Research progress of TiO 2 photocathodic protection to metals in marine environment*
2020-07-31WANGXiutongXUHuiNANYouboSUNXinDUANJizhouHUANGYanliangHOUBaorong
WANG Xiutong , XU Hui NAN Youbo SUN Xin DUAN Jizhou HUANG Yanliang HOU Baorong
1 Key Laboratory of Marine Environmental Corrosion and Bio-fouling, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Open Studio for Marine Corrosion and Protection, Pilot National Laboratory for Marine Science and Technology, Qingdao 266237, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
4 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
Abstract Corrosion protection has become an important issue as the amount of infrastructure construction in marine environment increased. Photocathodic protection is a promising method to reduce the corrosion of metals, and titanium dioxide (TiO 2) is the most widely used photoanode. This review summarizes the progress in TiO 2 photogenerated protection in recent years. Different types of semiconductors, including sulfi des, metals, metal oxides, polymers, and other materials, are used to design and modify TiO 2. The strategy to dramatically improve the efficiency of photoactivity is proposed, and the mechanism is investigated in detail. Characterization methods are also introduced, including morphology testing, light absorption, photoelectrochemistry, and protected metal observation. This review aims to provide a comprehensive overview of TiO 2 development and guide photocathodic protection.
Keyword: photocathodic protection; corrosion; titanium dioxide (TiO 2); photoelectrochemistry; metal
1 INTRODUCTION
As need for the marine economy increases, an increasing number of structures, including offshore platforms, marine pipelines, and marine wharfs, have been built with large quantities of steel and other metals. Steel has been the most popular construction material because of its low cost, high strength and good machinability. In marine environments, the corrosion of steel is very severe because of the presence of oxygen, sunshine, microorganisms, Cl-and other ions.
In a survey project led by Hou from the Institute of Oceanology, Chinese Academy of Sciences (IOCAS), it was found that the cost of corrosion in China was approximately 310 billion USD in 2014, equivalent to 3.34% of the GDP (Hou et al., 2017; Hou, 2019). In the shipbuilding industry, approximately 9% of income is spent on corrosion protection and repair. However, many factors can play roles in the corrosion of metals in seawater, such as temperature, dissolved oxygen, salinity, and microorganisms, which cause deterioration and failure of metal materials with long exposure times (Melchers, 2003). Among these factors, the effect of Cl–is especially remarkable; even metals with high corrosion-resistance performance, such as stainless steel, can be affected by the high concentration of Cl–, which induces pitting-type corrosion (Tsutsumi et al., 2006, 2007).
To reduce the corrosion of metals in marine environments, many methods have been proposed, such as coating, corrosion inhibitors and cathodic protection (CP). Among these methods, CP has been widely used for metals with high efficiency, and it has a long research history of almost 200 years since its fi rst application in the protection of copper ship sheeting (Davy, 1824).

Fig.1 Scheme and electron transfer of sacrifi cial anode cathodic protection (SACP) (a) and photocathodic protection (b)
Corrosion is a process in which electrons flow from the metal, and CP technology is a method to decrease the metal potential; thus, redox reactions on metal surfaces are inhibited, and the corrosion rate is reduced. For traditional CP, two main methods have been designed for the marine industry. The fi rst is sacrifi cial anode cathodic protection (SACP), which uses an active metal with a negative corrosion potential such as magnesium, zinc and aluminum to provide electrons, and the anodes are consumed during the protection. The second method is impressed current CP (ICCP), which uses a rectifi er or potentiostat as an external power source to provide current to keep the metal in the proper potential range. For an SACP system, the quantity of sacrifi cial anodes depends on the dimensions and service life of the structure; however, the production of the anodes requires a considerable amount of energy consumption, and the anodes dissolve in seawater and thus cause pollution and harm to the marine ecology. For the ICCP system, stable power supplies and complicated maintenance devices are needed, which would be difficult in some offshore conditions, and large amounts of energy are also necessary during the duty life of the structure.
In recent years, photocathodic protection has been proposed as a new type of technology that utilizes green and sustainable solar energy to provide the current to protect metals from corrosion. A semiconductor can utilize light and produce electrons that are transferred to the metal to reduce the corrosion rate. Photocathodic protection is similar to sacrifi cial anode CP (Park et al., 2001), and the mechanism is illustrated in Fig.1.
Yuan and Tsujikawa (1995) investigated the photoeffect of TiO2coatings on copper substrates under ultraviolet illumination for the fi rst time. In the last three decades, a considerable amount of effort has been devoted to photocathodic protection for metals by using many types of semiconductors, such as TiO2, SrTiO3, g-C3N4, In2O3and ZnO (Yuan et al., 1994; Huang et al., 2000; Ohko et al., 2001; Liu et al., 2007; Bu et al., 2011, 2013; Li et al., 2014, 2015b; Sun et al., 2014, 2015; Zhang et al., 2015a; Yang and Cheng, 2018). Among these materials, TiO2is the most widely used material and serves as a photoanode due to its low cost, high stability, nontoxicity and easy availability.
Since Fujishima and Honda (1972) established a method of water photolysis in which TiO2is subjected to light irradiation, the photocatalytic performance of TiO2and other semiconductors has been investigated in many fi elds, such as wastewater treatment (Nagaveni et al., 2004), sterilization (Matsunaga et al., 1988), dye-sensitized solar cells (O'Regan and Grätzel, 1991), self-cleaning (Wang et al., 1998; Balaur et al., 2005), and biomedical fi elds (Oh et al., 2005). When the energy of the irradiation light is higher than the bandgap (Eg), there are three steps in the photocatalysis: fi rst, the electrons and holes separate, where the electrons are excited from the valence band (VB) to the conduction band (CB) and the holes remain in the VB; second, the excited carriers, including electrons and holes, migrate to the surface; third, the carriers react with electron donors (D) and electron acceptors (A) (Guo et al., 2019). This process is shown in Fig.2. The carrier transmission process under light irradiation includes the generation and transportation of electrons and holes.
However, TiO2, with a relatively wide bandgap (approximately 3.2 eV), can absorb only ultraviolet light, which makes up approximately 5% of sunlight. On the other hand, during migration, recombination occurs on the surface and in the bulk. These two drawbacks have limited the applications of TiO2in photocatalysis and energy harvesting (Cai et al., 2019).

