Rediscovery of the abyssal species Peniagone leander Pawson and Foell, 1986 (Holothuroidea: Elasipodida: Elpidiidae): the fi rst record from the Mariana Trench area*
2020-07-31GONGLinLIXinzhengXIAONingHELishengZHANGHaibinWANGYong
GONG Lin , LI Xinzheng , , XIAO Ning , , HE Lisheng , ZHANG Haibin , WANG Yong
1 Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266200, China.
3 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
4 University of Chinese Academy of Sciences, Beijing 100049, China
5 Department of Life Science, Institute of Deep-sea Science and Engineering, Chinese Academy of Sciences, Sanya 572000, China
Abstract The elpidiid holothurian Peniagone leander Pawson and Foell, 1986 is recorded for the fi rst time from the Mariana Trench area at a depth of 5 571 m. The type description of this species was based on only in-situ photographs. P. leander is re-described and illustrated based on a preserved material and in-situ photographs taken on the seabed. The 16S rRNA was also obtained from the specimen and submitted to GenBank. This is the fourth discovery of this species, extending its bathymetric range in the western Pacifi c Ocean.
Keyword: Elpidiidae; abyssal zone; deep-sea holothurian; benthopelagic species; new record
1 INTRODUCTION
The genus Peniagone Théel, 1882 with 32 known species (WoRMS, 2019) is the most species-rich in the Elpidiidae family. The species of Peniagone are widely distributed in the Atlantic, Pacifi c, Indian and Antarctic Oceans. They have a rather wide vertical distribution, from 400 m ( Peniagone vignoni Hérouard, 1901, recorded in the Antarctic Ocean) to 8 300 m ( Peniagone azorica Marenzeller von, 1892, recorded in the Kermadec Trench), and many are benthopelagic (Pawson and Foell, 1986; Gebruk, 2008; Cross et al., 2012; Rogacheva et al., 2012, 2013). The expanded mouth tentacles and fused posterior tube feet are adaptations to the benthopelagic lifestyle (primarily for swimming) of the genus (Gebruk, 2008; Rogacheva et al., 2013).
The large swimming holothurian Peniagone leander Pawson and Foell, 1986 was fi rst described based on in-situ photographs taken in the Clarion-Clipperton Fracture Zone (14°45′N–13°50′N, 127°39′W–125°49′W), eastern central Pacifi c, at a depth of approximately 4 400–5 000 m. The observation was used to describe a new species on the basis of the photographs alone, without details from ossicles since no specimens were obtained. P. leander is known from the abyssal zone, has rarely been encountered and is very fragile similar to most swimming holothurians. P. leander was observed and sampled during the French Nodinaut cruise in 2005 (Fifi s and Scolan, 2005). Although a specimen of P. leander was sampled during the cruise, its description was not published. Fujikura et al. (2008) reported the species from the Japan Trench and Nankai Trough areas at a depth of 3 764–4 836 m. This was the third discovery of this species, and the fi rst record of it in the western Pacifi c.
In June 2016, a biodiversity survey cruise of the Mariana Trench was organized by the Institute of Deep-sea Science and Engineering, Chinese Academy of Sciences (IDSSE), using the R/V Xiangyanghong 9 and manned submersible Jiaolong. During the cruise, three individuals of P. leander were observed. A whitish specimen (individual #1) was collected by the mechanical arm of the submersible Jiaolong. Another whitish specimen (individual #2) and a light violet specimen (individual #3) were only observed on the video. In this study, we re-describe P. leander, giving details of ossicles which were lacking in the original description. This is the fi rst record of the species from the Mariana Trench area.
2 MATERIAL AND METHOD
2.1 Specimen preservation
The specimen was collected by the mechanical arm of manned submersible Jiaolong at a depth of 5 571 m from the Mariana Trench area (11°48′N, 142°07′E). After collection, the specimen was preserved in 95% ethanol and deposited at the Institute of Deep-sea Science and Engineering, Chinese Academy of Sciences.
