Role of Long Non-coding RNAs in Reprogramming to Induced Pluripotency
2020-07-29ShahzinaKanwalXiangpengGuoCarlWardGiacomoVolpeBaomingQinMiguelEstebanXichenBao
Shahzina Kanwal ,Xiangpeng Guo ,Carl Ward ,Giacomo Volpe ,Baoming Qin ,Miguel A.Esteban ,6,*,Xichen Bao
1Joint School of Life Sciences,Guangzhou Medical University and Guangzhou Institutes of Biomedicine and Health,Guangzhou 511436,China
2Key Laboratory of Regenerative Biology and Guangdong Provincial Key Laboratory of Stem Cells and Regenerative Medicine,Guangzhou Institutes of Biomedicine and Health,Chinese Academy of Sciences,Guangzhou 510530,China
3Laboratory of RNA,Chromatin,and Human Disease,Guangzhou Institutes of Biomedicine and Health,Chinese Academy of Sciences,Guangzhou 510530,China
4Guangzhou Regenerative Medicine and Health Guangdong Laboratory(GRMH-GDL),Guangzhou 510005,China
5Laboratory of Metabolism and Cell Fate,Guangzhou Institutes of Biomedicine and Health,Chinese Academy of Sciences,Guangzhou 510530,China
6Institute for Stem Cells and Regeneration,Chinese Academy of Sciences,Beijing 100101,China
7Laboratory of RNA Molecular Biology,Guangzhou Institutes of Biomedicine and Health,Chinese Academy of Sciences,Guangzhou 510530,China
KEYWORDS Mesenchymal-to-epithelial transition;X chromosome reactivation;Apoptosis;Proliferation
Abstract The generation of induced pluripotent stem cells through somatic cell reprogramming requires a global reorganization of cellular functions.This reorganization occurs in a multi-phased manner and involves a gradual revision of both the epigenome and transcriptome.Recent studies have shown that the large-scale transcriptional changes observed during reprogramming also apply to long noncoding RNAs(lncRNAs),a type of traditionally neglected RNA species that are increasingly viewed as critical regulators of cellular function.Deeper understanding of lncRNAs in reprogramming may not only help to improve this process but also have implications for studying cell plasticity in other contexts,such as development,aging,and cancer.In this review,we summarize the current progress made in profiling and analyzing the role of lncRNAs in various phases of somatic cell reprogramming,with emphasis on the re-establishment of the pluripotency gene network and X chromosome reactivation.
Introduction
High-throughput sequencing techniques have demonstrated that mammalian genomes are ubiquitously transcribed[1].The transcription of large numbers of non-coding RNAs(ncRNAs)[2]might help to explain interspecies differences despite limited variations in the number and sequence of coding genes.ncRNAs are classified based on their length into small RNAs(<200 nucleotides),e.g.,microRNAs(miRNAs)and piwi-interacting RNAs (piRNAs), and long ncRNAs(lncRNAs,>200 nucleotides)[3,4].
lncRNAs are typically transcribed by RNA polymerase II,although this process occurs with different modalities and origins.They include circular RNAs(circRNAs)[5],enhancer RNAs(eRNAs)[6],antisense transcripts[7],and long intergenic ncRNAs(lincRNAs)[8,9].These lncRNAs recruit epigenetic regulators to chromatin and serve as scaffolds to stabilize protein complexes or as decoys for proteins and miRNAs[10-12].These diverse regulatory modes are due to the complementary base pairing of lncRNAs with both DNA and other RNAs,the ability of lncRNAs to interact with proteins,and the localization of lncRNAs in different cellular compartments(e.g.,cytoplasm,nucleus,or mitochondrion)[13,14].Interestingly,a large fraction of lncRNAs also associate with ribosomes and, paradoxically, some previously annotated lncRNAs produce small peptides with biological functions[15,16].The latter raises relevant questions regarding the true nature of some lncRNAs,but the general significance of these findings requires further investigation.
Although the functional relevance of lncRNAs has not yet been systematically explored,some are known to regulate key aspects of normal and pathological cellular functions such as proliferation[17],metabolism[18,19],and epithelial cytoarchitecture[20].The recent discovery that lncRNAs display cellspecific and development-specific expression patterns has also suggested a pivotal role of lncRNAs in cell fate determination[21-23].In support of this idea,suppression of several embryonic stem cell(ESC)-specific lincRNAs influences pluripotency maintenance/exit and early lineage commitment[21].Similarly,the heart-associated lncRNA Braveheart serves as a key player in the activation of the core vascular gene network during mouse cardiac cell fate specification[22].In addition,lincRNA Yin Yang 1(linc-YY1),which is highly conserved in humans,regulates myogenesis through dislodgement of the YY1/polycomb repressive complex on target promoters,resulting in the activation of muscle gene expression[23].
