A new acyclic peroxide from Aspergillus nidulans SD-531, a fungus obtained from deep-sea sediment of cold spring in the South China Sea*
2020-07-31FengyiLIXiaomingCHILupingMENGLinghongWANGBingui
LÜ Fengyi , LI Xiaoming , CHI Luping , MENG Linghong , WANG Bingui ,
1 Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, and Laboratory of Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China 2 College of Earth Science, University of Chinese Academy of Sciences, Beijing 100049, China
3 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
Abstract A new acyclic peroxide derivative asperoxide A ( 1), along with 13 known compounds, namely, microperfuranone ( 2), 9-hydroxymicroperfuranone ( 3), gibellulin A ( 4), lecanoric acid ( 5), terrequinone A ( 6), sterigmatocystin ( 7), isosecosterigmatocystin ( 8), arugosin C ( 9), curvularin ( 10), 3,3'-diindolylmethane ( 11), austinol ( 12), austin ( 13), and dehydroaustin ( 14), were isolated and identifi ed from the culture extract of Aspergillus nidulans SD-531, a fungus obtained from the deep-sea sediment of cold spring in the South China Sea. Their structures were determined based on detailed interpretation of nuclear magnetic resonance (NMR) spectroscopic and mass spectrometry data analysis. All the isolated compounds were evaluated for antimicrobial activities against human and aquatic bacteria as well as plant pathogenic fungi. Compounds 1– 8, 10, and 11 exhibited antimicrobial activities against some of the tested strains with minimum inhibitory concentration (MIC) values ranging from 2 to 64 μg/mL. Compounds 4 and 6 displayed strongest activities among the tested samples and might be used as promising molecules for the development of natural antimicrobial agents.
Keyword: acyclic peroxide; Aspergillus nidulans; cold spring fungus; antimicrobial activity
1 INTRODUCTION
Peroxide-containing compounds are widely occurred in the nature, and more than 600 peroxides have been isolated and structurally characterized from natural resources (Dembitsky et al., 2007). Some of these peroxides possess various biological properties including antibacterial, cytotoxic, antimalarial, anticancer and so on, which are shown as an important source of leads for development of new drugs or drug candidates (Dembitsky, 2008; Gandhi et al., 2017). The fi rst reported marine natural peroxide was plakortin, an antibiotic isolated from the Caribbean sponge Plakortis halicondrioides (Higgs and Faulkner, 1978). During our recently initiated program on identifying new bioactive secondary metabolites from cold-spring-derived fungi, we performed chemical investigations on the culture extract of the Aspergillus nidulans SD-531, which was isolated from the sediment of cold spring in the South China Sea in September 2017. As a result, a new acyclic peroxide, asperoxide A ( 1), together with 13 known compounds, namely, microperfuranone ( 2), 9-hydroxymicroperfuranone ( 3) (Fujimoto et al., 2006), gibellulin A ( 4) (Bunbamrung et al., 2015), lecanoric acid ( 5) (Narui et al., 1998), terrequinone A ( 6) (He et al., 2004), sterigmatocystin ( 7) (Wu et al., 2014), isosecosterigmatocystin ( 8) (Yang et al., 2018), arugosin C ( 9) (Hawas et al., 2012), curvularin ( 10) (Deng et al., 2015), 3,3'-diindolylmethane ( 11) (Roy et al., 2013), austinol ( 12), austin ( 13), and dehydroaustin ( 14) (Hayashi et al., 1994), were isolated and identifi ed from the culture extract of the fungus (Fig.1). The structures of Compounds 1– 14 were established on the basis of spectroscopic analysis and all these compounds were examined for antimicrobial activity. This paper describes the isolation, structural elucidation and bioactivity evaluation of these compounds.

Fig.1 Chemical structures of Compounds 1–14 isolated from A. nidulans
2 MATERIAL AND METHOD
2.1 General experimental procedure
1D-NMR (1H-NMR,13C-NMR, Distortionless Enhancement by Polarization Transfer (DEPT)) and 2D-NMR (1H-1H Correlation Spectroscopy (COSY), Heteronuclear Multiple Quantum Correlation (HMQC), Heteronuclear Multiple Bond Coherence (HMBC)) were recorded on a Bruker Avance-500 MHz spectrometer (Bruker Biospin Group, Karlsruhe, Germany). Mass spectra were determined on an API QSTAR Pulsar 1 mass spectrometer. Analytical high performance liquid chromatography (HPLC) analysis was carried out using a Dionex HPLC system, equipped with P680 pump, ASI-100 automated sample injector, and UVD340U multiple wavelength detector controlled by Chromeleon software (version 6.80) (Dionex, Sunnyvale, CA, USA). Optical rotations were measured on an Optical Activity AA-55 polarimeter (Optical Activity Ltd, Cambridgeshire, UK). Ultraviolet (UV) spectra were recorded on a Lengguang Gold S54 spectrophotometer (Shanghai Lengguang Technology Co. Ltd., Shanghai, China). Thin-layer chromatography (TLC) was performed using precoated Si gel GF254 plates (Merck, Darmstadt, Germany). Column chromatography (CC) was performed with Si gel (200–300 mesh, Qingdao Haiyang Chemical Co.), Sephadex LH-20 (Pharmacia) and Lobar LiChroprep RP-18 (40–63 μm, Merck).
