Flux Growth of Tungsten Oxychloride Li23CuW10O40Cl5
2020-07-28LIShufangZHAOShuangLIManrong
LI Shufang, ZHAO Shuang, LI Manrong
Flux Growth of Tungsten Oxychloride Li23CuW10O40Cl5
LI Shufang, ZHAO Shuang, LI Manrong
(Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, School of Chemistry, Sun Yat-Sen University, Guangzhou 510275, China)
Mixed anion compounds can generate the emergence of novel properties that differ from those with mono-type anion due to the difference of electronegativities, ionic radii, polarizabilities, and oxidation states between unlike anions. Abundant research has been conducted on metallic mixed-anion materials with potential application in electronics, detectors of moisture, gas sensors, electrodes for solar batteries,The flux method has been widely applied for mixed-anion crystal growth, which based on metathetical reaction with appropriate metal-salts flux under mild conditions. It is meaningful to synthesize the mixed anion compounds by the flux method. Singlecrystals of tungsten oxychloride Li23CuW10O40Cl5were preparedCuCl2flux-growth method by two steps, which using high quality and phase-pure polycrystalline Li4WO5as precursor. The crystal structure was determined by single-crystal X-ray diffraction analysis, which indicates that Li23CuW10O40Cl5crystallizes in P63/mcm space group (= 1.02846(3) nm,=1.98768(9) nm,=1.82076(11) nm3, and=2). There are crystallographically independent five Li, two W, one Cu, two Cl, and five O atoms in the unit cell, where W(1) atoms are coordinated with one Cl and five O atoms in a distorted octahedra geometry, while W(2) atoms are connected with four O atoms in a tetrahedral coordination. The Cu atoms are connected with six O atoms forming [CuO6] octahedra. Thus, the crystal structure of the titled compound consists of [CuO6] and [W(1)O5Cl] octahedra, and [W(2)O4] tetrahedra. The successful synthesis oftungsten oxychloride Li23CuW10O40Cl5through flux-growth method is meaningful for explore new mixed anion compounds in future.
tungsten oxychloride; CuCl2flux; crystal structure; X-ray diffraction
Mixed anion compounds, especially their unique structures and excellent physical properties, have been extensively studied and have great applications in military and civilization. They have attracted abundant attention since the difference of electronegativities, ionic radii, polarizabilities, and oxidation states between unlike anions can generate the emergence of novel properties that differ from those with mono-type anion[1-4]. The active research of metallic mixed-anion materials with potential application in electronics, detectors of moisture, gas sensors, electrodes for solar batteries,has been realized in several types of crystalline and thin film materials[5]. It is meaningful to search a suitable method to synthesize aforementioned compounds. The main hotpot to research this kind of compounds lies in how to control the arrangement of anions to refine their electronic structures, such as the two-dimensional quantum antiferromagnetism in Sr2CuO2Cl2with trans-configuration of Cl ions in the CuO4Cl2octahedra[6].
Recently, it has been reported that incorporation of halides into oxides can strikingly change their electronic structures and modify the physical properties[7-8]. Several novel transition-metal oxychlorides have been reported to date, such as MnSb4O6Cl2[9], PbCu2(SeO3)2Cl2[10], Cu3Bi(SeO3)2O2Cl[11], FeTe2O5X (X=Cl, Br)[12], SrCu2(SeO3)2Cl2[13], SmSb2O4Cl[14], and MSb2O3(OH)Cl (M=Co, Fe, Mn)[15]. Above-mentioned materials show novel structures and special magnetic properties due to their diversity structural which was more helpful to generate the magnetic ordering during low temperature. This was especially manifested in layered transition metal oxyhalide FeTe2O5X (X=Br, Cl), where the layers are built by [FeO6] octahedra, and then forming the [Fe4O16]20–units which were linked[Te4O10X2]6–anionic groups. The magnetic properties are reported within a cluster approach of antiferromagnetically coupled tetramers including spin frustration and a ferromagnetic inter- tetramer interaction[12].
The flux method widely applied for mixed-anion crystal growth is based on metathetical reaction with appropriate metal-salts flux under mild conditions. Experimentally, zone melting and hydrothermal synthesis methods are all comparatively complex and expensive for growing single crystals, and the flux method is at present one of the most economic and convenient methods for mixed anions compounds[16]. In this study, we report the single crystal growth of tungsten oxychloride Li23CuW10O40Cl5in copper chloride (CuCl2, melting point of 498 ℃) flux, and analyze its crystal in details.