Here,we summarize the current knowledge of lncRNA profiling and functions in an extreme scenario of cell fate transition,somatic cell reprogramming.
lncRNAs are new players in somatic cell reprogramming
Mammalian somatic cells can be reprogrammed to an ESC state and this has revolutionized stem cell research[24,25].Originally, the reprogramming factor cocktail contained SOX2,KLF4,OCT4(encoded by Pou5f1 or POU5F1 gene in mice or humans,respectively),and c-MYC(SKOM),but other combinations of exogenous factors are also effective[26,27]. More recently, mouse reprogramming has been achieved using only chemicals [28]. Remarkably, induced pluripotent stem cells(iPSCs)provide a priori unlimited number of individual-specific stem cells that can be used for in vitro disease modeling, drug screening, and potential cell-based therapies[29,30].Although producing iPSCs from different cell sources and mammalian species is in general no longer an issue[31-34],the underlying mechanisms remain unclear and clarifying them is important for improving iPSC quality[35].
Reprogramming occurs through a stepwise process that involves reorganization of most cell functions,culminating in the reactivation of the pluripotency gene program[36,37].This conversion is characterized by several roadblocks and checkpoints.For example,reprogramming cells must overcome the senescence/apoptosis barrier to acquire the ability to proliferate indefinitely[38,39],switch their metabolism from oxidative phosphorylation to glycolysis [40], and undergo a mesenchymal-to-epithelial transition(MET)[41,42].By the end of these events,under standard conditions,only a small percentage of the original population activates the endogenous pluripotency gene circuitry.
Since the first demonstration of somatic cell reprogramming,multiple regulatory factors have been identified.Among these,miRNAs play essential roles in creating or removing reprogramming roadblocks[43,44].For example,components of miRNA cluster 302-367 suppress Tgfbr2 to neutralize the pro-mesenchymal effects of TGFβ cytokines secreted by somatic cells or present in serum, facilitating the MET[45,46].miRNA cluster 302-367 also targets genes encoding chromatin regulators(e.g.,BAF170)that prevent the activation of pluripotency genes in the late phase of reprogramming[46].Conceivably,lncRNAs,which are present in much higher numbers than miRNAs and are in principle more versatile players in cell regulation,could be important regulators of reprogramming too.In this regard,lncRNAs also experience phase-dependent changes during reprogramming,and their regulation shares the same epigenetic mechanisms that drive mRNA changes in reprogramming[47,48].These findings suggest a coordinated role between protein-coding and ncRNAs in reorganizing cellular functions.Studying lncRNAs is,therefore,important to understand reprogramming as a whole(Table 1).Such knowledge may also contribute to clarifying the role of lncRNAs in other contexts such as development,aging,and cancer.
p53-regulated lncRNAs in reprogramming
Proliferation facilitates the appearance of stochastic events needed for directing chromatin reorganization into a pluripotent state during reprogramming[38].However,somatic cells have a limited life span/proliferation capacity,and reprogramming is a stressful process involving activation of senescence/-cell death pathways.Accordingly,suppressing p53,p16Ink4a,or p19Arfenhances reprogramming efficiency by accelerating proliferation and/or reducing apoptosis[39,49,50].
Interestingly,several p53-regulated lncRNAs have strong impacts on reprogramming efficiency(Figure 1).For instance,expression of lincRNA-RoR(regulator of reprogramming)[51]and lincRNA-p21[52,53]is induced by p53[54,55],which can influence reprogramming in positive and negative manner,respectively. Similarly, expression of p53-repressedLNCPRESS1(lncRNA p53-regulated and ESC-associated 1)is robustly induced during reprogramming and activates the pluripotency network[56].Given these circumstances,it would be useful to systematically profile lncRNAs during reprogramming in the presence or absence of p53 or other pro-senescence regulators.