2.2 Fungal material
The fungal strain SD-531 was isolated from the deep-sea sediment, collected in cold spring in the South China Sea, in September 2017. The strain was identifi ed as Aspergillus nidulans according to the ITS region sequence, while the information of the fungus SD-531 was deposited in GenBank with the accession no. MN901610. The strain is preserved at the Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences (IOCAS).
2.3 Fermentation, extraction, and isolation
The fungal strain A. nidulans SD-531 was statically cultivated on potato dextrose agar (PDA) medium at 28° C for 4 days. The fermentation was then carried out in 100 Erlenmeyer flasks (1 L) each containing 70-g rice, 0.2-g corn syrup, 0.3-g peptone, 0.5-g yeast powder, 0.6-g monosodium glutamate and 100-mL naturally sourced and fi ltered seawater. The fresh mycelia of A. nidulans SD-531 was inoculated into the flasks and cultivated for 30 days at room temperature.
The fermented rice substrate was extracted four times with EtOAc and the organic solvent was removed under reduced pressure to yield crude extract (90 g). The crude extract was subjected to Si gel vacuum liquid chromatography (VLC) and was fractionated by different solvents of increasing polarity from petroleum ether (PE) to MeOH to yield 11 fractions (Frs. 1–11) based on TLC and HPLC analysis. Fr. 4 (3.8 g) was further separated by CC over Lobar LiChroprep RP-18 with a MeOH-H2O gradient (from 10:90 to 100:0) to yield six subfractions (Frs. 4.1–4.6). Fr. 4.3 (25.3 mg) was purifi ed by prep. TLC (plate: 20 cm×20 cm, developing solvents: CH2Cl2-MeOH, 40:1) to yield Compound 2 (6.3 mg). Fr. 4.5 (101.2 mg) was purifi ed by CC on Si gel eluting with a CH2Cl2-MeOH gradient (from 100:1 to 30:1) to obtain Compound 9 (40.4 mg). Purifi cation of Fr. 5 (3.0 g) by CC over Lobar LiChroprep RP-18 with a MeOH-H2O gradient (from 10:90 to 100:0) yielded ten subfractions (Frs. 5.1–5.10). Fr. 5.1 (57.1 mg) was purifi ed by prep. TLC (plate: 20 cm×20 cm, developing solvents: PE-EtOAc, 2:1) to yield Compound 11 (8.4 mg). Fr. 5.5 (112.1 mg) was separated by CC on Si gel eluting with a CH2Cl2-MeOH gradient (from 80:1 to 10:1) and further fractionated by CC on Sephadex LH-20 (MeOH) to give Compound 5 (12.0 mg). Fr. 5.6 (130.9 mg) was purifi ed by CC on Si gel eluting with a CH2Cl2-MeOH gradient (from 100:1 to 40:1) to yield Compounds 1 (3.6 mg), 3 (15.4 mg), 10 (28.3 mg) and 12 (5.3 mg). Fr. 5.7 (164.3 mg) was purifi ed by CC on Si gel eluting with a CH2Cl2-MeOH gradient (from 100:1 to 60:1) to afford Compound 13 (91.2 mg). Fr. 5.8 (117.3 mg) was separated by CC on Si gel eluting with a CH2Cl2-MeOH gradient (from 100:1 to 40:1), then purifi ed by CC on Sephadex LH-20 (MeOH) to give Compound 4 (3.4 mg). Fr. 5.9 (205.3 mg) was fractionated by CC on Si gel eluting with a CH2Cl2-MeOH gradient (from 100:1 to 60:1) to obtain Compound 6 (18.0 mg). Fr. 5.1 (205.9 mg) was separated by CC on Si gel eluting with a CH2Cl2-MeOH gradient (from 100:1 to 40:1) to give Compound 7 (11.0 mg). Fr. 6 (3.2 g) was further separated by CC over Lobar LiChroprep RP-18 with a MeOH-H2O gradient (from 10:90 to 100:0) to yield nine subfractions (Frs. 6.1–6.9). Fr. 6.5 (112.2 mg) was purifi ed by prep. TLC (plate: 20 cm×20 cm, developing solvents: CH2Cl2-MeOH, 20:1) to yield Compound 14 (3.7 mg). Purifi cation of Fr. 8 (5.1 g) by CC over Lobar LiChroprep RP-18 with a MeOHH2O gradient (from 10:90 to 100:0) yielded three subfractions (Frs. 8.1–8.3). Fr. 8.1 (44.4 mg) was purifi ed by prep. TLC (plate: 20 cm×20 cm, developing solvents: CH2Cl2-MeOH, 10:1) to afford Compound 8 (7.9 mg).