1 Experimental
1.1 Materials and methods
The tungsten oxychloride Li23CuW10O40Cl5was synthesized through flux method in open-end quartz tubes. And the synthesis of Li23CuW10O40Cl5adopt a two-step process. Firstly, precursor polycrystalline Li4WO5were synthesized by reacting high-purity reagents in solid- state method. Li2CO3and WO3were mixed with the mole ratio of 2:1. Then these raw materials were placed in a covered alumina crucible and calcinated at 890 ℃ for 12 h as described in reference[17]. Then, single crystal of Li23CuW10O40Cl5was grown from CuCl2flux and precursor Li4WO5. Li4WO5polycrystalline samples and ten times excess CuCl2was loaded into an open-end quartz tube, and put into a vertical pit furnace. The raw mixture was heated at 873 K for 2 d, and then cooling down to 573 K with a cooling rate of 5 K/h before shutting down the furnace.
Finally, the reaction products were washed by hot demineralized water to eliminate the fluxing agents. After subsequent drying at 353 K, yellow, block shaped single crystals of the desired products were grown and suitable for subsequent single-crystal X-ray diffraction measurements.
1.2 Single-crystal structure determination
Block-shaped single crystal of Li23CuW10O40Cl5was selected for single-crystal diffraction measurements. The R-AXIS Spider CCD diffractometer were used to collect data equipped with the graphite monochromated Mo Kradiation (λ=0.071073 nm) at 293 K.
The structure of Li23CuW10O40Cl5was determined through direct method and refined by full-matrix least- squared methods on2with SHELXL package[18]. ADDSYM/PLATON was performed to studied with the final structure for additional symmetry, and no other missed or higher symmetry was found[19]. Crystallographic data (including structure factors) for the structures in this study have been deposited with the Cambridge Crystallographic Data Centre, CCDC, 12 Union Road, Cambridge CB21EZ, UK.Copies of the data can be obtained free of charge on quoting the depository numbers CCDC-1952905.
2 Results and discussion
The tungsten oxychloride Li23CuW10O40Cl5synthesized from CuCl2flux in a vertical pit furnace crystallizes hexagonally in space group of P63/mcm with the unit cell parameters of=1.02846(3) nm,=1.98768(9) nm, and=2. There are crystallographically independent five Li, two W, one Cu, two Cl, and five O atoms in the unit cell, respectively, where W(1) atoms are coordinated with one Cl and five O atoms in a distorted octahedra geometry, while W(2) atoms are connected with four O atoms in a tetrahedral coordination. The Cu atoms are connected with six O atoms forming [CuO6] octahedra.
Every two W(2)O4tetrahedra are in reverse symmetry along thedirection (Fig. 1 and 3). Three [W(1)O5Cl] octahedra are connected with each othersharing one Cl atom, and three O atoms with [CuO6] octahedra to form the [W(1)6CuO24Cl2] unit (Fig. 2). The three-dimensional (3D) structure of Li23CuW10O40Cl5is assembled by the [W(1)6CuO24Cl2] units sharing O and Cl atoms with Li. The Li atoms in Li23CuW10O40Cl5present four kinds of environments (Fig. 4): Li(1) atoms are connected with two Cl atoms and four O atoms and Li(2) with one Cl atom and five O atoms, while Li(3) and Li(4) atoms are coordinated with six O atoms, and Li(5) atoms are surrounded by three Cl and four O atoms.
In Li23CuW10O40Cl5, the W−O distances ranging from 0.1778(9) to 0.2154(5) and the bond lengths of W−Cl are 0.2141(5) nm, which are comparable to those in Ba3WO5Cl2[20], K2W3O10[21], WCl6[22], and WOCl4[23], the Cu-O distances are 0.1992(8) nm in good agreement with Ba3Cu2O4Cl2[23], and BaCuSi2O6[24], from bond valence sums (BVS, Table 3) calculations in Li23CuW10O40Cl5, Cu displays +2 formal oxidation states according to charge balance.
The Li−O and Li−Cl distances range from 0.2012(6) to 0.249(3) nm and 0.248(3) to 0.295(4) nm, which are closed to those in Li2MnCl4[25], Li2ZnCl4[26], LiWCl6[27], Li2CaTa2O7[28]. The crystallographic data and structuralrefinements for Li23CuW10O40Cl5are summarized in Table 1.