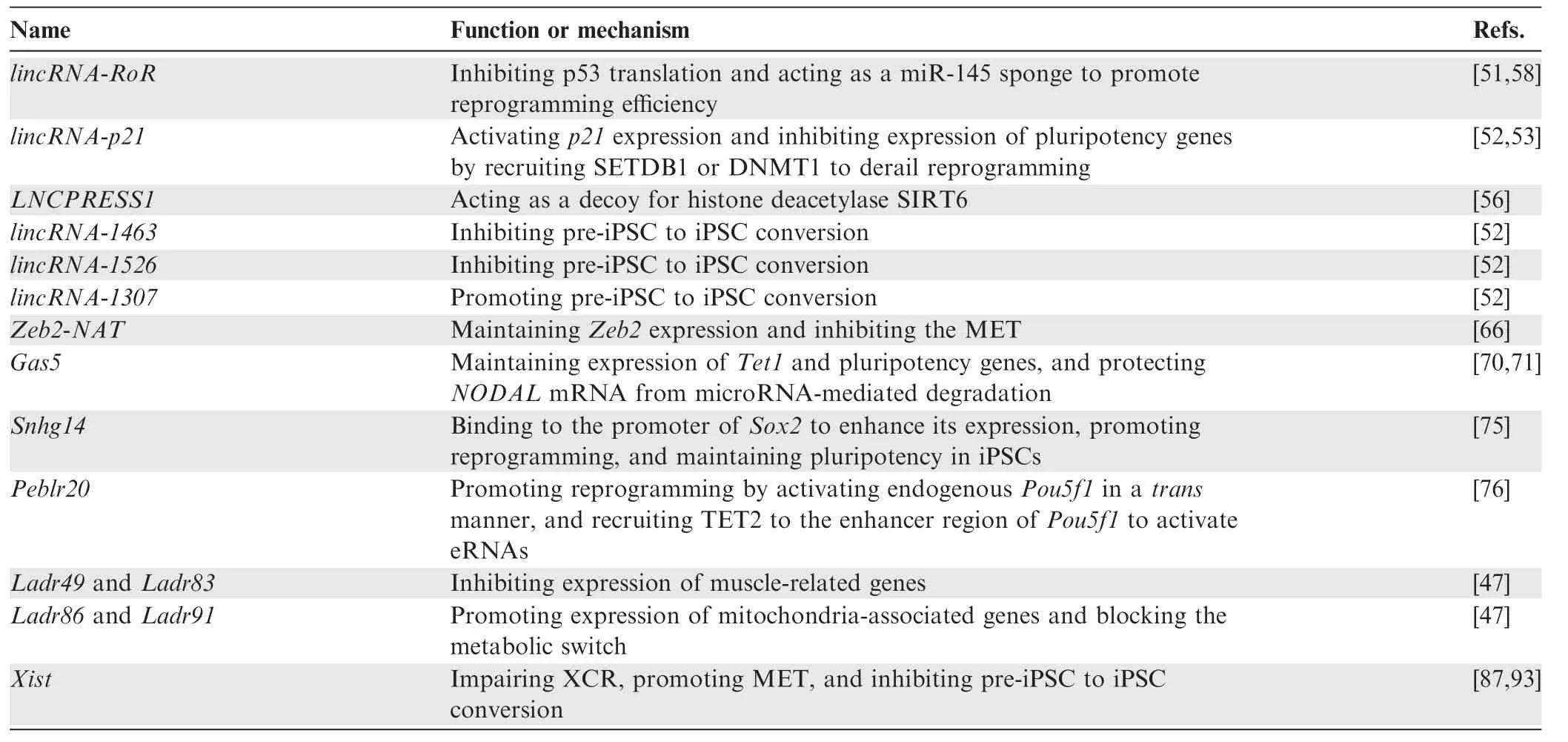
Table 1 lncRNAs and their functions in somatic cell reprogramming
lincRNA-RoR
lincRNA-RoR(2603 nucleotides)is located on chromosome 18q21.31.Its expression is induced by p53[54]and facilitates human reprogramming by suppressing the p53-mediated transcriptional response to oxidative stress and DNA damage[51].However,overexpression or knockdown of lincRNA-RoR does not influence cell growth at the early stages of reprogramming.In cancer cells, lincRNA-RoR suppresses p53 translation through heterogeneous nuclear ribonucleoprotein I(hnRNP I),a classical RNA-binding protein(RBP)involved in splicing[54].In this regard,a fraction of hnRNP I is localized in the cytoplasm,where it promotes the translation of p53 through binding to internal ribosome entry sites.Apart from suppressing the p53 pathway, lincRNA-RoR sequesters prodifferentiation miRNAs,including miR-145,to maintain the expression of the core pluripotency transcription factors OCT4,SOX2,and NANOG in human ESCs[57,58].Although these two latter mechanisms have not yet been tested in reprogramming,it is likely that they also contribute to the effects of lincRNA-RoR in this process.Notably,lincRNA-RoR is more highly expressed in human iPSCs than in ESCs[51],which may be a consequence of a selective advantage conferred during reprogramming.