2.4 Spectral data
Asperoxide A ( 1): white powder; C12H26O4(Mw 234.18);1H and13C NMR data, Table 1; HRESIMS m/ z 235.190 9 [M+H]+(calcd for C12H27O4, 235.190 4), m/ z 257.172 9 [M+Na]+(calcd for C12H26O4Na, 257.172 3).
Microperfuranone ( 2): white powder; C17H14O3(Mw 266.09); [α]D25-5.6 ( c 0.18, MeOH); UV (MeOH) λmax(log ε) 282 (3.72) nm;1H NMR (DMSO- d6, 500 MHz) δH: 3.87 (2H, brs, H-12), 5.93 (1H, brs, H-5), 7.20 (1H, m, H-16), 7.21 (1H, m, H-15), 7.21 (1H, m, H-17), 7.29 (1H, m, H-14), 7.29 (1H, m, H-18), 7.45 (1H, m, H-9), 7.46 (1H, m, H-8), 7.46 (1H, m, H-10), 7.47 (1H, m, H-7), 7.47 (1H, m, H-11), 8.05 (1H, brs, OH-5);13C NMR (DMSO- d6, 125 MHz) δC: 31.9 (CH2, C-12), 97.0 (CH, C-5), 126.8 (CH, C-16), 128.5(CH, C-8), 128.5 (CH, C-10), 128.7 (CH, C-14), 128.7 (CH, C-18), 128.7 (CH, C-15), 128.7 (CH, C-17), 128.8 (CH, C-9), 128.8 (CH, C-7), 128.8 (CH, C-11),129.4 (C, C-3), 136.3 (C, C-13), 159.3 (C, C-4), 170.2 (C, C-2).

Table 1 1 H and 13 C NMR data for Compound 1 (DMSO- d 6) (Supplementary Figs.S2–S6)
9-Hydroxymicroperfuranone ( 3): white powder; C17H14O4(Mw 282.09); [α]D250 ( c 0.12, MeOH); UV (MeOH) λmax(log ε) 280 (3.74) nm;1H NMR (DMSO- d6, 500 MHz) δH: 3.85 (2H, brs, H-12), 5.88 (1H, s, H-5), 6.84 (1H, m, H-8), 6.84 (1H, m, H-10), 7.22 (1H, m, H-16), 7.24 (1H, m, H-15), 7.24 (1H, m, H-17), 7.25 (1H, m, H-14), 7.25 (1H, m, H-18), 7.30 (1H, m, H-7), 7.30 (1H, m, H-11), 7.90 (1H, brs, OH-5), 9.69 (1H, m, OH-9);13C NMR (DMSO- d6, 125 MHz) δC: 31.8 (CH2, C-12), 97.3 (CH, C-5), 115.3 (CH, C-8), 115.3 (CH, C-10), 119.9 (CH, C-6), 126.6 (CH, C-16), 128.5 (C, C-3), 128.6 (CH, C-14), 128.6 (CH, C-18), 128.7 (CH, C-15), 128.7 (CH, C-17), 130.1 (CH, C-7), 130.1 (CH, C-11), 156.8 (C, C-4), 157.9 (C, C-9), 170.5 (C, C-2).
Gibellulin A ( 4): brown powder; C14H12O5(Mw 260.07); [α]D25+185.7 ( c 0.07, MeOH); UV (MeOH) λmax(log ε) 235 (4.27) nm, 287 (3.37) nm;1H NMR (DMSO- d6, 500 MHz) δH: 2.03 (3H, s, H-7'), 2.13 (3H, s, H-7), 6.22 (1H, s, H-3'), 6.24 (1H, s, H-2), 6.33 (1H, s, H-4), 8.42 (1H, brs, OH), 8.78 (1H, brs, OH), 9.07 (1H, brs, OH);13C NMR (DMSO- d6, 125 MHz) δC: 14.1 (CH3, C-7'), 20.5 (CH3, C-7), 107.1 (CH, C-2), 110.8 (CH, C-3'), 111.5 (CH, C-4), 113.4 (C, C-2'), 127.3 (C, C-6), 130.9 (C, C-6'), 132.5 (C, C-1'), 132.6 (C, C-3), 141.2 (C, C-4'), 142.1 (C, C-1), 145.0 (C, C-5).
Lecanoric acid ( 5): brown powder; C16H14O7(Mw 318.07); [α]D25+144.8 ( c 0.15, MeOH); UV (MeOH) λmax(log ε) 211 (4.34) nm, 269 (3.99) nm, 302 (3.77) nm;1H NMR (DMSO- d6, 500 MHz) δH: 2.35 (3H, s, H-8), 2.39 (3H, s, H-8'), 6.22 (1H, s, H-3), 6.22 (1H, s, H-5), 6.56 (1H, s, H-5'), 6.59 (1H, s, H-3'), 10.04 (1H, brs, OH-4), 10.34 (1H, brs, OH-2);13C NMR (DMSO- d6, 125 MHz) δC: 21.2 (CH3, C-8'), 21.3 (CH3, C-8), 100.5 (CH, C-3), 107.3 (CH, C-3'), 108.0 (C, C-1), 109.9 (CH, C-5), 114.5 (CH, C-5'), 116.3 (C, C-1'), 139.8 (C, C-6'), 140.2 (C, C-6), 152.2 (C, C-4'), 159.7 (C, C-2'), 160.2 (C, C-2), 161.1 (C, C-4), 167.1 (C, C-7), 170.7 (C, C-7').