Fig.2 Schematic diagram of excitation transfer processes in photocatalysis
There are three types of TiO2often used in photoelectrochemistry: rutile, anatase, and brookite. Different TiO 2 types can affect the charge transfer and bandgap levels and rutile and anatase are most widely used. Many methods have been used to increase the visible light response and reduce the recombination of TiO2, such as energy band engineering, morphology control, nanoassembly, electronic structure calculations and molecular dynamics simulations (Linsebigler et al., 1995; Tong et al., 2012). Among these methods, material doping is the most commonly used, and it includes metal doping, such as V, Cr, Mn, Fe and Ni (Paramasivam et al., 2012); nonmetal doping, such as N, C, S, B, P and F (Fujishima et al., 2008); metal oxide doping, such as niobates, tantalates, vanadates and germanates (Tong et al., 2012); and narrow bandgap semiconductor coupling (Chen et al., 2012). In addition to the strong catalytic property and high charge mobility, cost and stability are also important parameters of the new type of material for the photoanode (Kudo and Miseki, 2009).
Structures with different dimensionalities (0D, 1D, 2D, 3D) and different energy facets (001, 101) can exhibit special properties (Li and Wu, 2015), as proposed in Fig.3. The appropriate nanostructure has high solar energy conversion and results in better photocatalytic activities. Nanoparticles, as 0D structures, usually have a large surface area and are used as photocatalysts in the suspended state, and the small particle size can increase the number of active sites. 1D structures such as nanotubes, nanobelts and nanowires mostly have large length-diameter ratios, which can enhance charge transport along the longitudinal direction. 2D nanomaterials such as nanosheets and fi lms can reduce the distance between photoexcited carriers and increase photon absorption due to the large area. 3D nanostructures can adopt unique arrangements using precise and self-assembly strategies, and specifi c performance can be obtained.
However, in addition to the dimensions of the nanostructure, the facet design is also important; three-dimensional hierarchical spheres of anatase TiO2with exposed TiO2(001) facets and single-crystal nanosheets have superior photoactivity (Yang et al., 2009; Chen et al., 2010), as illustrated in Fig.4.
In this review, we summarize the recent progress in the application of TiO2in the fi eld of photocathodic protection. The modifi cation and mechanism are discussed, and characterization and analysis techniques are also introduced.
2 TiO 2 MODIFICATION AND APPLICATION
TiO2tends to be modifi ed by semiconductors in the following three ways: increasing the separation efficiency of photogenerated electron-hole pairs, decreasing the conduction band potential of the semiconductor and changing the redox potential of the electrolyte. A sufficiently negative CB potential is required for photoelectrochemical CP. Hitherto, based on these strategies, researchers have synthesized TiO2nanotube photoanodes and modifi ed them by increasing the surface area, doping, sensitizing quantum dots, and combining different semiconductors to extend their functionalities in the visible range. Coupling with narrow-bandgap semiconductors, metals and nonmetals are used for photocathodic protection, and the protected metal tends to be polarized into a safe range under visible light (Li et al., 2017a; Bu et al., 2018).
2.1 TiO 2 preparation
There are several methods to synthesize TiO2, such as the hydrothermal process, the sol-gel method, liquid phase deposition, and anodization. During the hydrothermal process of TiO2, pH, and temperature are the key factors determining morphology, and different materials lead to distinct properties. Liu et al. (2018) employed a TiCl4solution as the Ti source with a pH of 5–6 at 180°C and obtained TiO2nanoparticles. Zhang et al. (2010) selected titanium foil with a porous TiO2fi lm as the source of Ti to obtain a three-dimensional TiO2NT network under hydrothermal conditions, and the structure could be useful in photocatalysis and other fi elds. Lan et al. (2018) assembled a uniform and ordered TiO2nanosheet by the hydrothermal-induced solventconfi ned method, and the assembly is illustrated in Fig.5.
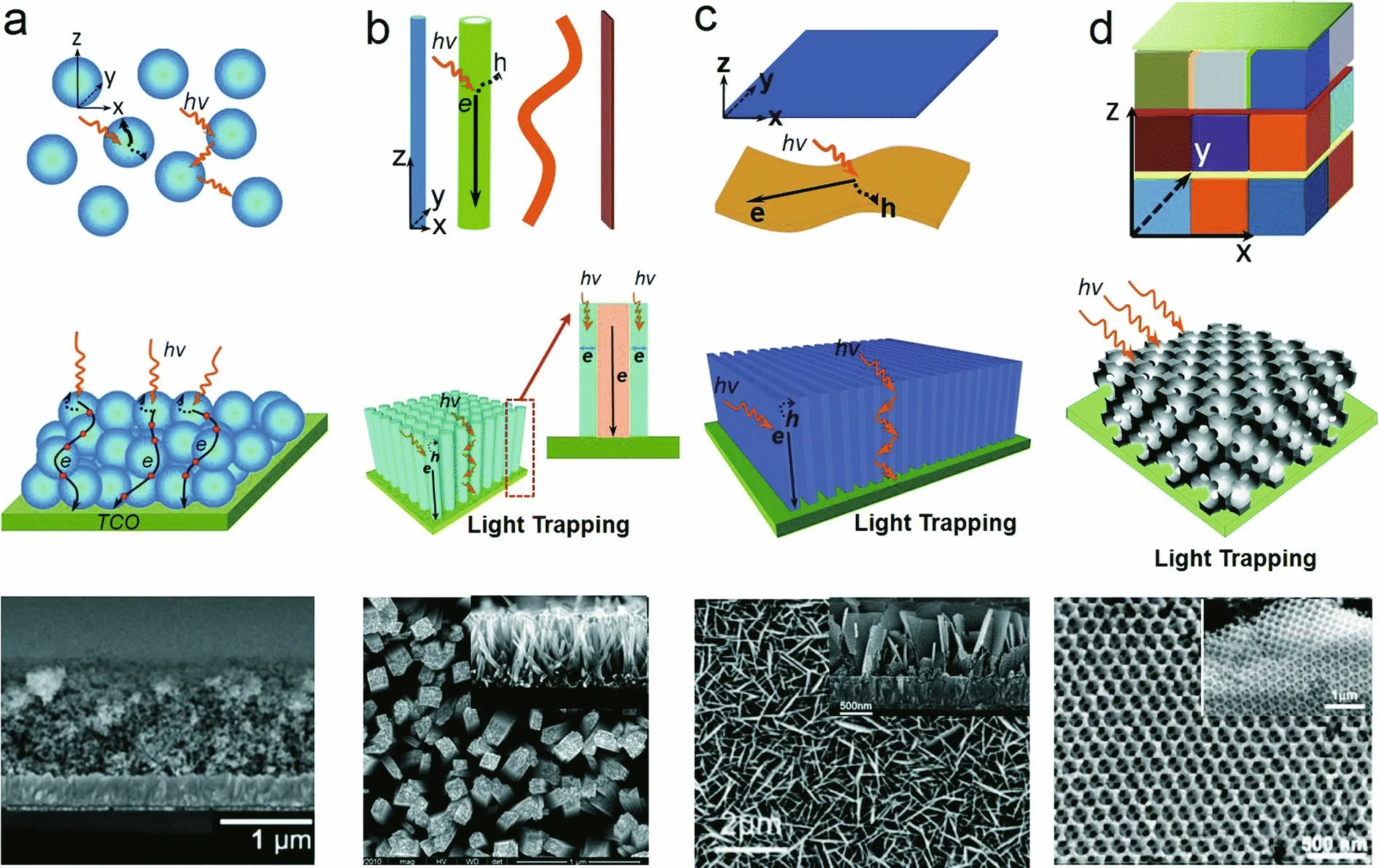
Fig.3 SEM images of light trapping and structuring with different dimensions

Fig.4 Anatase TiO 2 spheres (a) and single-crystal nanosheets with an exposed TiO 2 (001) facet (b)
Through the sol-gel method, TiO2fi lm can be synthesized by aging of a TiO2sol liquid, coating this liquid on the metal surface and then heating. Shen et al. (2005) used tetra-n-butyl titanate and ethyl acetoacetate as raw materials to synthesize a TiO2sol and then distributed the sol on the steel substrate using the dip-coating method. The coatings showed good corrosion resistance. In the sol-gel method, it is easy to control the coating thickness; however, the fi lms usually crack during heating. To avoid this, Shen et al. (2005) soaked the TiO2fi lm in boiling water for 10 min, and the cracks decreased with less heating time. Zhu et al. (2010) also developed a combined sol-gel and hydrothermal method and prepared a 3D titanium nanowire network. Liquidphase deposition is a useful method in which a TiO2fi lm can be prepared on an ITO substrate surface, and the substrate is placed vertically at a low temperature (less than 100°C). Lei et al. (2012) used (NH4)2TiF6and H3BO3as precursor bath solutions. After drying naturally and annealing in air, TiO2is obtained.

Fig.5 Schematic illustration of the fabrication of TiO 2 nanosheets via the hydrothermal method
Anodization is an effective method to prepare nanotubes, and it has been widely used in the preparation of TiO2. In general, titanium foil materials need to be cleaned in polishing solutions containing acetone, ethanol, and deionized water. During anodization, titanium foil and platinum sheets are connected to the positive and negative poles of the DC power supply, respectively. The structure of the nanotubes depends on the electrolyte, temperature, potential, etc. Recently, two-step anodization has been proposed and applied, including two processes with different applied powers and times. The fi rst step is to obtain a glossy surface that could provide a better growth environment for the nanotubes in the next anodization step. Finally, annealing is used to increase the crystallinity, and the time and temperature can vary based on the proportion of rutile and anatase TiO2(Roy et al., 2011).
2.2 Sulfi de modifi ed TiO 2
As mentioned above, modifi cation is the most widely used method to improve the photocatalytic performance of TiO2, and many materials are used to modify and dope TiO2. Among these materials, sulfi de compounds are very popular because of their superior performance.
CdS is a type II-VI semiconductor with a bandgap of 2.4 eV, and its photoelectron transmission capacity is preeminent. Li et al. (2011) coated CdS nanoparticles onto TiO2nanotube arrays, and the composite could protect the metals under UV and visible light. However, CdS is sensitive to photocorrosion, and Boonserm et al. (2017) investigated the reaction during the measurement and found that corrosion of the nanocomposite fi lm decreased the photocurrent. However, it is difficult to simultaneously achieve both high electron transmission efficiency and superior redox capacity.
ZnS has some properties similar to those of CdS, such as nontoxicity and chemically stable performance. It has been reported that a ZnS coating can protect CdS and CdTe from photocorrosion (Lin et al., 2010; Zhang et al., 2015a). Construction of Z-scheme photocatalytic systems consisting of two semiconductors as photoexcitation systems, with the constructor located at the interface to reduce the contact resistance, is increasing in popularity. Zhu et al. (2019) employed CdS to modify TiO2nanowires, prepared a ZnS shell to protect CdS from photocorrosion and added Ag as an electron transporter between CdS and TiO2. Upon coupling with the composite, the potential of 403SS decreased to -1 174 mV (vs. SCE), and the photocurrent reached 19.97 mA/cm2. The absorption edge was nearly 700 nm, and the visible absorption range increased because the energy of the incident ray was absorbed by the noble metal. Moreover, surface plasmon resonance might be triggered. CdTe (Eg=1.47 eV) is an ideal photoelectric conversion material that has a very high optical absorption coefficient (>104/cm) and good overlap with the solar spectrum (Li et al., 2015a). The light absorption range of the CdTe/TiO2composite fi lm can be expanded to the visible region by adjusting the size of the quantum dots, which can capture electrons and generate multiple electron-hole pairs (e--h+) under illumination. The photoelectric conversion efficiency was very high after CdTe quantum dots were used to modify TiO2. However, the stability of CdTe is the main problem hindering practical application. To improve the stability of CdTe QDs, ZnS shells were deposited on CdTe/TiO2composite fi lms. The results showed that the ZnS/CdTe/TiO2composite fi lm had excellent photoelectrochemical properties (Zhang et al., 2015a), and the mechanism is illustrated in Fig.6.