lincRNA-p21
lincRNA-p21 (3121 nucleotides, located on chromosome 6p21.2)resides 5 kb upstream of p21.It was originally discovered as an executioner of the p53-mediated apoptotic response[55].Expression of lincRNA-p21 is induced by p53 and impairs mouse reprogramming,but different cis or trans modes of action have been proposed[52,53].The cis-acting model is based on the observation that conditional excision of the lincRNA-p21 promoter and first exon reduces the expression of p21 mRNA,resulting in enhanced proliferation and reprogramming efficiency based on alkaline phosphatase activity(an early marker of reprogramming)[53].However,it is important to note that removal of the lincRNA-p21 locus leads to the loss of multiple enhancers that control the transcription of nearby genes,including p21 itself,independently of lincRNA-p21[59].In fact,removal of the lincRNA-p21 locus alters the expression of nearby genes even in tissues with no detectable lincRNA-p21 transcript.Conversely,the trans-acting model of lincRNA-p21 in reprogramming proposes that lincRNA-p21 impairs reprogramming independently of proliferation by binding to pluripotency loci(e.g.,Nanog)and blocking their reactivation[52].In this model,lincRNA-p21 represses pluripotency loci by recruiting the histone 3 lysine 9(H3K9)methyltransferase SETDB1 or the maintenance DNA methyltransferase DNMT1.Binding of lincRNA-p21 to these epigenetic regulators is mediated by the classical RBP hnRNP-K.Hence,suppressing hnRNP-K also results in enhanced reprogramming efficiency, although it is unlikely that it acts exclusively through lincRNA-p21. The idea that expression of p53-induced lncRNAs such as lincRNA-p21 prevents reprogramming independent of proliferation or apoptosis is attractive,as this may have implications for the control of cell fate when stem cells are under stress or in aging and in p53-negative cancers.
In a screen for lncRNAs modulating the conversion of preiPSCs to iPSCs,lincRNA-p21 was identified as a negative regulator of reprogramming[52].Pre-iPSCs are stable but incompletely reprogrammed clones,and their conversion to iPSCs is commonly used as a proxy for the late phase of reprogramming[60].The same screen also identified lincRNA-1463 and lincRNA-1526 as negative regulators of the conversion of pre-iPSCs to iPSCs.However,it is unclear whether expression of these two lncRNAs is also regulated by p53.
LNCPRESS1
Another screening study conducted on differentiating human ESCs led to the discovery of LNCPRESS1(832 nucleotides,located on chromosome 7q22.1). LNCPRESS1 is a p53-repressed lncRNA that positively regulates the pluripotency gene network in human ESCs[56].LNCPRESS1 acts as a decoy for the histone deacetylase SIRT6,thereby enriching the activating H3K56/K9 acetylation marks at pluripotency loci. Interestingly, expression of LNCPRESS1 is strongly induced during reprogramming[56],suggesting a potential role of LNCPRESS1 in improving reprogramming efficiency.
Other lncRNAs regulating reprogramming
In addition to lncRNAs regulated by p53, several other lncRNAs have been identified which can regulate somatic cell reprogramming through MET,metabolic switch,or reactivation of the pluripotency gene network(Figure 1).ESCs are epithelial-like,therefore,reprogramming of mesenchymal-like cells such as fibroblasts to iPSCs unavoidably involves the acquisition of an epithelial phenotype.This occurs in the early phase of reprogramming through MET [41,42,61]. Since pluripotent cells and somatic cells have different metabolic profiles, reprogramming, logically, also requires metabolic remodeling.iPSCs exhibit a metabolic shift from oxidative phosphorylation to glycolysis, which is associated with a decreased number and complexity of mitochondria.Importantly,blocking MET or the metabolic switch derails reprogramming [41,42,62], but not every reprogramming intermediate that successfully passes through these checkpoints achieves full activation of the pluripotency gene network. Likewise, even when somatic cells have been successfully reprogrammed to iPSCs,their DNA methylation patterns often do not faithfully reflect those of ESCs.For example, iPSCs can show aberrant DNA methylation of imprinted regions,which alters the expression of lncRNAs encoded by those regions[63-65].