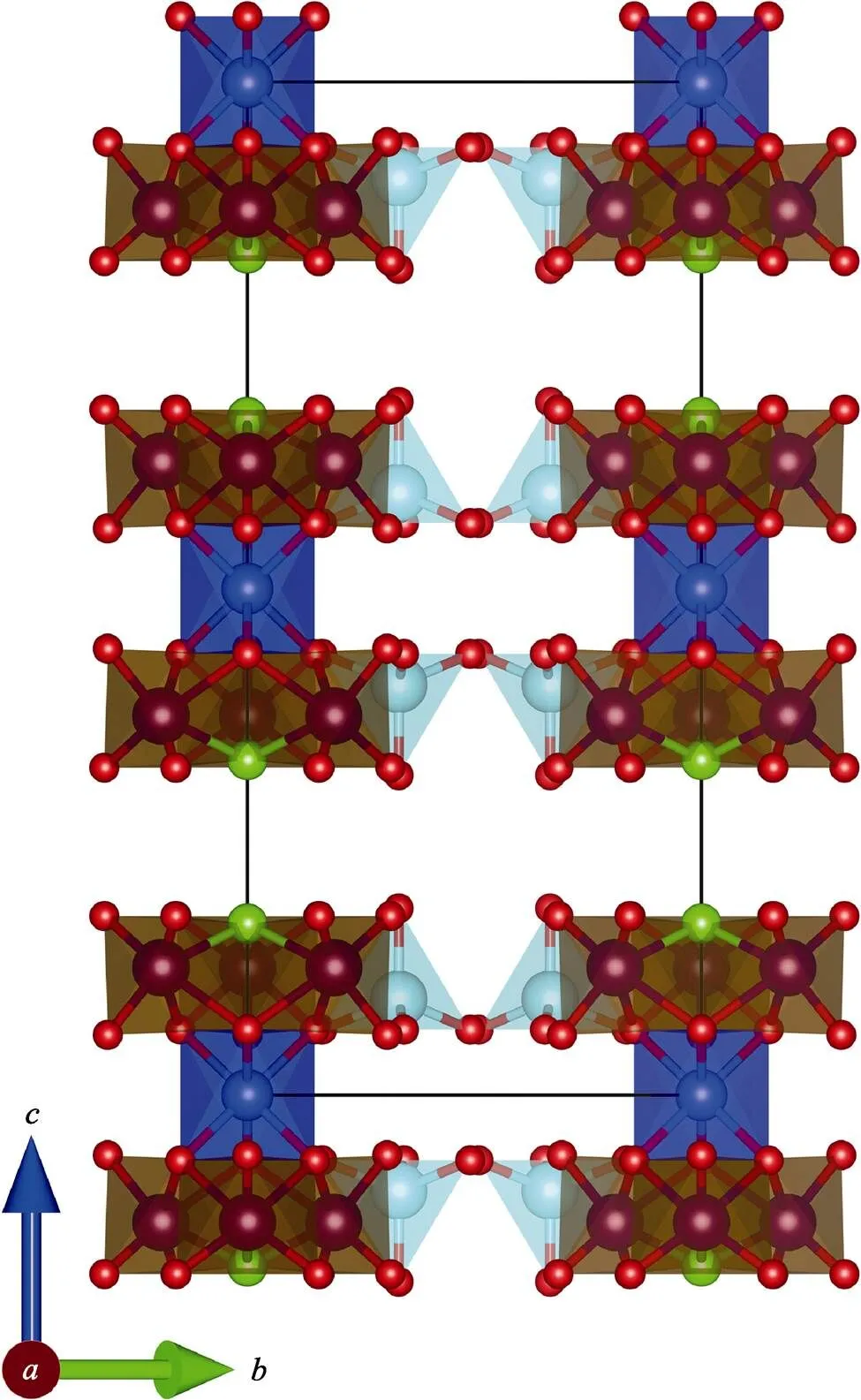
Fig. 1 View of Li23CuW10O40Cl5 along a direction, the Li atoms are omitted for clarity Cu: blue; W(1): brown; W(2): cyan; Cl: Green; O: red
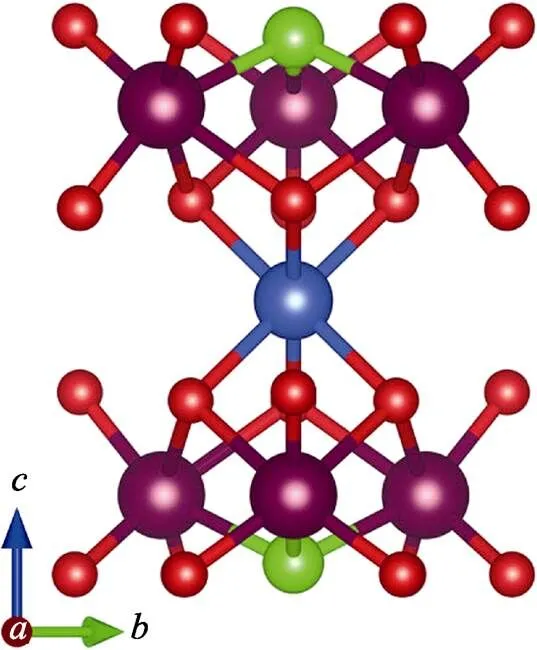
Fig. 2 [W(1)6CuO24Cl2] unit in Li23CuW10O40Cl5 Cu: blue; W(1): brown; Cl: Green; O: red
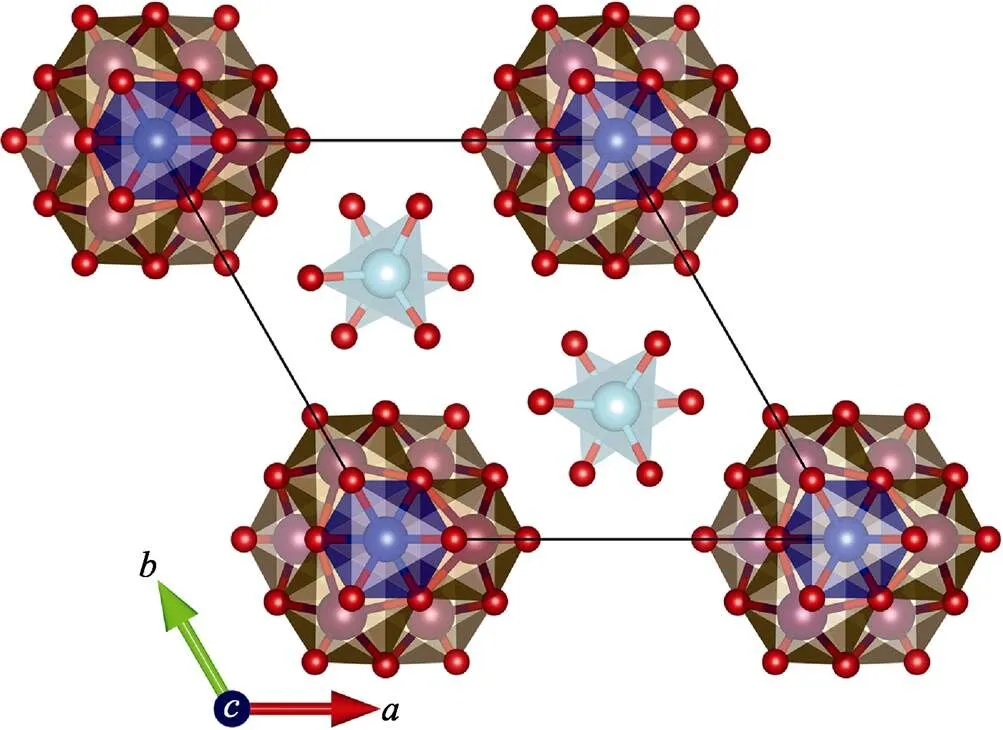
Fig. 3 View ofLi23CuW10O40Cl5along c axis, the Li atoms are omitted for clarity Cu: blue; W(1): brown; W(2): cyan; Cl: Green; O: red
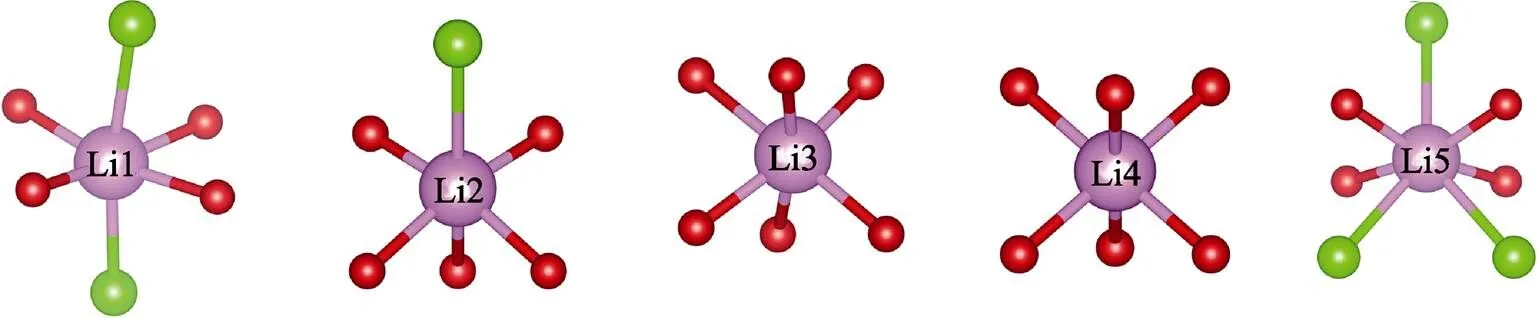