Fig.6 Mechanism of CdTe/GR/TiO 2 (a) and ZnS/CdTe/TiO 2 (b) composite for corrosion protection
Sb2S3is also used to modify TiO2because of its narrow bandgap. Some experiments have been performed, and the results show that the bandgaps of Sb2S3/Sb2O3/TiO2and Sb2O3/TiO2are approximately 2.02 eV and 3.32 eV, respectively, revealing that Sb2S3reduces the bandgap. It is also known that Sb2S3is a p-type semiconductor, and when it combines with an n-type semiconductor, for example, TiO2and Sb2O3, the Fermi level of different materials flattens; at the same time, a built-in electric fi eld forms, which can drive electron transfer (Li et al., 2018b). Similarly, the bandgap of Ni3S2is 2.5 eV, making this material also appropriate to modify TiO2. Nan et al. (2019) found that the photocurrent increased to 53.1 μA/cm2, which is higher than that of pure TiO2. In a double electrolytic cell working with a three-electrode system coupled with Ni3S2, the potential of 304SS decreased to a minimum of -720 mV (vs. SCE) with 0.1 mol/L Na2S and 0.2 mol/L NaOH as the hole scavenger solution and 3.5 wt.% NaCl as the corrosive solution, simulating a marine environment. Compared with TiO2, the composite has stronger absorption in the visible region. In addition, the UV-Vis spectroscopy showed that the absorption is redshifted, indicating that the forbidden band energy decreases; the mechanism is shown in Fig.7.
The direct bandgap of SnS is approximately 1.4 eV, and the optical absorption coefficient α is larger than 104/cm. SnS is nontoxic, inexpensive and environmentally compatible. Sn is one of the most promising materials because of its good photoconductivity and nonlinear optical response. SnS has been proven to have good photocatalytic performance in the application of photocatalysis. It has been reported that SnS can be used as a sensitizer of TiO2. Therefore, it is of great signifi cance to apply SnS/TiO2composites to photocathode protection (Shao et al., 2018).
The CB of Ag2S (0.3 eV) is higher than that of TiO2(0.1 eV), and the VB of Ag2S (0.7 eV) is lower than that of TiO2(3.1 eV). Therefore, nontoxic Ag2S cosensitized with TiO2can improve the separation of photogenerated electron-hole pairs and accelerate the transfer of carriers. The Ag2S/TiO2composite has higher visible light utilization than pure TiO2. Under visible light, the photochemical properties of the Ag2S/TiO2composite are better than those of pure TiO2, so the composite can be used to protect stainless steel (Ning et al., 2017; Yang et al., 2019). Bi2S3is an attractive material due to its narrow bandgap (Eg=1.3 eV) and high photoelectric conversion efficiency. It can absorb almost all visible light from the solar spectrum. There is a strong interface electron fi eld between Bi2S3nanoparticles and TiO2nanotube arrays. The electric fi eld increases the separation of photogenerated carriers and then reinforces the photoelectrochemical properties (Hu et al., 2017; Li et al., 2017a; Guan et al., 2018b). The narrow bandgap doping mechanism is shown in Fig.8 (Li et al., 2017a; Yang et al., 2019). MnS has excellent photoelectric performance and is mainly used as a buffer material, photoelectric device and magnetic component of many important diluted magnetic semiconductors. Although MnS has a wide bandgap (3.7 eV), as a p-type semiconductor, it can form a p-n heterojunction with TiO2(n-type semiconductor). The inherent fi eld in the p-n heterojunction can reduce the recombination of photogenerated electrons and holes, which is benefi cial to the photoelectric properties of materials (Ge et al., 2015).