2.2 Morphological observation
For scanning electron microscope (SEM) examinations of ossicles, small pieces of tissue from dorsal body wall, ventral body wall and tentacles of the holothurian were macerated in sodium hypochlorite solution, and thereafter rinsed several times in distilled water before being transferred to 95% ethanol and air dried. The ossicles were then transferred to an aluminum stub, coated with gold, and observed under SEM (Hitachi S-3400N) at the Institute of Oceanology, Chinese Academy of Sciences (IOCAS).
2.3 Molecular analysis
Total genomic DNA was extracted from a small piece of tissue using a Tissue DNA Kit (OMEGA Qingdao, China) according to the manufacturer’s instructions. Partial sequences of 16S rRNA genes was amplifi ed with primers16S-AR/BR (Kessing et al., 1989) with the following program: initial denaturation for 5 min at 94°C, followed by 30 cycles of denaturation for 30 s at 94°C, 30 s at 48°C, 1 min at72°C, and a fi nal extension for 5 min at 72°C. Polymerase chain reaction (PCR) amplifi cation was carried out with a total reaction volume of 25 μL, containing 12.5-μL Premix TaqTM (TaKaRa, Japan), 8.5-μL DNase free ddH2O, 1-μL each primer, 2-μL template DNA. The 16S rRNA sequence was submitted to GenBank with an accession number of MN567717.

Table 1 Details of specimens and GenBank accession number used in this study
Considering that only three species of Peniagone have 16S rRNA sequences in GenBank and two more species were classifi ed only to the genus level, we added COI sequences to give a more comprehensive phylogenetic tree to determine the taxonomic status of P. leander. 16S rRNA and COI sequences of all species in the Elpidiidae, including 5 species from Peniagone and 5 additional species from fi ve genera in the family Elpidiidae, were downloaded from GenBank for phylogenetic analysis. Mitochondrial sequence data of Peniagone sp. YYH-2013 and Scotoplanes sp. TT-2017 were included here and Psychropotes longicauda was used as the out-group (Table 1).
The homologous sequences, including 9 sequences of 16S rRNA and 8 sequences of COI genes, were aligned using MUSCLE 3.8 (Edgar, 2004) under the default parameters. The best-fi t model of DNA substitution for each partitioned dataset were assessed by ModelTest v3.7 (Posada and Crandall, 1998). Finally, the concatenated dataset consisted of 1 044 bp (16S/COI=459/585 bp), the alignment gaps were represented as ‘-’ and the missing data were marked with ‘?’.
RAxMLGUI v1.5 (Silvestro and Michalak, 2012) was used to conduct the maximum likelihood (ML) analysis with the GTRGAMMA substitution model for all partitions in the concatenated dataset. Mrbayes 3.2 (Huelsenbeck and Ronquist, 2001) was used to conduct the Bayesian inference (BI) analysis, Markov Chains were run for 2 million generations, with sampling every 100 generations. The fi rst 25% of trees were discarded as burn-in, and the remaining trees were summarized in 50% majority rule consensus tree to estimate the posterior probabilities. Finally, we used Tracer v1.7 (Rambaut et al., 2018) to diagnose the effective sample size (ESS) values for all sampled parameters to make sure convergence was reached.
3 TAXONOMY
Order Elasipodida Théel, 1882
Suborder Psychropotina Hansen, 1975
Family Elpidiidae Théel, 1879
Genus Peniagone Théel, 1882
Peniagone leander Pawson and Foell, 1986 (Figs.1–3)
Peniagone leander Pawson and Foell, 1986: 293–299, Figs. 1–8, Clarion-Clipperton Fracture Zone, 14°45′N–13°50′N, 127°39′W–125°49′W, 4 400– 5 000 m; Fifi s and Scolan, 2005: 72–74, Fig. Peniagone sp.1, Clarion-Clipperton Fracture Zone, 13°55.63′N, 130°12.20′W, 4 800 m; Fujikura et al, 2008: 288, Fig. 22.60, Japan Trench, 4 836 m, Nankai Trough, 3764 m, coordinates unknown.
3.1 Type locality
Eastern central Pacifi c, 4 400–5 000 m.
3.2 Material examined
IDSSE Lab-2016-0629, 1 adult (length 230 mm), Mariana Trench area, fi ne sand, St. JL-Dive121, 11°48′N, 142°07′E, 5 571 m, 29 June 2016.