Terrequinone A ( 6): purple powder; C32H30N2O3(Mw 490.23); [α]D25+500.0 ( c 0.01, MeOH); UV (MeOH) λmax(log ε) 223 (5.60) nm, 273 (5.13) nm, 362 (4.19) nm;1H NMR (DMSO- d6, 500 MHz) δH: 1.23 (3H, s, H-10), 1.42 (3H, s, H-13'), 1.42 (3H, s, H-14'), 1.53 (3H, s, H-11), 3.11 (1H, dd, J=12.4, 6.1 Hz, H-7a), 3.21 (1H, dd, J=12.4, 6.1 Hz, H-7b), 4.94 (1H, m, H-8), 5.01 (2H, brd, J=17.4 Hz, H-12'), 6.06 (1H, dd, J=17.4, 10.6 Hz, H-11'), 6.89 (1H, t, J=7.4 Hz, H-7'), 7.03 (1H, d, J=7.4 Hz, H-8'), 7.05 (1H, d, J=7.4 Hz, H-7''), 7.09 (1H, d, J=7.4 Hz, H-8''), 7.16 (1H, t, J=7.4 Hz, H-6'), 7.29 (1H, d, J=7.4 Hz, H-9'), 7.36 (1H, d, J=7.4 Hz, H-6''), 7.46 (1H, d, J=2.5 Hz, H-2''), 7.48 (1H, d, J=7.4 Hz, H-9''), 10.22 (1H, brs, NH-3'), 10.86 (1H, brs, NH-3''), 11.54 (1H, brs, OH-2);13C NMR (DMSO- d6, 125 MHz) δC: 17.5 (CH3, C-10), 25.4 (CH3, C-11), 26.5 (CH3, C-13'), 27.3 (CH3, C-14'), 27.6 (CH2, C-7), 38.8 (C, C-10'), 101.4 (C, C-1'), 106.6 (C, C-1''), 110.7 (CH, C-9'), 110.8 (CH2, C-12'), 111.8 (CH, C-9''), 117.0 (C, C-3), 118.3 (CH, C-7'), 118.6 (CH, C-6'), 119.4 (CH, C-7''), 119.6 (CH, C-6''), 120.5 (CH, C-8'), 121.4 (CH, C-8''), 122.8 (CH, C-8), 126.7 (CH, C-2''), 127.0 (C, C-5''), 128.3 (C, C-5'), 132.3 (C, C-9), 134.2 (C, C-6), 135.2 (C, C-4'), 135.9 (C, C-4''), 142.3 (C, C-2'), 144.8 (C, C-5), 145.6 (CH, C-11'), 153.4 (C, C-2), 183.4 (C, C-1), 187.4 (C, C-4).
Sterigmatocystin ( 7): yellow needles (MeOH); C18H12O7(Mw 340.06); [α]D25-381 ( c 0.21, CHCl3); UV (MeOH) λmax(log ε) 206 (4.39) nm;1H NMR (DMSO- d6, 500 MHz) δH: 3.99 (3H, s, H-18), 4.79 (1H, dt, J=7.0, 1.9 Hz, H-15), 5.44 (1H, t, J=2.5 Hz, H-16), 6.43 (1H, s, H-11), 6.50 (1H, t, J=2.5 Hz, H-17), 6.75 (1H, d, J=8.2, Hz, H-4), 6.81 (1H, d, J=2.6, Hz, H-6), 6.83 (1H, d, J=1.9 Hz, H-14), 7.49 (1H, t, J=8.2 Hz, OH-5), 13.21 (1H, s, OH-3);13C NMR (DMSO- d6, 125 MHz) δC: 48.2 (CH, C-15), 56.9 (CH3, C-18), 90.6 (CH, C-11), 102.4 (CH, C-16), 106.0 (C, C-13), 106.1 (C, C-9), 106.7 (CH, C-6),109.1 (C, C-2), 111.4 (CH, C-4), 113.2 (CH, C-14), 135.8 (C, C-5), 145.5 (CH, C-17), 154.2 (C, C-8), 155.1 (C, C-7), 162.4 (C, C-3), 163.4 (C, C-12), 164.7 (C, C-10), 181.5 (C, C-1).