Fig.7 Mechanism of Ni 3 S 2/TiO 2 photocathodic protection for 304SS
ZnIn2S4is a ternary sulfur compound with a narrow bandgap of 2.34–2.48 eV, which is signifi cantly narrower than that of TiO2. It is widely used as a visible light-responsive photocatalyst. In addition, the potential at the bottom of the CB of ZnIn2S4is -0.74 eV, and the potential at the top of VB is more positive than the oxygen-producing potential. Therefore, ZnIn2S4is a promising photoanode material in photoelectrochemistry. As an n-type semiconductor, it can form a heterojunction electric fi eld at the interface when it is combined with TiO2. It is obvious that ZnIn2S4can promote the separation of electrons and holes, and the TiO2/ZnIn2S4composite has enhanced charge transfer and photocatalytic degradation properties (Li et al., 2018a, 2019). AgInS2is a nontoxic and environmentally friendly visible photosensitizer, and it is an ideal photoelectric conversion material to replace toxic cadmium sulfi de. It has potential for application in the fi eld of photoelectrochemical conversion and photocatalysis. The bandgap of AgInS2is between 1.87 and 2.03 eV, and the CB and VB of AgInS2are 1.08 eV and 0.83 eV, respectively, with unique absorption in the visible and near-infrared regions. Sensitization of the ordered TiO2NTs by AgInS2QDs can signifi cantly improve the PEC conversion efficiency in the visible region, and the ternary structure of AgInxSy-sensitized TiO2also enhances visible light photocatalytic activity (Sun et al., 2018a).
Selenide and telluride are similar to sulfi de, as Se and Te belong to the VIA family, and these compounds manifest semiconductor properties and have already been applied in the fi eld of photoelectrochemistry. For example, ZnSe has attracted great interest due to its small bandgap (2.7 eV) and outstanding photoelectric properties (Zhang et al., 2015b). NiSe2-doped TiO2NTs broaden the response range of visible light, and CdSe has a bandgap of 1.6–1.8 eV and can thus absorb visible light (Li et al., 2015b; Wang et al., 2016a). It has been reported that Bi2Se3is an ideal n-type semiconductor with a bandgap of 0.35 eV, and it is a distinctive topological insulator with a conductive surface and dielectric body structure. Hence, the absorption coefficient of Bi2Se3is high in the visible and near-infrared regions (Wang et al., 2018a). CdTe is also used in the protection of metals, and Wang (Yang et al., 2016b) coated CdTe on TiO2nanotubes using the potentiodynamic deposition method. The potential of stainless steel upon coupling decreased to -850 mV (vs. SCE), and the composite promoted the absorption of visible light.
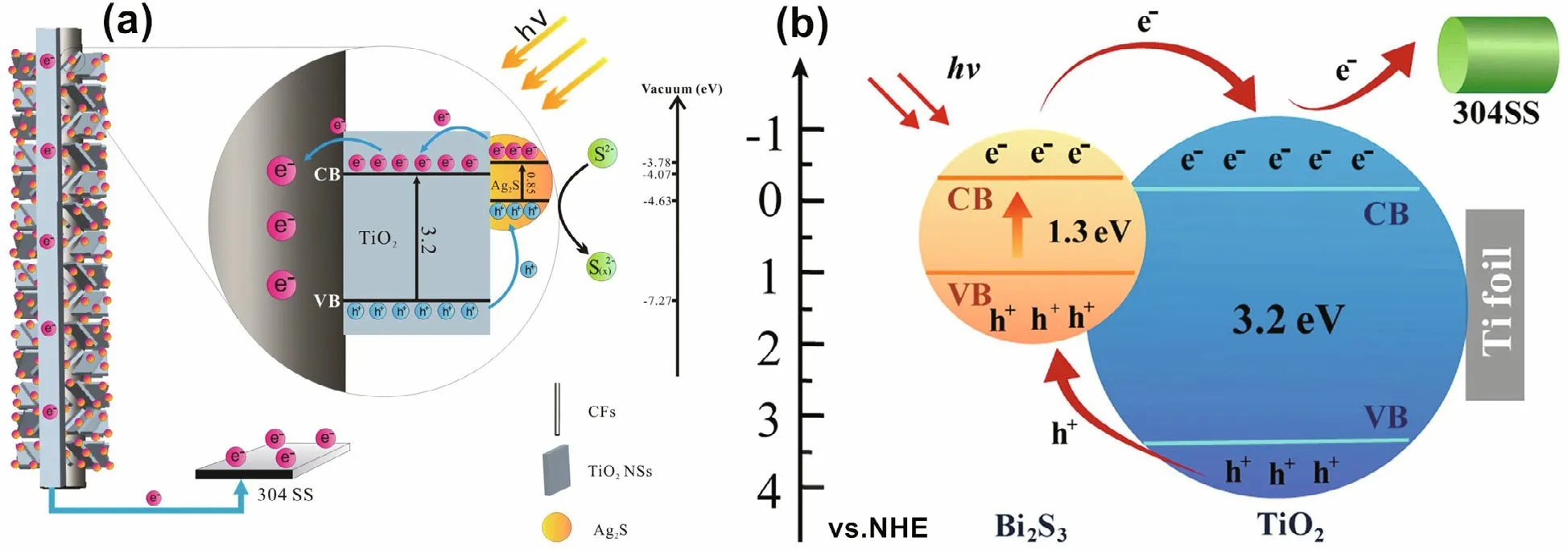
Fig.8 Schematic illustration of Ag 2 S QDs sensitized CFs-TiO 2 (a) and Bi 2 S 3/TiO 2 doping (b)
2.3 Metal-doped TiO 2 material
To improve the photoelectrochemical response, Au, Ag and Ni were doped into TiO2to prepare widelight-response composite materials. However, the doping of noble metals may introduce additional defects into the lattice structure of the TiO2substrate, increase the recombination rate of photogenerated carriers, and lead to the loss of photoelectrochemical activity. Therefore, an appropriate element content needs to be maintained.
Ag is one of the most important noble metals used to decorate TiO2nanotubes. The surface plasmon resonance (SPR) effect of Ag nanoparticles on TiO2can prolong the visible light response and improve the absorption capacity of TiO2. SPR occurs when the oscillation frequency of the incident electromagnetic fi eld matches that of the free electron under visible light (Ma et al., 2020). The energy of the incident ray is absorbed, and the absorption of visible light extends due to the proper distribution of the Ag nanoparticles (Guan et al., 2019), as shown in Fig.9. In addition, the high Schottky barrier between Ag nanoparticles and TiO2materials can prevent the recombination of photogenerated electrons and holes, thus promoting the electron transfer process. Therefore, the combination of Ag nanoparticles on TiO2NTs is a promising way to improve the corrosion protection performance of 304SS under visible light (Li et al., 2014). Another noble metal, Au, is also exploited in the same fi eld. There have been some reports about Au and TiO2composites. Zhu et al. (2013) proposed that the Au/TiO2compound with ZnS could prevent electrons from flowing from the substrate/Au surface into the Au/solution owing to the potential barrier, as the conduction band (1.85 eV vs. NHE) is higher than the Fermi level of Ag (+0.5 eV vs. NHE).
Research on Fe doping has been carried out as well; iron can enter the lattice of TiO2, thus destroying the integrity of TiO2and generating defect dots, which plays an important role in provoking the separation of photoelectrons and holes in promoting photocatalytic activity (Li et al., 2007). It has been found that iron ions can improve the photocatalytic activity of TiO2, and TiO2doped with iron can be used as an energy storage agent, which can maintain corrosion resistance for a long time in the dark (Liu et al., 2014; Momeni et al., 2018).
When Ni is substituted for Ti atoms, oxygen vacancies are produced in TiO2, which promote the transfer of photoinduced electrons, improve the photoelectric conversion efficiency of TiO2under visible light, and improve the corrosion resistance. Sun et al. (2013) found that Ni doping introduced an impurity energy level that was higher than the TiO2valence band, thus decreasing the conduction band, which hence remained negative. Therefore, Ni-doped TiO2has a visible light response, and Ni doping is conducive to the photoelectrochemical corrosion resistance. With the increase in the Ni doping amount, the main doping mode is gap doping. However, the content of oxygen vacancies decreases during this process. Excessive Ni will form recombination centers of electrons and holes in TiO2, which will worsen the visible light response performance.
Vanadium is an inhibitor used in paint. Thus, Chen et al. (2019) prepared V-doped TiO2through a sol-gel solution. In the composite, V atoms entered the TiO2lattice, which enhanced the anti-corrosion capability. Cerium nitrate is also a corrosion inhibitor applied in aqueous corrosive media. Li et al. (2012) exploited cerium nitrate to synthesize a Ce-doped TiO2fi lm. Due to the suppression of Ti4+, the transfer capacity of photogenerated electron-hole pairs increased, and recombination decreased. The absorption edge of the composite thus redshifted. However, metal doping increased the efficiency of electron transfer to the protected metal, and the mechanism is shown in Fig.10.

Fig.9 TEM images of the TiO 2 (a), low (b) and high (c) magnifi cations of Ag/TiO 2, and CQDs/Ag/TiO 2 composite (d)
2.4 Metal-oxides/TiO 2
Metal-oxides usually have unique structures and appropriate bandgaps to enhance the light absorption of TiO2to achieve high photocatalytic performance.
ZnO has a photovoltaic effect and a bandgap similar to that of TiO2,and the electron mobility in zinc oxide is approximately three times that of TiO2. High electron mobility can effectively reduce the secondary recombination of photoinduced electronhole pairs, thus signifi cantly improving the lifetime of the carriers. Therefore, ZnO has a higher photoelectric conversion potential, and the potential of ZnO is more negative than that of TiO2. Xu et al. (2014) designed a ZnO/TiO2composite using a simple hybrid sol-gelpowder method (Xu et al., 2014). The OCP of 304SS coupled with the ZnO/TiO2fi lm was up to -730 mV (vs. SCE) in 3 wt.% NaCl at an annealing temperature of 500°C, and the absorption of the composite fi lm redshifted. Multilayered ZnO/TiO2coatings exhibited anticorrosion performance on 304SS (Boukerche et al., 2019). However, ZnO has a wide energy gap of 3.37 eV, which still needs to be ameliorated in the synthesis and structure.