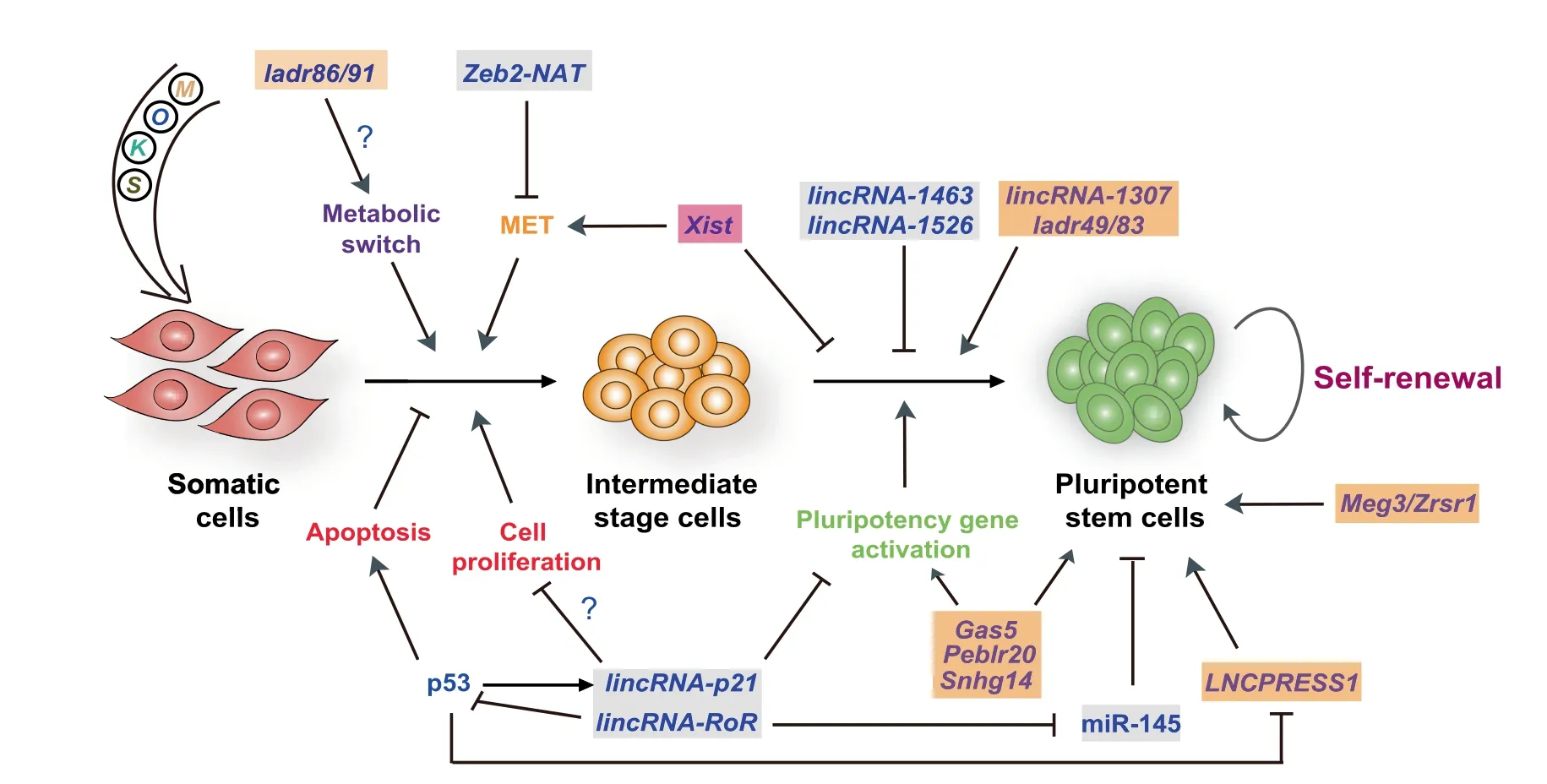
Figure 1 lncRNAs regulating somatic cell reprogramming
Zeb2-NAT
Zinc finger E-box binding homeobox 2-natural antisense transcript(Zeb2-NAT,430 nucleotides,located on chromosome 2q22.3)is a natural antisense transcript for the transcription factor ZEB2 [66]. ZEB2 is a master regulator of the epithelial-to-mesenchymal transition (EMT) that represses the expression of many epithelial genes[67].Zeb2-NAT is involved in maintaining Zeb2 expression by preventing the splicing of the Zeb2 5′-untranslated region(5′-UTR)[66].Interestingly,Zeb2-NAT is highly expressed in fibroblasts from aged mice[68],which are known to be less amenable to reprogramming[69].The reprogramming efficiency of these aged fibroblasts is enhanced when expression of Zeb2-NAT is reduced. Given that aging promotes the accumulation of DNA damage,thereby activating p53,it is plausible that expression of Zeb2-NAT is also induced by p53 in aged fibroblasts and during reprogramming.However,this speculation has not yet been tested.