Isosecosterigmatocystin ( 8): yellow powder; C18H18O8(Mw 362.10); [α]D25-15.8 ( c 0.19, MeOH); UV (MeOH) λmax(log ε) 231 (3.67) nm, 251 (3.51) nm, 326 (3.01) nm;1H NMR (CDCl3, 500 MHz) δH: 3.16 (2H, m, H-4'), 3.53 (1H, m, H-1'a), 3.59 (1H, m, H-2'), 3.69 (3H, s, H-11), 3.93 (1H, m, H-3'), 3.98 (1H, m, H-1'b), 5.84 (1H, s, H-3), 6.51 (1H, d, J=8.2 Hz, H-6), 6.70 (1H, d, J=8.2 Hz, H-8), 7.39 (1H, t, J=8.2 Hz, H-7);13C NMR (CDCl3, 125 MHz) δC: 40.9 (CH, C-2'), 55.0 (CH3, C-11), 61.4 (CH2, C-1'), 65.7 (CH2, C-4'), 71.4 (CH, C-3'), 98.7 (C, C-4a), 99.8 (CH, C-3), 105.4 (CH, C-8), 107.1 (C, C-10a), 107.5 (C, C-1), 109.0 (CH, C-6), 134.0 (CH, C-7), 154.5 (C, C-9a), 157.4 (C, C-8a), 160.2 (C, C-4), 161.5 (C, C-2), 161.5 (C, C-5), 178.1 (C, C-10).
Arugosin C ( 9): yellow powder; C25H28O6(Mw 424.19); [α]D25+33.3 ( c 0.23, MeOH); UV (MeOH) λmax(log ε) 202 (4.95) nm, 219 (4.61) nm, 271 (4.33) nm, 305 (3.36) nm;1H NMR (DMSO- d6, 500 MHz) δH: 1.13 (3H, s, H-22), 1.13 (3H, s, H-23), 1.68 (3H, s, H-17), 1.68 (3H, s, H-18), 2.15 (3H, s, H-24), 3.23 (2H, d, J=7.2 Hz, H-14), 2.25 (1H, m, H-20), 3.95 (1H, t, J=11.4 Hz, H-19a), 4.36 (1H, dd, J=11.4, 3.7 Hz, H-19b), 5.22 (1H, d, J=5.7 Hz, H-25), 5.27 (1H, t, J=7.2 Hz, H-15), 6.41 (1H, d, J=8.3 Hz, H-9), 6.82 (1H, s, H-4), 7.31 (1H, d, J=8.3 Hz, H-10), 9.89 (1H, brs, OH-3), 13.46 (1H, brs, OH-12);13C NMR (DMSO- d6, 125 MHz) δC: 15.8 (CH3, C-24), 17.5 (CH3, C-18), 25.4 (CH3, C-17), 27.2 (CH2, C-14), 28.0 (CH3, C-23), 28.2 (CH3, C-22), 49.1 (CH, C-20), 65.5 (CH2, C-19), 69.2 (C, C-21), 74.4 (CH, C-25), 108.9 (CH, C-9), 112.4 (C, C-13), 119.5 (CH, C-4), 121.5 (C, C-7), 121.8 (C, C-2), 122.1 (C, C-11), 122.1 (CH, C-15), 131.9 (C, C-16), 132.9 (C, C-5), 136.9 (CH, C-10), 144.8 (C, C-6), 151.8 (C, C-3), 158.5 (C, C-8), 160.1 (C, C-12), 196.2 (C, C-1).
Curvularin ( 10): yellow powder; C16H20O5(Mw 292.13); [α]D25-12.5 ( c 0.80, MeOH); UV (MeOH) λmax(log ε) 222 (1.35) nm, 273 (0.69) nm, 306 (0.51) nm;1H NMR (DMSO- d6, 500 MHz) δH: 1.07 (3H, d, J=6.3 Hz, H-16), 1.17 (1H, m, H-11a), 1.17 (1H, m, H-13a), 1.33 (1H, m, H-11b), 1.33 (1H, m, H-13b), 1.33 (1H, m, H-14a), 1.46 (1H, m, H-12a), 1.53 (1H, m, H-14b), 1.63 (1H, m, H-12b), 2.68 (1H, m, H-10a), 2.97 (1H, m, H-10b), 3.59 (1H, d, J=15.6 Hz, H-2a), 3.71 (1H, d, J=15.6 Hz, H-2b), 4.82 (1H, m, H-15), 6.17 (1H, d, J=2.1 Hz, H-4), 6.27 (1H, d, J=2.1 Hz, H-6), 9.88 (1H, brs, OH-5), 9.88 (1H, brs, OH-7);13C NMR (DMSO- d6, 125 MHz) δC: 20.1 (CH3, C-16), 22.3 (CH2, C-11), 23.3 (CH2, C-13), 26.2 (CH2, C-12), 31.6 (CH2, C-14), 38.7 (CH2, C-2), 42.9 (CH2, C-10), 71.4 (CH, C-15), 101.6 (CH, C-6), 110.9 (CH, C-4), 119.6 (C, C-8), 135.2 (C, C-3), 157.4 (C, C-7), 159.2 (C, C-5), 170.2 (C, C-1), 206.0 (C, C-9).