Fig.10 Schematic mechanism of the CQDs/Ag/TiO 2 composite fi lm as a photoanode for photocathodic protection
As an n-type semiconductor, α- Fe2O3has been verifi ed to absorb almost 40% of the solar spectrum, but α-Fe2O3also has a short carrier diffusion length, high electron hole binding rate and poor electron mobility (Xue et al., 2020). Cui and Pei (2019) prepared TiO2nanotubes modifi ed with Fe2O3particles. Theoretically, the bandgap of the Fe2O3/TiO2composite is 2.2 eV; thus, it has a wider spectrum response than pure TiO2. Furthermore, the photocurrents of pure TiO2and the Fe2O3/TiO2composite are 90 and 400 μA/cm2, respectively. Therefore, nontoxic Fe has good performance in photocathode protection (Deng et al., 2015). V2O5can store electrons and cations, and TiO2/V2O5composite materials can serve as photoelectrodes for light energy conversion and storage (Zhou et al., 2012).
The indirect bandgap energy of In2O3is 2.8 eV, so In2O3is an effective semiconductor material with a visible light response. In2O3has a good band structure, with a CB bottom of -0.63 eV, a VB of +2.17 eV and a conduction band potential that is more negative than the corrosion potential of steel. Therefore, In2O3is a potential anti-corrosion anode under visible light irradiation. In addition, there are many oxygen vacancies in In2O3that can affect the photoelectrochemical conversion. Keeping the structure unchanged, oxygen vacancies can improve the photoelectric conversion performance and effectively expand the visible light absorption area (Sun and Chen, 2015).
Bi2O3(p-type semiconductor) is the simplest Bibased oxide. Due to its excellent properties, such as high dielectric constant, refractive index and photoluminescence, it has been widely used in a wide range of photoelectrochemical applications. There are six polymorphic forms of Bi2O3: α-Bi2O3, β-Bi2O3, γ-Bi2O3, δ-Bi2O3, ε-Bi2O3and ω-Bi2O3(triclinic). Among these polymorphs, β-Bi2O3has a unique tetragonal crystal structure, resulting in a narrow bandgap (2.3–2.8 eV) and providing a transport channel for photogenerated electrons and holes. Therefore, β-Bi2O3is a suitable candidate for TiO2nanotube modifi cation. More importantly, if p-Bi2O3and n-TiO2are integrated into composite materials, a p-n heterojunction will be formed at the interface, which can signifi cantly improve the separation efficiency of photogenerated electrons and holes (Guan et al., 2018a). The slow discharging of 304SS and the potential variation of 403SS are shown in Fig.11. After light exposure, it can be seen that the potential of both 304SS coupled with TiO2/V2O5and 403SS coupled TiO2/Bi2O3composites increase slowly in the dark, and both values are lower than the original corrosion potential of metals.
2.5 Polymer and special structure design
Some inhibitors are now applied for photogenic CP, such as polypyrrole (PPy) and polyaniline (PANI), which are both types of conductive polymers. Conducting polymers are conductive and have conjugated sequences of double and single bonds, and they participate in corrosion protection by forming compact and protective oxide fi lms on the surface of the substrate.
PANI has benzenoid and quinonoid units and has a good ability to transport holes into n-type semiconductors. PANI is one of the most attractive conductive polymer materials and is widely used in the fi eld of photoelectrochemistry (Zhang et al., 2017b). PANI exhibits the properties of p-type semiconductors. Combining PANI with p-type TiO2increases the fi nal photoelectric fi eld. In addition, an appropriate amount of PPy is helpful for photosensitization, heterojunction formation and the electron pool effect. Under white light irradiation, PPy provides sufficient photocathodic protection to Q235 carbon steel. Many researchers have fabricated PPy on TiO2composites (Lenz et al., 2003; Cui et al., 2015; Ren et al., 2016) to form synergistic interactions and increase the protective properties relative to those of the individual materials. Moreover, the addition of TiO2nanoparticles altered the surface of PPy to introduce more sites of interaction with the corrosion product. Polyacrylate was also applied in TiO2coating through the liquid-phase deposition method at 80°C (Lei et al., 2013) and formed hierarchical superstructures due to the interaction between these materials. The composite showed a higher photocurrent and lower potential than the original material; thus, polyacrylate could offer photocathodic protection to the metal.
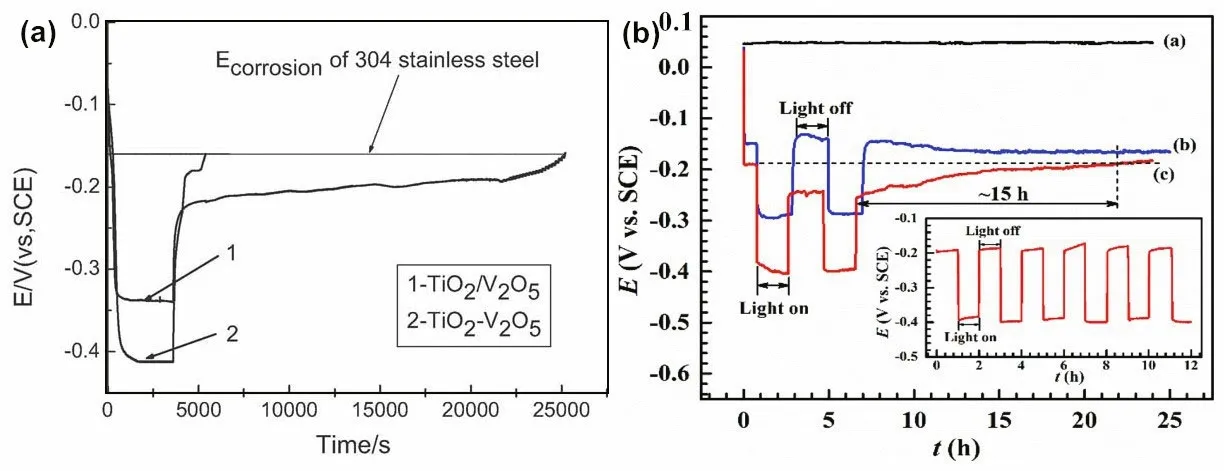
Fig.11 Discharge progress of TiO 2/V 2 O 5 composite coupling with 304SS (a) and potential variation of 403SS coupled with the β-Bi 2 O 3-TiO 2 NTA fi lm (b)
SrTiO3is a well-known solid semiconductor material with high photoelectric activity. The bandgaps of SrTiO3and TiO2are similar, approximately 3.2 eV, and SrTiO3is a p-type semiconductor with a perovskite structure. It was found that the SrTiO3/TiO2composite could increase the charge separation rate and thus improve the photoelectrochemical properties (Zhu et al., 2014; Bu et al., 2018).
The structure of layered hydroxides (LDHs) consists of cation metal layers and interlayers of a charge-balanced anion. Researchers have been inspired, and some follow-up work is being executed. Based on zinc oxide, LDHs have a strong absorption rate of visible light. In iron-based LDHs, an oxygen bridge decreases the recombination of electron-hole pairs and extends the effective diffusion distance of the holes. In addition, after heat treatment, semiconductor properties are exhibited by ZnAlFe-LDH materials (Wang et al., 2018b). Therefore, LDHs are applicable to TiO2modifi cation.
Crystalline tin dioxide (SnO2) is an important n-type semiconductor. Its photoelectrochemical properties are similar to those of TiO2,and the electron mobility of SnO2is higher than that of TiO2for single crystals and the corresponding nanostructures. The conduction band edge of SnO2is lower than that of TiO2, so the conduction band of SnO2can be used as an electron pool to preserve electrons in the case of coupling of photogenerated electrons. Thus, the SnO2coating can be used as a kind of energy storage material, and the SnO2/TiO2electrode has a good CP effect on 304SS in the dark (Subasri and Shinohara, 2003; Li et al., 2014; Hu et al., 2015a, b).
Co(OH)2/TiO2has been reported to have improved electrochemical properties relative to pure TiO2because Co(OH)2has a good ability to capture photogenic holes and provide additional reaction sites. To accelerate the transfer velocity of electrons, the binary mixture requires other components; for example, Xie et al. (2018) and Lu et al. (2020) added graphene to the mixture.
The above modifi cation is mainly focused on improving the photoelectric conversion efficiency, and there are some modifi cations that provide CP without light by introducing semiconductors with charge storage capacity into TiO2photoanodes, such as WO3(Guan et al., 2018b). WO3has a narrow bandgap of 2.6 eV and is responsive to visible light. In particular, due to the electrochemical reduction of WO3, it has the ability to store energy, which makes it possible to protect metals in the dark. When TiO2is illuminated, electrons are excited from the VB to the CB. There are two pathways for electrons injection into the metal and acceptance by WO3,which is reduced to consequently tungsten bronze (MxWO3, M=H, Li, Na, etc.; x ≤1) and in this process, the electrons were stored (Tatsuma et al., 2001). Zhou et al. (2009) prepared a TiO2/WO3bilayer coating that provided 6-h photocathodic protection after 1 h of irradiation. In addition to WO3/TiO2coating, WO3can be deposited as nanoparticles on TiO2nanotube arrays, resulting in synergistic effects between 1D and 3D nanostructures (Sun et al., 2018b). Yu et al. (2018) combined CP with superhydrophobicity by constructing TiO2nanoparticles and WO3nanosheet compounds. In the complex of a substrate with a TiO2/WO3coating, Yu et al. (2018) found that some amorphous particles were present and were likely to prevent pitting corrosion. The antisepsis method prevents electron consumption; WO3can preserve electrons, and the mechanism is discussed. Therefore, WO3is considered to be a good way to modify TiO2(Jing et al., 2016), and the process is illustrated in Fig.12.