3.3 Diagnosis
Body ovoid, not flattened. Colour in life light violet, pink to reddish-brown. Anus dorsal. Ten tentacles. Velum broad, composed of two pairs of dorsal papillae; the central pair fused; the outer pair very short. Two pairs of short, pointed dorsal papillae posterior to the velum. Tube feet, 2 short free in the posterior part of the body and 4 posteriormost fused forming a lobe. Dorsal ossicles with straight or slightly curved arms up to 0.5 mm in length and slightly curved apohyses 0.15–0.3 mm in length; both arms and apophyses spinous. Ventral ossicles primary crosses, with four arms 0.16–0.4 mm long, slightly bent; short apophysies on each arm; occasionally irregular ossicles are present on ventrum.
3.4 Description of the material from the Mariana Trench area
Body cylindrical to ovoid, 230 mm in length (observed on fresh material on board), body width approximately 110 mm. Dorsal side with conspicuous transverse wrinkles, ventral side convex, bulbous posteriorly. Colour in vivo whitish to light violet; colour in alcohol whitish. Skin from transparent to semi-transparent.
Tube feet 2 pairs of small free in the posterior part of the body and 4 pairs in the posteriormost portion of the ventral sole, decreasing slightly in length towards the mid-ventral line (Fig.2a & b). Posteriormost tube feet fused forming a swimming lobe.
Velum short, broad, consisting of two pairs of almost completely fused dorsal papillae. The central pair of papillae longer than the outer pair. Another two pairs of small, free and pointed papillae posterior to the velum. These papillae inconspicuous.
Tentacles Ten. Tentacle stalks cylindrical, fairly long (58 mm in a 230-mm-long individual). The terminal discs bilobed on the aboral margin.
Ossicles Ossicles of dorsal skin: Peniagone-type, consisting of a central stem 0.14–0.36 mm long with four downwardly straight or slightly curved arms and four slightly curved apophyses (Fig.3a–c); both arms and apophyses spinous. Some variations occurred in the arm angle of curvature (in some ossicles arms slightly more curved than others). Maximum height of Peniagone-type ossicle 0.8 mm.
Ventral ossicles flattened crosses with a short stem 0.1–0.15 mm long and highly spinous arms bearing four, short blunt spinous apophyses; arms 0.16–0.4 mm long, apophyses 0.03–0.1 mm long (Fig.3d–e). Side-branches common on the arms of ventral ossicles (Fig.3f).
Tentacles containing curved spinous rods as well as large spinous primary crosses (Fig.3g–k), measuring up to 0.6 mm.
3.5 Remarks
Based on the body shape, velum morphology, number and position of dorsal papillae, number and arrangement of tube feet, we can confi rm that the individuals from the Mariana Trench area belong to Peniagone leander. However, the specimens from the type locality differ from those from the Mariana Trench area by having a brownish color with a less transparent body wall and a much more conspicuous lateral ridge. In the Mariana Trench area individuals, the body color in life ranges from whitish to light violet, and the body wall is translucent or transparent, with the internal organs visible through the body wall. An important characteristic missing in the original description is one additional pair of free tube feet developed close to the central ventral ambulacrum. These tube feet can be seen on the in-situ images in Pawson and Foell (1986) and Fujikura et al. (2008). Descriptions of ossicles of P. leander are given for the fi rst time.
3.6 GenBank accession number
MN567717 (16S rRNA).
3.7 Molecular data
The phylogenetic trees constructed using BI (Fig.4) and ML (Fig.5) analyses were generally congruent however the genera Rhipidothuria and Elpidia showed different topological structure. The phylogeny shows that Peniagone leander and other Peniagone species form a separate clade within the family suggesting the monophyly of the genus, with support values >0.7 for both the ML and BI analyses.
3.8 Distribution
Eastern central Pacifi c, 4 400–5 000 m; Nankai Trough and Japan Trench areas, 3 700–4 840 m; Mariana Trench area, 5 571 m.