3, 3′-Diindolylmethane ( 11): white powder; C17H14N2(Mw 246.12); UV (MeOH) λmax(log ε) 204 (5.09) nm, 207 (5.07) nm, 277 (4.51) nm;1H NMR (DMSO- d6, 500 MHz) δH: 4.43 (2H, s, H-8), 6.91 (1H, t, J=7.3, Hz, H-5), 6.91 (1H, t, J=7.3, Hz, H-5'), 7.00 (1H, t, J=7.3 Hz, H-6), 7.00 (1H, t, J=7.3 Hz, H-6'),7.14 (1H, s, H-2), 7.14 (1H, s, H-2'), 7.29 (1H, d, J=8.0 Hz, H-7), 7.29 (1H, d, J=8.0 Hz, H-7'),7.53 (1H, d, J=7.3 Hz, H-4), 7.53 (1H, d, J=7.3 Hz, H-4'),10.81 (1H, s, NH-1) 10.81 (1H, s, NH-1');13C NMR (DMSO- d6, 125 MHz) δC: 34.4 (CH2, C-8), 110.9 (CH, C-2), 110.9 (CH, C-2'), 117.6 (C, C-3), 117.6 (C, C-3'), 119.0 (CH, C-7), 119.0 (CH, C-7'), 120.3 (CH, C-6), 120.3 (CH, C-6'), 123.1 (CH, C-5), 123.1 (CH, C-5'), 127.9 (CH, C-4), 127.9 (CH, C-4'), 132.7 (C, C-3a), 132.7 (C, C-3a'), 136.0 (C, C-7a), 136.0 (C, C-7a').
Austinol ( 12): white powder; C25H30O8(Mw 458.19); [α]D25+441 ( c 0.17, DMSO); UV (MeOH) λmax(log ε) 248 (3.91) nm;1H NMR (DMSO- d6, 500 MHz) δH: 1.12 (3H, d, J=6.3 Hz, H-10'), 1.27 (3H, s, H-12), 1.31 (3H, s, H-15), 1.37 (1H, m, H-6a), 1.45 (3H, s, H-14), 1.53 (1H, m, H-6b), 1.57 (3H, s, H-9'), 1.63 (3H, s, H-13), 3.25 (1H, m, H-7a), 3.28 (1H, m, H-7b), 4.41 (1H, q, J=6.3 Hz, H-5'), 4.57 (1H, s, H-11), 5.14 (3H, s, H-1'a), 5.63 (1H, s, H-1'b), 5.80 (1H, d, J=5.6 Hz, OH-11), 6.00 (1H, d, J=9.8 Hz, H-2), 6.67 (1H, s, OH-6'), 6.73 (1H, d, J=9.8 Hz, H-1);13C NMR (DMSO- d6, 125 MHz) δC: 11.8 (CH3, C-10'), 13.9 (CH3, C-13), 20.6 (CH3, C-9'), 22.7 (CH3, C-14), 23.8 (CH3, C-12), 25.9 (CH3, C-15), 26.2 (CH2, C-6), 26.3 (CH2, C-7), 41.5 (C, C-8), 45.7 (C, C-5), 62.8 (C, C-7'), 73.7 (CH, C-11), 78.7 (CH, C-5'), 80.8 (C, C-6'), 85.3 (C, C-3'), 85.7 (C, C-4), 116.2 (CH2, C-1'), 118.3 (CH, C-2), 135.8 (C, C-9), 137.8 (C, C-2'), 138.4 (C, C-10), 148.0 (CH, C-1), 163.2 (C, C-3), 169.1 (C, C-4'), 171.8 (C, C-8').

Fig.2 Key COSY and HMBC correlations of Compound 1
Austin ( 13): white powder; C27H32O9(Mw 500.20); [α]D25+350 ( c 0.20, CHCl3); UV (MeOH) λmax(log ε) 243 (3.99) nm;1H NMR (CDCl3, 500 MHz) δH: 1.17 (3H, s, H-12), 1.27 (3H, d, J=6.3 Hz, H-10'), 1.35 (3H, s, H-15), 1.50–1.70 (2H, m, H-6), 1.52 (3H, s, H-14), 1.59 (3H, s, H-9'), 1.84 (3H, s, H-13), 2.00 (3H, s, H-17), 3.11 (1H, m, H-7a), 3.13 (1H, m, H-7b), 3.76 (1H, s, OH-6'), 4.41 (1H, q, J=6.3 Hz, H-5'), 5.46 (1H, s, H-1'a), 5.70 (1H, s, H-1'b), 6.00 (1H, s, H-11), 6.05 (1H, d, J=9.8 Hz, H-2), 6.64 (1H, d, J=9.8 Hz, H-1);13C NMR (CDCl3, 125 MHz) δC: 11.5 (CH3, C-10'), 15.5 (CH3, C-13), 20.3 (CH3, C-9'), 20.8 (CH3, C-17), 23.7 (CH3, C-12), 22.6 (CH3, C-14), 26.1 (CH3, C-15), 26.7 (CH2, C-7), 27.1 (CH2, C-6), 42.2 (C, C-8), 46.7 (C, C-5), 63.0 (C, C-7'), 74.9 (CH, C-11), 78.9 (CH, C-5'), 80.8 (C, C-6'), 84.3 (C, C-3'), 85.7 (C, C-4), 132.9 (C, C-9), 118.2 (CH2, C-1'), 120.3 (CH, C-2), 137.7 (C, C-2'), 143.8 (C, C-10), 146.8 (CH, C-1), 163.9 (C, C-16), 168.6 (C, C-3), 170.2 (C, C-4’), 171.0 (C, C-8').