Fig.12 Schematic diagram of the photocathodic protection of WO 3@TiO 2-coated 316 SS under illumination
CeO2has widespread application in organic-dyefree solar cells, and it has been reported that the bandgap of CeO2is shifted by 80 nm compared to that of TiO2. Some researchers have started to employ it in photocathodic protection. Subasri et al. (2006) found that the CeO2/TiO2bilayer coating had better photocathodic protection than the coating of CeO2alone, although CeO2had better conductivity. For the bilayer coating, the outer TiO2coating accepts light to generate photoelectrons, and photoelectrons spread to the substrate across CeO2. Subasri et al. (2006) also found that the protection of Cu lasted for 40 h after the light was turned off.
Zuo et al. (2018) prepared three kinds of nanotubes with different morphologies and used a schematic diagram to simulate and depict the behavior of incident light and reflected light. The flower-like nanostructure has the most voids where light has more probability to reflect and scatter. For the nanorod structure, the regular array and straight-pipe structure have less obstruction to stop incident rays escaping from the surface. Thus, with sufficient voids, flowerlike TiO2showed the best photocathodic protection, highest photocurrent, lowest potential and smallest charge transfer resistance. Li et al. (2010) also prepared a flower-like N-TiO2fi lm with a two-level nanostructure and found that the composite showed good CP even in the dark. The different nanostructures with LDHs, nanoflowers, spheres, and N-doped flower-like doping for TiO2are illustrated in Fig.13.
Special methods such as hydrogenation and UV radiation strategies are also used to modify the TiO2nanostructure. Wei et al. (2016) tried to improve the photoelectrochemical properties via hydrogenated TiO2nanotubes (H-TiO2). After hydrogenation, the surface structure of H-TiO2was disordered, and oxygen vacancies and Ti3+were introduced. As a result, the photocurrent density was 1.20 mA/cm2at 0.7 V under simulated light. Zhang et al. (2017a) irradiated the surface of a TiO2nanotube array fi lm with UV light. In subsequent photoelectric tests, the photocurrent increased by 50% compared with that of pristine TiO2, and the corrosive potential negatively shifted to -678 mV (vs. SCE) in 3.5 wt.% NaCl and 0.01 mol/L NaOH. The reason for this phenomenon is that UV treatment contributed to the formation of hydroxyl groups on the TiO2surface, and the presence of hydroxyl groups accelerated the recombination of photogenerated electrons and holes descend and the water oxidation reaction. The open circuit potential variation of 304SS coupled with TiO2fi lm with the treatment of hydrogenation and UV irradiation is shown in Fig.14.
3 CHARACTERIZATION OF TiO 2 PHOTOCATHODIC PROTECTION
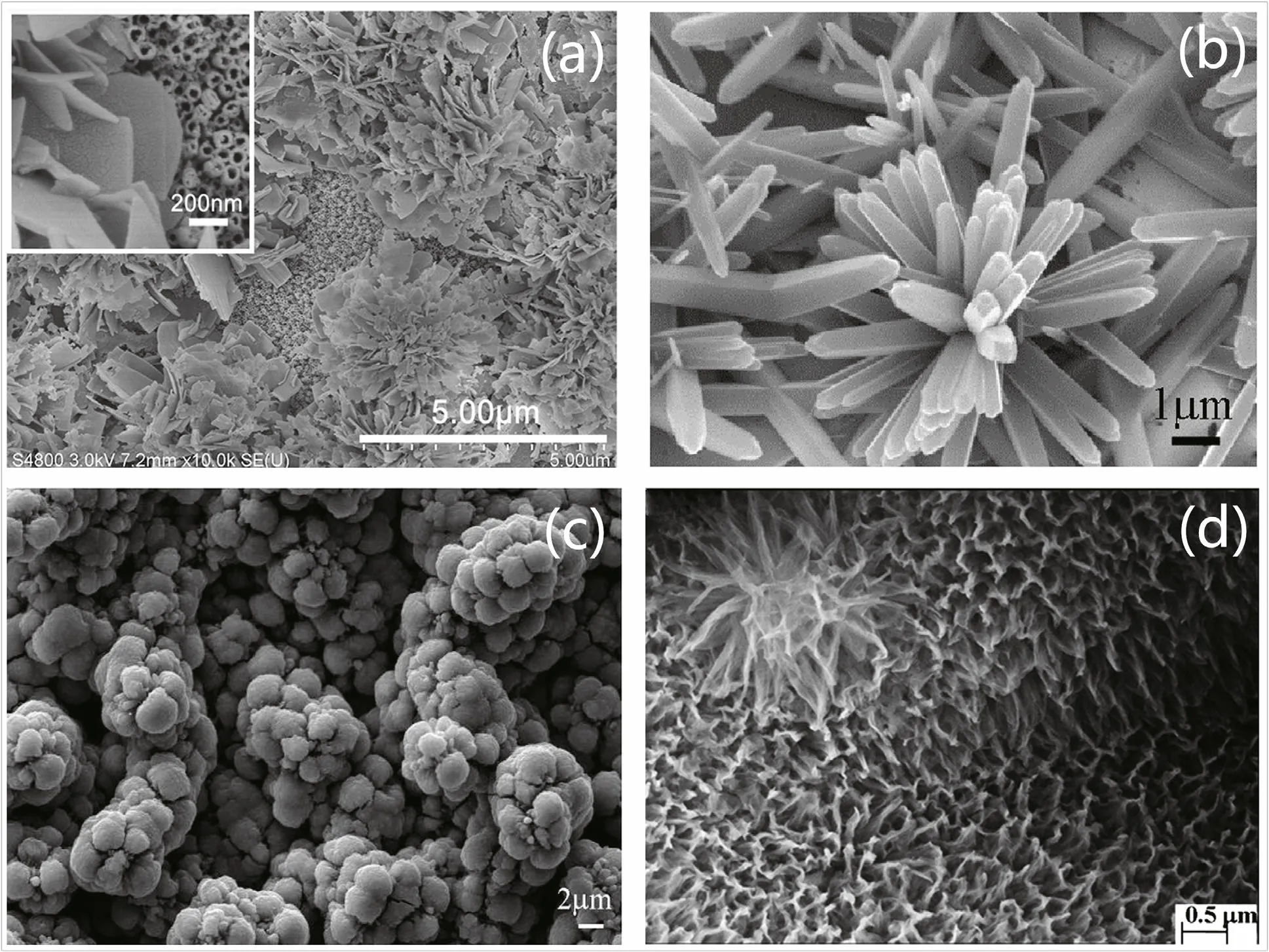
Fig.13 Nanostructure with LDHs (a), nanoflower (b), sphere-like (c), N-doped flower-like doping with TiO 2 (d)
The photocathodic protection performance of TiO2and semiconductor-doped composites on metal surfaces is the most important concern for researchers. Characterization of the materials not only focuses on the physical and chemical properties, such as photocatalytic properties, but also includes the protection effect for metals under different conditions, such as the protection potential of a metal during long-term continuous activity.
3.1 Morphology and structure characterization
Observing the morphology is usually the fi rst step when investigating the surface of TiO2nanocomposites. The structure is an important factor that determines the properties of materials doped with TiO2. From scanning electron microscopy (SEM) images, some characteristics can be known, such as the thickness of the surface fi lm, the size of the nanoparticles and nanotubes, and the interspace of clusters (Lenz et al., 2003). In Fig.15, carbon fi bers (CFs) as substrates and TiO2nanosheets with the (001) facet can be seen clearly in the FESEM images (Yang et al., 2019).
Transmission electron microscopy (TEM) can also be used to observe the surface morphologies of the surface fi lms. Specifi cally, the crystallization of the microcrystal plane and the lattice fringe spacing can be determined from the TEM images. In addition, TEM, including high-resolution TEM (HRTEM) and selected-area electron diffraction (SAED), can distinguish the internal structure of crystals and growth direction, and it has a higher defi nition than SEM. From the TEM and HRTEM images in Fig.16, the distribution of ZnIn2S4nanosheets on the TiO2nanotube structure is very clear, as are the lattice spacing and the corresponding facet (Li et al., 2019).
X-ray diffraction (XRD) is a technology that satisfi es Prague’s law to confi rm whether TiO2is crystalline or amorphous, determine the specifi c phase structure qualitatively, and utilize the crystallization direction to verify the element composition (Yuan and Tsujikawa 1995; Ma et al., 2020). Ding et al. (2019) confi rmed through XRD that TiO2was an amorphous phase and caused no obvious essential change in the g-C3N4spectrum except for increasing the peak intensity and shifting the peaks to the larger degree. Through XRD analysis, Li et al. (2017b) concluded that Ag existed in a cubic phase, and as the TiO2peak remained unchanged, the Ag atom did not enter the TiO2lattice and replace the Ti. From the typical binding energy of the XPS spectroscopy, it could be concluded that TiO2was successfully fabricated. Similarly, Liu et al. (2019) also analyzed the characteristic signal of Ti4+and Sn4+, which verifi ed that TiO2and SnO2were successfully fabricated.