Gas5
Expression of the lncRNA growth arrest specific 5(Gas5,656 nucleotides,located on chromosome 1q25.1)is controlled by pluripotency transcription factors.Gas5 plays a pivotal role in reprogramming and self-renewal of mouse ESCs by maintaining the expression of genes encoding key pluripotency factors and ten-eleven translocation 1(Tet1)[70].The effect of Gas5 on Tet1 expression suggests that Gas5 regulates active DNA demethylation in reprogramming.Its human ortholog,GAS5,also controls human ESC pluripotency by protecting NODAL mRNA from miRNA-mediated degradation[71],a mechanism that may also participate in human reprogramming.GAS5 is also a well-known regulator of cell proliferation and apoptosis in various cell contexts,including breast cancer,lung cancer,and differentiating mouse ESCs[70,72,73].
Ladr lncRNAs
Single-cell transcriptomic analysis of mouse reprogramming has unveiled numerous lncRNAs that are activated or repressed during this process[47].Among them,knockdown of two lncRNAs activated during reprogramming 49 and 83(Ladr49 and Ladr83),showed modest effects on reprogramming efficiency,but led to the upregulation of muscle-related genes in reprogramming intermediate cells.Interestingly,these two lncRNAs have been previously shown to physically associate with polycomb repressive complex 2(PRC2)[21,74].On the other hand,depletion of Ladr86 and Ladr91,which show upregulated expression in reprogramming, represses mitochondria-associated genes,suggesting a role in the metabolic switch during reprogramming.
Promoter/enhancer-interacting lncRNAs
A recently devised approach chromatin-RNA in situ reversetranscription sequencing(CRIST-seq)took advantage of the specificity of the clustered regularly interspaced short palindromic repeats(CRISPR)/CRISPR-associated 9(Cas9)system for DNA to profile pluripotency-specific lncRNAs that interact with the promoter of the core pluripotency genes Sox2 and Pou5f1[75].Among the identified lncRNAs interacting with the Sox2 promoter, 59 of them were differentially expressed in reprogramming.Notably,overexpression of one of these differentially expressed lncRNAs,Sox2 promoterinteracting lncRNA 14(Spilr14,also known as Snhg14),led to significant enhancement of reprogramming efficiency,whereas its knockdown caused loss of pluripotency in iPSCs.Another lncRNA, Pou5f1 enhancer-binding lncRNA 20(Peblr20),was identified utilizing a strategy combining RNA reverse transcription-associated capture sequencing (RATseq)and RNA sequencing[76].Peblr20 is expressed at higher levels in iPSCs than in mouse embryonic fibroblasts and promotes reprogramming by activating endogenous Pou5f1 in trans through the recruitment of TET2 to the enhancer region,which enhances the expression of eRNAs.
Imprinted lncRNAs
Maternally expressed 3(Meg3,also known as Gtl2)is localized at the imprinted Dlk1-Dio3 gene cluster on mouse chromosome 12qF1,which also encodes numerous miRNAs[77,78].ncRNAs harbored in this region are maternally expressed in mammals.Interestingly,they are strongly repressed in most mouse iPSCs compared to ESCs,which is responsible for the failure to support the development of all-iPSC mice[63,79].This inability can be reversed through reactivation of the imprinted Dlk1-Dio3 locus in reprogramming using the histone deacetylase inhibitor valproic acid or ascorbic acid(vitamin C, Vc) [63,80,81]. Vc facilitates the conservation of imprinting at this locus by interfering with reprogramming factor-induced loss of H3K4 methylation,which prevents the recruitment of DNA methyltransferase 3A (DNMT3A).Importantly,MEG3,which is encoded in the DLK1-DIO3 locus,is also frequently silenced in human iPSCs[82].Similarly,hypomethylation of the maternally expressed imprinted lncRNA zinc finger(CCCH type),RNA binding motif,and serine/arginine rich 1 (Zrsr1) is associated with reduced pluripotency in mouse iPSCs,which could not be rescued by treating reprogramming cells with Vc or PD0325901 and CHIR99021(inhibitors of MEK1/2 and GSK3,respectively)[64].It remains to be clarified how these imprinted lncRNAs regulate the reprogramming process.