Dehydroaustin ( 14): white powder; C27H30O9(Mw 498.19); [α]D25+138 ( c 0.13, CHCl3); UV (MeOH) λmax(log ε) 233 (3.51) nm;1H NMR (CDCl3, 500 MHz) δH: 1.30 (3H, s, H-12), 1.41 (1H, m, H-6a), 1.43 (3H, s, H-14), 1.48 (3H, s, H-15), 1.56 (3H, s, H-9'), 1.57 (3H, d, J=6.3 Hz, H-10'), 1.65 (1H, m, H-7a), 1.65 (1H, m, H-7b), 2.03 (3H, s, H-17), 2.09 (1H, m, H-6b), 5.29 (1H, q, J=6.3 Hz, H-5'), 5.65 (1H, s, H-11), 5.74 (3H, brs, H-13), 5.87 (1H, d, J=4.1 Hz, H-1'a), 5.88 (1H, d, J=9.8 Hz, H-2), 6.13 (1H, d, J=4.1 Hz, H-1'b), 6.96 (1H, d, J=9.8 Hz, H-1);13C NMR (CDCl3, 125 MHz) δC: 13.7 (CH3, C-10'), 17.2 (CH3, C-12), 19.3 (CH3, C-9'), 20.9 (CH3, C-17), 24.0 (CH3, C-14), 25.8 (CH3, C-15), 26.7 (CH2, C-7), 27.2 (CH2, C-6), 44.2 (C, C-8), 50.6 (C, C-5), 64.5 (C, C-7'), 74.3 (CH, C-11), 76.6 (CH, C-5'), 82.8 (C, C-3'), 85.1 (C, C-6'), 86.3 (C, C-4), 90.6 (C, C-9), 115.0 (CH2, C-1'), 116.2 (CH, C-2), 125.8 (CH3, C-13), 137.5 (C, C-2'), 139.5 (C, C-10), 151.1 (CH, C-1), 163.6 (C, C-3), 167.6 (C, C-4'), 168.7 (C, C-16), 169.3 (C, C-8').
2.5 Antimicrobial assay
The antimicrobial activities against six aquatic pathogens ( Aeromonas hydrophilia, EdwardsieIla ictarda, Edwardsiella tarda, Vibrio alginolyticus, Vibrio harveyi and Vibrio parahaemolyticus), and a human pathogenic bacterium ( Escherichia coli), as well as a plant-pathogenic fungus ( Colletot r ichum gloeosporioides Penz), were determined by a serial dilution technique using 96-well microtiter plates. Tested compounds and the positive controls (chloramphenicol for bacteria and amphotericin B for fungi) were dissolved in DMSO to give a stock solution.
3 RESULT AND DISCUSSION
Compound 1 was obtained as white powder with its molecular formula of C12H26O4based on high resolution electrospray ionization mass spectroscopy (HRESIMS) data (Supplementary Fig.S1), indicating no degrees of unsaturation. It showed a blue spot on TLC after spraying with anisaldehyde reagent. The1H and13C NMR data (Table 1) revealed six singlet methyls, three methylenes (with one oxygenated) and three oxygenated quaternary carbons.
The structure of Compound 1 was deduced from exhaustive analyses of the COSY and HMBC spectra. The correlations from H-3 to H-4 and from H-1' to 1'-OH were observed in the COSY spectrum, and a full set of all possible2J and3J HMBC correlations from H-1/H-7 to C-2 and C-3, from H-3 to C-1 and C-2, from H-4 to C-5, C-6 and C-8, from H-6/H-8 to C-5, from H-1' to C-2', C-3' and C-4', from H-3'/H-4' to C-1' and C-2', and from OH-2 to C-1 and C-7 allowed the construction of C-1 through C-8 and C-1' through C-4' (Fig.2). The peroxide bond between C-5 and C-2' was evident from chemical shifts for C-5 and C-2' at δC79.8 and 80.6, respectively (Ibrahim et al., 2008). On the basis of the above evidence, the structure of Compound 1 was determined, and the trivial name asperoxide A was assigned to this compound.