Fig.14 OCP of 304SS connected with hydrogenation (a) and UV-treated TiO 2 NTs (b)

Fig.15 FESEM images of the carbon fi bers (a) and carbon fi bers-TiO 2 composites (b–d)
In addition to the above measurements, X-ray photoelectron spectroscopy (XPS) is also a common technology to characterize TiO2fi lms. XPS uses indefi nite carbon to calibrate the binding energy (Yuan and Tsujikawa, 1995; Cui et al., 2015; Hu et al., 2017; Nan et al., 2019). By analyzing the binding energy in the diffraction spectroscopy, the element doping and the cohesion between different materials can be determined, as shown in Fig.17.

Fig.16 TEM image (a) and HRTEM image (b) of the ZnIn 2 S 4/TiO 2 sample
3.2 Photoelectric property test
Photoelectric property tests, including UV-Vis DRS, photoluminescence (PL), and Raman spectroscopy, are signifi cant for photogenerated CP.
Ultraviolet-visible diffuse reflectance spectroscopy (UV-Vis DRS) is used to evaluate optical absorption. Through UV-Vis DRS, it was observed that the absorption edge of pure TiO2was 380 nm, and the bandgap was calculated to be 3.2 eV. In addition, compared with that of pure TiO2, the increasing absorption of CdS/TiO2indicated the validity of the modifi cation (Lin et al., 2010). Nan et al. (2019) converted the absorption spectrum of the Ni2S3/TiO2composite fi lm into Tauc plots through the Kubelka-Munk function and found that the band edge of the composite redshifted and the bandgap of Ni2S3decreased to 3.1 eV, as illustrated in Fig.18. After examined TiO2and its composite by UV-Vis DRS, Xie et al. (2019) found that different optical absorption performances for anatase and rutile TiO2as showed in Fig.18d.
A PL spectroscopy is also capable of certifi cating the usefulness of modifi cation. For instance, the CQDs/Ag/TiO2compound has a lower emission intensity than pure TiO2, indicating that fewer photogenerated carriers recombined and generated emission; that is, the conduction of CQDs and Ag contributes to the separation of electrons and holes (Guan et al., 2019). The PL spectroscopy can be analyzed with UV-Vis data to determine the light absorption, as shown in Fig.19.
Similarly, in fluorescence measurements, the fluorescence intensity represents the recombination amount of photoelectrons and holes. Therefore, it is usually used to investigate the photocathodic effect of doping compounds. Xu et al. (2020) noticed that the highest peak position in the fluorescence emission spectroscopy among all samples was approximately 425 nm, and the intensity gradually decreased with the introduction of CdSe, graphene and PANI, which greatly promoted photoelectron-hole separation. The TiO2/CdSe/PANI/graphene (TCPG) composite has a lower fluorescence intensity, which suggests a higher electron-hole pair separation efficiency. Cheng et al. (2017) obtained the fluorescence spectra of SiO2/TiO2fi lm, and it was obvious that the compound fi lm had lower fluorescence emission, and the spectra are shown in Fig.20.
In addition, Raman spectra can be used to determine whether the components vary under different treatments. For instance, Bamoulid et al. (2008) observed TiO2fi lms with different dip times through Raman spectroscopy, and the spectral signature of the surface remained unchanged; thus, the dipping treatment time would not trigger a composition change. Hu et al. (2017) discerned the presence of Bi2S3on TiO2nanotubes through a similar characteristic Raman peak of the specimen with Bi2S3deposited on TiO2and FTO.
3.3 Electrochemical test
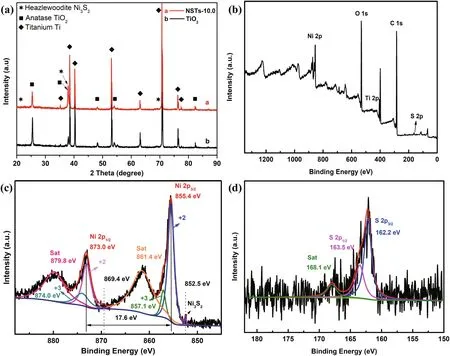
Fig.17 XRD patterns (a); XPS survey spectra (b); corresponding high-resolution Ni 2p and S 2p XPS spectroscopy of Ni 3 S 2-doped TiO 2 (c & d)
To investigate and verify the photogenerated CP effect of the photoanodes, electrochemical tests are necessary to examine the potential and current of stainless steel and the stability of the photoanode. The experiments, including OCP, i-t, EIS, and Mott-Schottky tests, are usually carried out on an electrochemical workstation. The photoelectrochemical cell is usually designed with three electrodes, a working electrode (WE), a counter electrode and SCE as the reference electrode (RE) (Park et al., 2013, Li et al., 2018b), as depicted in Fig.21.
3.3.1 OCP and i-t tests
The open circuit potential test and i-t are used to examine the potential and current density with time, respectively. If the potential range is safe for stainless steel, photoanode coupling with a metal can provide protection, and testing the potential for a long time can determine the stability of the photoanode. The OCP and i-t represent the photogenerated charge separation efficiency. In addition, the more negative the OCP is, the higher the i-t, and better the protection. OCP and i-t are usually tested by a three-electrode system on an electrochemical workstation system, such as PARSTAT 4000, CHI660B and Gamry Reference 600. In this system, generally, to simulate a marine environment, 0.5 mol/L (3.5 wt.%) NaCl is placed in the corrosion cell, and the coated metal, SCE and Pt foil are all placed in the NaCl solution as working, reference and counter electrodes, respectively. If the metal is guarded by an independent photoanode, connection remains unchanged and the metal is coupled with the photoanode. In the threeelectrode system, to further promote the efficiency of electron and hole separation, Na2S and NaOH as holetrapping agents are added to the photoanode cell.

Fig.18 UV-Vis DRS absorption spectra (a) and Tauc plots of Ni 3 S 2 nanosheets/TiO 2 (b); UV-Vis DRS absorption spectra of ZnWO 4/TiO 2 (c), and Co(OH) 2-modifi ed TiO 2 (d)

Fig.19 UV-Vis diffuse reflectance spectroscopy (a) and PL spectra of CQDs/Ag/TiO 2 composite fi lm (b)

Fig.20 Fluorescence spectroscopy of the TCPG composite (a) and SiO 2-TiO 2 fi lm on 304SS (b)

Fig.21 Photoelectrochemistry cell with three-electrode setup
The OCP values of a series of compounds can be compared to determine the composition with optimal photogenerated protection. As shown in the SiO2/TiO2compound coupled with 304SS, 10% SiO2has the most negative potential (-642 mV vs. SCE), 170 mV lower than that of pure TiO2(Cheng et al., 2017). The potential for electron transport indicated that SiO2was instrumental in enhancing the electrochemical properties of TiO2. Park et al. (2013) tested the OCP of coated stainless steel over a long time, and the restoration time of the compound was approximately 6 h after 3 h of irradiation. Under light irradiation, WO3and TiO2were excited, electrons were transferred to the metal, and the metal with the WO3/TiO2coating exhibited a lower potential range of 0.5 V and 0.7 V (vs. SCE) in 3.5 wt.% NaCl. Compared with pure TiO2, WO3enhanced the photoelectrons and increased the protection time after the simulated light was turned off. After turning off the light, the potential increased slowly to the previous potential in the dark. The transient photocurrent was used to directly evaluate the efficiency of photoelectron and pair separation. Cui et al. (2015) investigated the photocurrent density of 304SS coupled with a PPy/TiO2nanofi lm photoanode. While exposed to the light, the current of PPy/TiO2increased to 60.5 μA/cm2, and there was no apparent change in the pure TiO2photoanode. Thus, it could be determined which material has a photocathodic protection effect (Wang et al., 2016a), as shown in Fig.22.
In addition to being used for comparison among composites, OCP can monitor the potential under onoff cycles and long-term illumination. However, the long-term stability and continuous protection performance in the dark are two important factors that affect photocathodic protection, as shown in Fig.23. Hence, long-term OCP measurements are necessary to evaluate the photoelectrochemical properties. Liang et al. (2017) coupled WO3/TiO2with 403SS and found that the effective CP lasted for 19 h. Li et al. (2014) set the illumination time as 2 h per cycle, and after turning off the light, the potential of 304SS coupled with Ag and SnO2cosensitized TiO2photoanodes remained below the corrosion potential for more than 8 h, which exhibited good durability.