X chromosome reactivation during reprogramming
In the late stage of reprogramming,another important process involving lncRNAs is X chromosome reactivation (XCR)(Figure 2). During the development of mice and other mammals, one of the two X chromosomes in females is randomly silenced in somatic cells shortly after implantation to maintain the dosage equivalence between the sexes[83,84].X chromosome inactivation(XCI)is initiated by the expression of X-inactive specific transcript(Xist),a 17,918-nucleotide lncRNA localized in the X chromosome inactivation center(XIC)[85].Xist coats the X chromosome,which gradually removes RNA polymerase II and active histone marks such as H3K4 trimethylation.This is followed by a sequential gain of diverse repressive marks including H3K27 trimethylation (H3K27me3), macroH2A.1 histone(macroH2A),and DNA methylation on the inactivated X chromosome(Xi).Consequently,Xi is silenced throughout life with remarkable stability.Despite possessing a stable nature,Xi can be reactivated in specific contexts such as primordial germ cell differentiation,somatic cell nuclear transfer(SCNT),and somatic cell reprogramming[86].Due to its ease and reproducibility, reprogramming provides an unprecedented tool for characterizing the events involved in XCR[87,88].
A relevant question in the field of reprogramming is the order of the epigenetic events leading to XCR. Highresolution single-cell time course analyses of reprogramming using immunofluorescence and RNA fluorescence in situ hybridization(FISH)[87,89]demonstrate that XCR occurs in the following order:(1)recruitment of H3K27 methyltransferase EZH2 to Xi(XiEZH2+)in E-cadherin(CDH1)positive(CDH1+)cells,(2)activation of NANOG in XiEZH2+cells,(3)loss of EZH2,H3K27me3,Xist coating,and macroH2A1 on Xi in NANOG+cells,and(4)removal of DNA methylation on Xi and activation of the transcribed antisense to Xist(Tsix).Therefore,except for DNA demethylation,the events of XCR in reprogramming follow the inverse order of the events of XCI in development. Notably, the removal of DNA methylation during XCR in reprogramming is TETindependent and hence passive,and the expression of Tsix is paradoxically dispensable.
Conspicuously,despite the initiation of XCR in NANOG+cells,full reactivation of the core pluripotency circuitry precedes XCR in reprogramming.Accordingly,XCR serves as one of the crucial standards for bona fide mouse iPSC identification[90].This is consistent with the observation that the pluripotency factor PRDM14 represses Xist expression[91].Although ectopic expression of Xist impairs XCR in reprogramming,its depletion does not affect XCR or reprogramming efficiency [87]. This is inconsistent with the observations in SCNT, the efficiency of which is greatly improved following Xist elimination[92].A possible explanation could be that Xist plays opposite roles at different stages of somatic cell reprogramming,as SCNT has no obvious phases.In support of this idea,Xist depletion impairs the MET in the early phase of reprogramming but significantly improves the conversion of pre-iPSCs to iPSCs[93].Interestingly,the reprogramming booster Vc[81]promotes XCR in reprogramming by preventing the relocalization of macroH2A onto Xi[93].This is likely mediated by the boosting effect of Vc on H3K27me3 demethylases[94,95].It remains to be determined whether Vc also acts through unrelated mechanisms,for example by decreasing the levels of N6-methyladenosine(m6A)deposition on Xist,as Vc is also a cofactor for another two dioxygenases,the alpha-ketoglutarate-dependent dioxygenase fat mass and obesity-associated protein (FTO) and AlkB homolog 5(ALKBH5),which are responsible for erasing this epitranscriptomic mark[96-98].
Notably,the aforementioned mechanisms have been investigated in mouse cell reprogramming,but it is unclear whether the same principles apply to human cells.In this regard,XCR is either absent or unstable in female donors when human iPSCs are generated using standard culture conditions[99],but is present in reprogramming to naı¨ve pluripotency[100].Further studies are needed to understand the differences and similarities between XCR in mouse and human cell reprogramming.