The antimicrobial activities of Compounds 1– 14 against human and aquatic bacteria as well as plant pathogenic fungi were tested. Compound 1 displayed activity against the aquatic pathogens A. hydrophilia, E. tarda, V. harveyi and V. parahaemolyticus with MIC values ranging from 16 to 64 μg/mL (Table 2). Moreover, Compound 4 showed potent inhibitory activity against the aquatic pathogen E. ictarda with MIC value of 2 μg/mL. In addition, this compound also showed activity against aquatic bacteria A. hydrophilia, E. tarda and V. harveyi as well as p lant-pathogenic fungus C. gloeosporioides Penz, each with MIC value of 4 μg/mL. While Compound 6 demonstrated activity against the human pathogenic bacterium E. coli, aquatic pathogen V. alginolyticus,and plant-pathogenic fungus C. gloeosporioides Penz, each with MIC value of 2 μg/mL. Compounds 2, 3, 5, 7, 8, 10, and 11 also have the inhibitory activities against various microbes, with MIC values ranging from 4 to 64 μg/mL (Table 2). Based on the above results, Compounds 4 and 6, which showed potent activities, might be used as leading compounds for further study to develop natural antimicrobial agents.
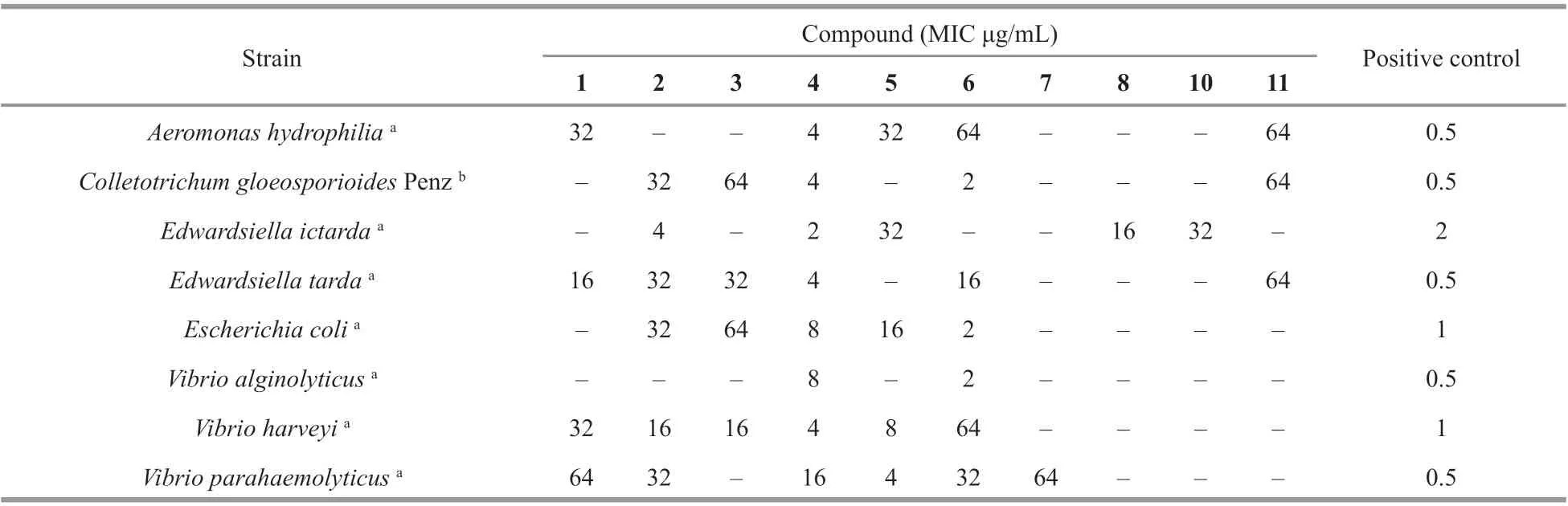
Table 2 Antimicrobial activities of Compounds 1–14 (MIC, μg/mL)
4 CONCLUSION
In this study, a new rarely described acyclic peroxide derivative, asperoxide A ( 1), along with 13 known secondary metabolites were isolated and identifi ed from the culture extract of cold-springderived fungus A. nidulans SD-531. The structures of these compounds were elucidated by detailed interpretation of NMR spectroscopic as well as mass spectrometry data analysis. Compounds 1–8, 10, and 11 exhibited potent antimicrobial activities against some of the tested strains with MIC values ranging from 2 to 64 μg/mL.
5 DATA AVAILABILITY STATEMENT
All the data supporting the results of this study are available within the article and the supplementary material.
杂志排行
Journal of Oceanology and Limnology的其它文章
- Review on observational studies of western tropical Pacifi c Ocean circulation and climate*
- Research progress of TiO 2 photocathodic protection to metals in marine environment*
- Smart anticorrosion coating based on stimuli-responsive micro/nanocontainer: a review*
- Status of genetic studies and breeding of Saccharina japonica in China*
- To be the best in marine sciences*
- A review of progress in coupled ocean-atmosphere model developments for ENSO studies in China*