Fig. 4 Coordination geometry Li atoms inLi23CuW10O40Cl5 Cl: Green ball; O: red ball; Li: pink ball

Table 1 Summary of crystallographic data and structure refinement parameters for Li23CuW10O40Cl5
,:unit cell dimensions;: volume;number of formula units per unit cell;calcd: calculated density;/mm–1: diffraction wavelength;range: theta range for data collection; GOFon2:Goodness of fit on2(: structure factors);1[>2()] :the residual factor for the observable diffraction point;2[>2()]: the weighted residual factor for the observable diffraction point;1(all data) :the residual factor for all diffraction points;2(all data) : the weighted residual factor for all diffraction points;1= Σ||F| –|F||/Σ|F|,2= Σ[((F2–F2)2)/ Σ[(F2)2]]1/2
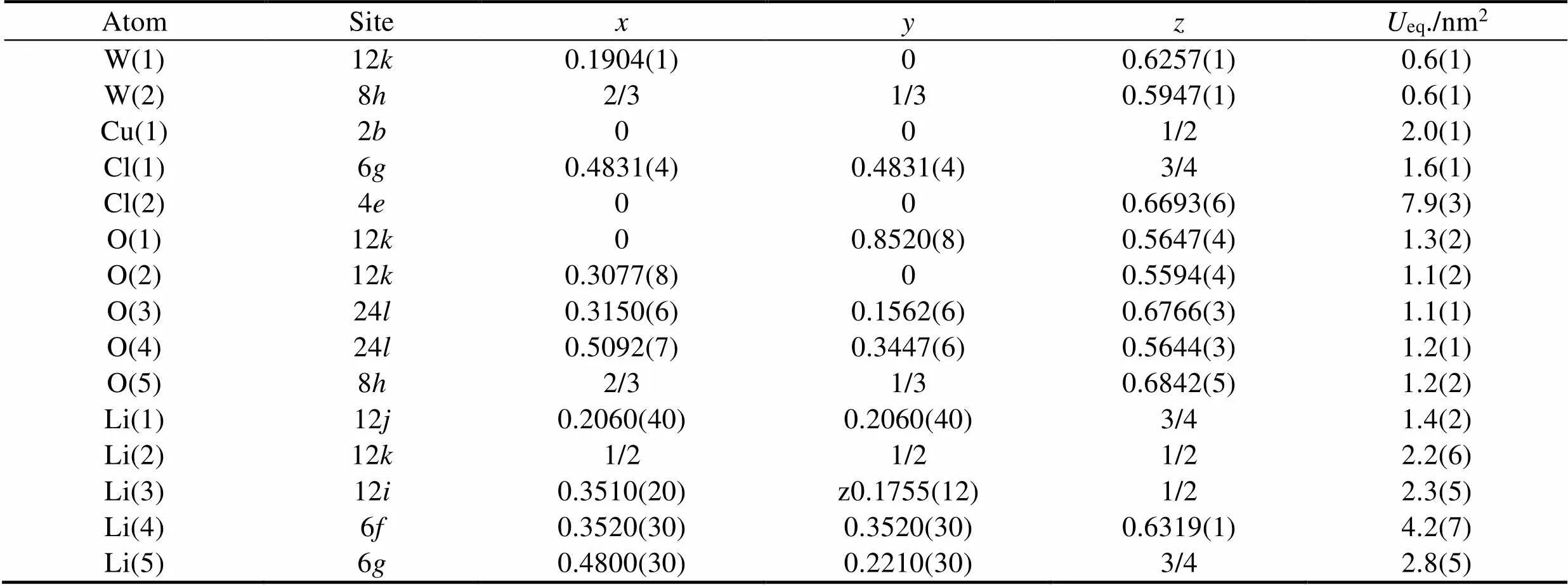
Table 2 Atomic coordinates and equivalent isotropic displacement parameters of Li23CuW10O40Cl5

Table 3 Selected bond lengths and atomic BVSfor Li23CuW10O40Cl5
Atomic coordinates and equivalent isotropic displacement parameters are listed in Table 2. Selected bond distances and atomic BVS are displayed in Table 3.