3.9 Relationship
Peniagone leander most closely resembles the other swimming sea cucumber, P. diaphana (Théel, 1882), in the morphology of its velum and number and arrangement of tube feet. Peniagone leander differs from P. diaphana by having a more inflated body, two pairs of dorsal papillae posterior to the velum, more or less conspicuous transverse wrinkles on the dorsal surface, and an extra pair of free tube feet close to the medioventral line. However, the ossicles in P. diaphana are slightly smaller, with arm lengths usually 0.2–0.4 mm (Hansen, 1975; Gebruk, 1990).
4 DISCUSSION
4.1 In-situ observation of Peniagone leander Pawson and Foell, 1986
Three individuals were observed in the video. The body of individual #1 was approximately parallel to the seafloor with its postanal fan touching the sediment surface and mouth tube above the seabed (Fig.1l). A total of 4 minutes elapsed from the time when we fi rst found the specimen to when we were able to collect it using the mechanical arm of the submersible. Within those 4 minutes, the specimen had moved its body slightly.
Individual #2 and individual #3 were hanging above the substrate (Fig.1k), and their bodies were undulating on the current as if they were at “rest”. Approximately 15 seconds later, individual #3 began to bend towards the seabed and expanded tentacles with bilobed aboral margins to ingest material from the seabed (Fig.1n–o). We tried to obtain individual #3 using a sediment push-core but failed.
As we operated a sediment push-core near individual #2, the sediments were stirred into the seawater, and individual #2 began to take off from the seabed approximately 4 minutes later. After leaving the seabed, it swam in an elegant pattern. The propulsion was accomplished by the stroke of both the mouth tube and posterior tube feet. When the mouth tube looked upward, the postanal fan flexed to the dorsal and the tentacles expanded, showing an “S” curve (Fig.1a). Then, the mouth tube and postanal fan flexed to the ventral, and the tentacles began to gradually fold (Fig.1b–e). Over approximately 5 seconds, the oral tentacles shifted position, from horizontal to vertical. Next, the mouth tube and postanal fan began to flex to dorsal and the tentacles began to gradually expand (Fig.1f–j). The oral tentacles shifted position from vertical to horizontal over approximately 5 seconds. Then, these movements were repeated in slow, rhythmic strokes.
Peniagone leander was not sensitive to the submersible’s lights, its disruption of the sediments or the sounds caused by operating the mechanical arm. They were not advanced swimmers and in the presence of danger, they need some time before they are able to take off from the seabed. Although we were unable to consistently observe the swimming posture of Peniagone leander, we consider it a frequent swimmer, unlike Peniagone diaphana, which is known as a preferential swimmer and can spend more than 12 h in seawater without contacting the substrate (Barnes et al., 1976).
4.2 Systematic status of Peniagone leander Pawson and Foell, 1986

Fig.1 Peniagone leander Pawson and Foell, 1986

Fig.2 Peniagone leander Pawson and Foell, 1986, IDSSE Lab-2016-0629
The phylogeny of the Elpidiidae, which is based on morphology, has been addressed repeatedly. Théel (1882) established the original classifi cation of Elpidiidae based on the external characteristics. Perrier (1902) primarily based his system on specimen ossicles. Hérouard (1923) regarded the genera of Elpidiidae as belonging to two evolutionary lineages: the fi rst branch was based on a three-radial ossicle type, whereas a four-radial (cross-shaped) ossicle type served as the basis for the second branch. Ekman (1926) divided the Elpidiidae into two subfamilies, Elpidiinae and Peniagoninae. Although based on completely different theories, the two subfamilies were identical to the two evolutionary lineages assumed by Hérouard (1923). Hansen (1975) rejected the views of Hérouard and Ekman. He concluded that the ossicles do not justify a division of the family into two subfamilies. Gebruk (1983) proposed a new view of the phylogenetic relationships within the Elpidiidae. The genera of the Elpidiidae may be divided into three evolutionary branches characterised by the formation of specifi c types of ossicles and the structure of the peripharyngeal calcareous ring. One of the evolutionary branches included Psychrelpidia and Rhipidothuria, which have typical cross-shaped ossicles only and paired gonads, lack a rectal caecum and have up to 8 pairs of arms on the segments of the peripharyngeal calcareous ring (in Psyhrelpidia). The second branch included Peniagone and Achlyonice, which have up to 12–15 pairs of thin long arms on the segments of the peripharyngeal ring (Hansen, 1975). The third branch included Scotoplanes, Protelpidia, Ellipinion, Amperima, Kolga, Irpa, and Elpidia, which have C-shaped spicules or very unusual ossicles and up to 4 or 5 pairs of arms on the segments of the peripharyngeal calcareous ring.