Perspectives
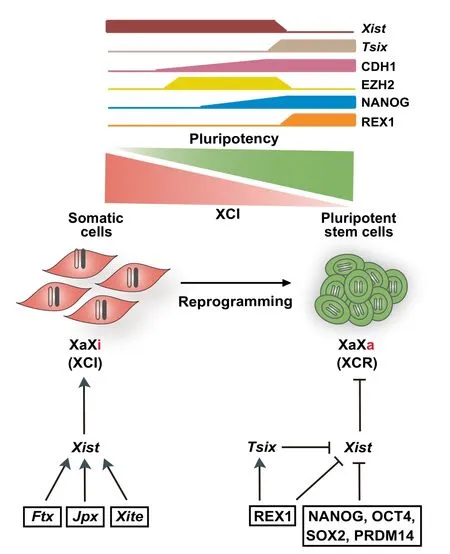
Figure 2 X chromosome reactivation in somatic cell reprogramming
It is becoming increasingly evident that lncRNAs are critical players in cell fate regulation.Few lncRNAs,mostly related to p53 or cell senescence,have been well studied thus far,but the repertoire of lncRNAs that regulate pluripotency/reprogramming is likely large.Systematic profiling is needed for functional characterization of these lncRNAs.This could be facilitated by developing highly efficient/deterministic reprogramming systems[101],as low-efficiency protocols are prone to cell heterogeneity and the cell reprogramming kinetics are asynchronous,which complicates the interpretations.In addition, specialized high-throughput RNA sequencing approaches such as global nuclear run-on sequencing(GROseq)and sequencing with RNase R treatment are necessary to enrich for certain lncRNAs such as eRNAs and circRNAs[102,103],respectively,since they are rarely detected using conventional RNA sequencing methodologies.In this regard,several circRNAs exhibit human iPSC/ESC-specific expression[104,105].Among them,circBIRC6 and circCORO1C positively regulate pluripotency maintenance and reprogramming[104]. circBIRC6 acts as a sponge for differentiationmediated miRNAs,whereas the mechanism underlying circCORO1C regulation is unknown.An important consideration is that functional lncRNA screens with short hairpin RNAs(shRNAs)or small interfering RNAs(siRNAs)have inevitable limitations because many lncRNAs are localized in the nucleus,where shRNAs/siRNAs cannot effectively silence transcripts.As a solution,CRISPR/Cas9-based screening systems assisted by comprehensive computer prediction algorithms could be used to increase on-target efficiency and minimize off-target effects.This approach requires eliminating the whole DNA fragment(rather than introducing a frame shift for coding genes),potentially in the promoter region,to suppress expression/functionality of lncRNAs without affecting the nearby genes[106].CRISPR/Cas13 and CRISPR interference are potential alternatives,as they can be used to edit RNA or repress gene expression without altering the genome[107,108].Collectively,the characterization of lncRNAs in reprogramming will not only help to uncover new layers of regulation for the diverse pathways modulating reprogramming but may also have implications in other types of cell fate transitions.
Competing interests
The authors have declared no competing interests.
Acknowledgments
We thank all members of the Esteban lab for their support.Research in the authors’laboratories is supported by the National Key R&D Program of China (Grant Nos.2016YFA0100701,2016YFA0100102,2018YFA0106903,and 2016YFA0100300);the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No.XDA16030502);the Youth Innovation Promotion Association of the Chinese Academy of Sciences(Grant No.2015294),the National Natural Science Foundation of China(Grant Nos.31671537, 31571524, and 31501192); the Natural Science Foundation of Guangdong Province (Grant No.2018B030306042),the Guangdong Province Science and Technology Program (Grant Nos. 2014A030312001,2016A050503037, 2016B030229007, and 2017B050506007),the Science and Technology Planning Project of Guangdong Province (Grant No. 2017B030314056), the Pearl River Science and Technology Nova Program of Guangzhou(Grant No.201610010107),and the Guangzhou Science and Technology Program(Grant No.201807010066),China.SK is supported by a President’s International Fellowship Initiative program from the Chinese Academy of Sciences and CW is supported by a Pearl River Overseas Young Talents Postdoctoral Fellowship.
ORCID
0000-0003-4694-136X(Kanwal S)
0000-0002-3323-1134(Guo X)
0000-0003-0889-9025(Ward C)
0000-0001-5000-6951(Volpe G)
0000-0001-7652-161X(Qin B)
0000-0002-1426-6809(Esteban MA)
0000-0003-0389-4233(Bao X)
杂志排行
Genomics,Proteomics & Bioinformatics的其它文章
- Acknowledgments to Reviewers 2019
- SinoDuplex:An Improved Duplex Sequencing Approach to Detect Low-frequency Variants in Plasma cfDNA Samples
- GPS 5.0:An Update on the Prediction of Kinase-specific Phosphorylation Sites in Proteins
- MSIsensor-pro:Fast,Accurate,and Matchednormal-sample-free Detection of Microsatellite Instability
- Procleave:Predicting Protease-specific Substrate Cleavage Sites by Combining Sequence and Structural Information
- Epitranscriptomic 5-Methylcytosine Profile in PM2.5-induced Mouse Pulmonary Fibrosis