Fig.22 Photocurrent spectra (a) and OCP of pure NiSe 2/TiO 2 nanocomposites (b)
3.3.2 EIS and Mott-Schottky measurement
Electrochemical impedance spectroscopy (EIS) shows the electrochemical properties of an electrode and the reaction process at an interface. The Nyquist plots can reflect the electrolyte resistance, coating resistance, coating capacitance, charge transfer resistance, and electrical double layer capacitance of a fi lm and reflect the electron-hole transfer efficiency and the conductance of the fi lm material. Comparing the resistance of different materials can be used to identify better CP. The diameter of the capacitive arc represents the resistance. A large diameter represents high resistance. The Nyquist plot represents a threeelement equivalent circuit model, in which the charge transfer resistance and the solution resistance can be represented by Rct and Rs. Furthermore, the small Rct represents a quick electron transfer speed. Zhang et al. (2013) and Guan et al. (2019) verifi ed that the CQDs/Ag/TiO2composite had a small Rct in the Nyquist plots, which means that a large number of excited photoelectrons flowed to the metal; thus, it was protective for 403SS.
Mott-Schottky measurements can be used to determine the energy band structure and semiconductor type, which provides guidelines for composing materials. Xu et al. (2020) determined the type of TiO2composites and calculated the flat band according to the slope of the Mott-Schottky spectroscopy, and the reason for the transmission change was further verifi ed; that is, materials with different flat band potentials can be used to construct an internal space electric fi eld that can drive the flow of electrons. The positive slope of the spectra of the different materials implied that pure TiO2and Ni2S3/TiO2are n-type semiconductors. According to the Mott–Schottky equation, the flat band Efbcan be calculated. The deposition of Ni2S3is clear. The flat band of the compound shifted negatively; thus, the Fermi level increased, and the conduction band shifted negatively, which indicated an easier and faster transfer efficiency (Nan et al., 2019). The EIS and Mott-Schottky analyses are shown in Fig.24.
3.3.3 i-v and Tafel test
In addition, there are also other analysis techniques, such as i-v and the Tafel method. In photoelectrochemical anticorrosion research, the Tafel polarization curve is used to recognize the corrosion potential and current. Linear sweep voltammetry can be used to measure the threshold bias potential, which is the crucial potential corresponding to the occurrence of photoanodes and related to the reducing ability. Li et al. (2018a) investigated i-v tests for TiO2and the Sb2S3/Sb2O3/TiO2composites and found that the composite had a more negative potential, stronger reduction capacity, higher photocurrent and more efficient transfer ability.
The Tafel curve reflects the polarization process, and the corrosion current density can be easily obtained from the curve. Zhou et al. (2009) tested the corrosion current and found that 304SS coupled with WO3/TiO2had a more negative potential than pure 304SS under illumination. Zuo et al. (2018) verifi ed that the flower-like nanostructure had the best corrosion protection performance among nanorods, nanospheres and flower-like nanostructures because it had the most negative potential and the highest current in the Tafel curve, as shown in Fig.25.

Fig.23 Long-term OCP variations of metal coupled with WO 3/TiO 2 (a) and Ag/SnO 2/TiO 2 (b)

Fig.24 Nyquist plots of AgInS 2/In 2 S 3-doped TiO 2 NTAs (a) and Mott-Schottky plots of Ni 3 S 2-doped TiO 2 (b)
3.4 Characterization of metal
Although there are many characterization and test methods to verify the protection effect of TiO2, it is important to view the real anticorrosion degree in a simulated marine environment and monitor the surface of stainless steel in real time.
Optical microscopy was used to observe the corrosion morphology change of metals after testing (Mahmoud et al., 2005). Direct observation can be used to verify the credibility of previous outcomes. Xu et al. (2020) analyzed the surface morphology of 304SS through optical microscopy after different corrosion times. Many corrosion pits could be seen on the surface of 304SS without protection. After coupling with TiO2photoanodes, the quantity and size of the pits decreased.
When testing the protection of ZnO and TiO2layers on 304SS, Boukerche et al. (2019) found through optical microscopy that ZnO and TiO2fi lms with different proportions had different colors, which provided the ability to discern the features of fi lms. Li and Fu (2013) observed the morphology after accelerated corrosion tests, which were conducted for 316L stainless steel substrates with chromium-doped TiO2coatings, and found that the photocathodic protection was increased by using chromium doping. Xie et al. (2019) compared the protection of Q235 steel with Co(OH)2-modifi ed TiO2to that of traditional sacrifi cial anodes in 3.5 wt.% NaCl solution for 15 days and investigated the macrocorrosion morphologies of the steel, as shown in Fig.26. The experiments proved that the photoanode composites provided effective supplemental protection for steel.

Fig.25 Photoinduced i-v curve of the Sb 2 S 3/Sb 2 O 3/TiO 2 composites (a) and Tafel polarization curves of 304 stainless steel coupled with different TiO 2 (b)
To explore the detailed morphology of the metal under the protection of photoanodes with light irradiation, SEM was employed for further investigation. Liu et al. (2014) found that the SEM images of 304SS showed that some corrosion pits emerged on the surface after dipping the samples in 0.5 mol/L NaCl solutions without photocathodic protection and that no pits were observed on the surface of 304SS coupled with an Fe-doped TiO2photoanode, as shown in Fig.27.
4 SUMMARY AND PERSPECTIVE
Photocathodic protection is a promising technology for metal corrosion in marine environment. The present review shows the application of TiO2and its modifi cation to prevent corrosion. Nevertheless, two defects limit the widespread application of TiO2: low absorption of visible light and quick recombination of photogenerated carriers. In the past 25 years, much progress has been achieved in this fi eld, and different nanostructures with doping, modifi cation and band construction have been developed to improve the photocatalytic performance of TiO2. There is still a large potential to promote the utilization of sunlight.
More characterization and analysis are needed to increase the understanding of the fundamental and kinetic processes of photocathodic protection. For example, density functional theory calculations can help design composite materials and determine the mechanism of the process. However, crucial problems still exist in the application of photocathodic protection. To effectively promote the separation of electrons and holes, sacrifi cial agents such as Na2S, NaOH and Na2SO4are usually added to the solution, and much effort has been made to substitute the sacrifi cial agents. Another challenge is achieving continuous protection in a dark environment. Although energy storage materials have increased protection without visible light irradiation, the development of new green energy materials with low cost is still needed. TiO2-based composite materials will play an important role in the photocathodic protection fi eld.
However, to improve the photocathodic protection performance, a wider range of new materials and new technologies should be investigated. For example, MXenes and MOFs can be constructed into 2D nanosheets (Guan and Han, 2019), and physical vapor deposition and chemical vapor deposition (Muratore et al., 2019) can also be used. Combining photocathodic protection with sacrifi cial anode protection or new energy harvesting technology, such as triboelectric nanogenerators, can harvest wave energy (Feng et al., 2016) and mechanical energy (Yang et al., 2020) and provide more effective protection for metals. Thus, it is expected that the combination of photocathodic protection and triboelectric nanogenerators will be of use in anticorrosion technologies.
5 DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.

Fig.26 Corrosion morphologies of Q235 unprotected (a), protected (b) by SACP and protected by Al photoanode composite (c)

Fig.27 SEM images of 304SS blank (a); no protection (b); and coupled with Fe-doped TiO 2 (c)
杂志排行
Journal of Oceanology and Limnology的其它文章
- Review on observational studies of western tropical Pacifi c Ocean circulation and climate*
- Smart anticorrosion coating based on stimuli-responsive micro/nanocontainer: a review*
- Status of genetic studies and breeding of Saccharina japonica in China*
- To be the best in marine sciences*
- A review of progress in coupled ocean-atmosphere model developments for ENSO studies in China*
- Experimental clearance rates of Aurelia coerulea ephyrae and medusae, and the predation impact on zooplankton in Jiaozhou Bay*