There are few works on the molecular phylogeny of the Elpidiidae, and this group has only recently been explored simply through molecular approaches recently (Miller et al., 2017). In our tree, we increased the family Elpidiidae by 11 species and 6 genera, and the tree was generally consistent with previous results. All the species of Peniagone clustered together into a strongly supported monophyletic clade (Bayesian posterior probabilities is 0.99, bootstrap value is 71%). Peniagone diaphana was a sister group to Peniagone leander and both of them have an ovoid or flatten body shape, almost reduced tube feet and weakly developed velum. Peniagone incerta was a sister group to Peniagone vignoni and both of them have an elongate or somewhat flattened body shape. In another clade, Scotoplanes was a sister group to Protelpidia, which was consistent with the foundings of Gebruk (1983) that Scotoplanes is most closely related to Protelpidia. The characteristics shared by the two genera included the presence of straight rodshaped ossicles with complex structures, number and localisation of dorsal papillae, and numbers of tube feet pairs. Amperima was a sister group to Scotoplanes + Protelpidia, which was also consistent with the fi ndings of Gebruk (1983), who found that Amperima has a close relationship with Scotoplanes and Protelpidia. All of these genera have ossicles with numerous small C-shaped elements. In the Bayesian tree, Rhipidothuria was a sister group to Elpidia, while in the ML tree, Rhipidothuria was a sister group to Scotoplanes + Protelpidia + Amperima. Gebruk (1983) suggested that Elpidia was located on the third evolutionary branch, but Elpidia was very distantly related to the other genera within the third branch based on molecular data. Rhipidothuria was most closely related to Psychrelpidia according to Gebruk (1983), and Psychrelpidia was not included in our analysis. We cannot know whether the formation of a clade containing Rhipidothuria and Psychrelpidia is supported by molecular data. Since the status of Elpidia is not consistent between molecular data and morphological characteristics and some of the closely related genera (based on morphology) were not included in the present analysis, more extensive taxon coverage and more molecular markers are required in further studies to elucidate the relationships among the genera of Elpidiidae. However, the phylogeny based on molecular data was roughly consistent with the morphology-based tree suggested by Gebruk (1983). Thus, specifi c morphological details of ossicles and peripharyngeal calcareous rings can be reliable synapomorphies for the elpidiid genera.

Fig.3 Images of ossicles from Peniagone leander Pawson and Foell, 1986
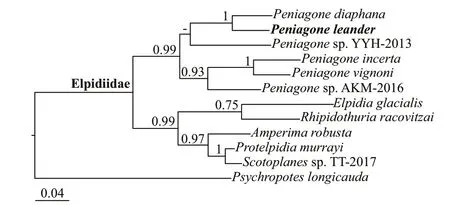
Fig.4 Phylogenetic tree obtained of Bayesian inference based on the combined data set of 16S rRNA and COI gene sequences

Fig.5 Phylogenetic tree obtained of ML based on the combined data set of 16S rRNA and COI gene sequences
5 DATA AVAILABILITY STATEMENT
The authors declare that the data supporting the fi ndings of this study are available within the article. The original data of measurements will be available on request from the fi rst author.
6 ACKNOWLEDGMENT
We are grateful to the crews of the R/V Xiangyanghong 9 for their support in collecting the deep-sea specimens during the cruises.
杂志排行
Journal of Oceanology and Limnology的其它文章
- Review on observational studies of western tropical Pacifi c Ocean circulation and climate*
- Research progress of TiO 2 photocathodic protection to metals in marine environment*
- Smart anticorrosion coating based on stimuli-responsive micro/nanocontainer: a review*
- Status of genetic studies and breeding of Saccharina japonica in China*
- To be the best in marine sciences*
- A review of progress in coupled ocean-atmosphere model developments for ENSO studies in China*
