may be related to the attachment of sea cucumbers. At low velocity conditions, there is no effect on the attachment of sea cucumber tube feet, but high velocity needed more than 10 s to attach. Analysis of the expression of TRPA1 from transcriptome data and FISH indicated that TRPA1 might play a role in flow perception and tube feet were important for sea cucumbers to keep themselves stable in the relative high flow by adhesion ability.
6 DATA AVAILABILITY STATEMENT
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
The authors thank all members of the research department of Shandong Oriental Ocean Sci-Tech Co., Ltd. We are grateful to the anonymous reviewers for their professional critique of the manuscript.
杂志排行
Journal of Oceanology and Limnology的其它文章
- Review on observational studies of western tropical Pacifi c Ocean circulation and climate*
- Research progress of TiO 2 photocathodic protection to metals in marine environment*
- Smart anticorrosion coating based on stimuli-responsive micro/nanocontainer: a review*
- Status of genetic studies and breeding of Saccharina japonica in China*
- To be the best in marine sciences*
- A review of progress in coupled ocean-atmosphere model developments for ENSO studies in China*