3 Conclusion
In summary, new single crystals Li23CuW10O40Cl5(1) have been successfully grown by flux growth method in open- end silica tubes. The crystal structure of Li23CuW10O40Cl5has been characterized by single crystal diffraction method. The 3D framework is built by [CuO6] octahedra, [W(1)O5Cl] octahedra and [W(2)O4] tetrahedra. The adjacent [W(1)O5Cl] octahedra are connected with each othersharing one Cl atom, and further sharing three O atoms with [CuO6] octahedra to form the [W(1)6CuO24Cl2] unit. The successful synthesis of Li23CuW10O40Cl5through flux-growth method is meaningful for explore new mixed anion compounds in the future work.
[1] RANMOHOTTI K G, JOSEPHA E, CHOI J,Topochemical manipulation of perovskites: low-temperature reaction strategies for directing structure and properties., 2011, 23(4): 442–460.
[2] ATTFIELD J P. Principles and applications of anion order in solid oxynitrides., 2013, 13(10): 4623–4629.
[3] CLARKE S J, ADAMSON P, HERKELRATH S J C,Structures, physical properties, and chemistry of layered oxychalcogenides and oxypnictides., 2008, 47(19): 8473–8486.
[4] KAGEYAMA H, HAYASHI K, MAEDA K,Expanding frontiers in materials chemistry and physics with multiple anions., 2018, 9: 772.
[5] KOVACHEVA D, PETROV K. Preparation of crystalline ZnSnO3from Li2SnO3by low-temperature ion exchange., 1998, 109(3/4): 327–332.
[6] KOROTIN M A, ANISIMOV V I. Electronic structure and antiferromagnetism in CaCuO2and Sr2CuO2Cl2., 1990, 10(1/2): 28–33.
[7] WU H, YU H, YANG Z,Designing a deep-ultraviolet nonlinear optical material with a large second harmonic generation response., 2013, 135(11): 4215–4218.
[8] WU H, PAN S, POEPPELMEIER, K R,K3B6O10Cl: a new structure analogous to perovskite with a large second harmonic generation response and deep UV absorption edge., 2011, 133(20): 7786–7790.
[9] ZIMMERMANN I, JOHNSSON M A. Synthetic route toward layered materials: introducing stereochemically active lone-pairs into transition metal oxohalides., 2014, 14(10): 5252–5259.
[10] BERDONOSOV P S, JANSON O, OLENEV A V,Crystal structures and variable magnetism of PbCu2(XO3)2Cl2with X=Se, Te., 2013, 42(26): 9547–9554.
[11] CONSTABLE E, RAYMOND S, PETIT S,Magnetic and dielectric order in the kagomelike francisite Cu3Bi(SeO3)2O2Cl., 2017, 96(1): 014413.
[12] BECKER R, JOHNSSON M, KREMER R K,Crystal structureand magnetic properties of FeTe2O5X (X = Cl, Br): a frustrated spin cluster compound with a new Te(IV) coordination polyhedron., 2006, 128(48): 15469–15475.
[13] BERDONOSOV P S, OLENEV A V, DOLGIKH V A. Strontium– copper selenite–chlorides: synthesis and structural investigation., 2009, 182(9): 2368–2373.
[14] GOERIGK F C, SCHLEID T. Composition and crystal structure of SmSb2O4Cl revisited-and the analogy of Sm1.5Sb1.5O4Br.., 2019, 645(17): 1079–1084.
[15] GENG L, LI Q, LU H,Sb-based antiferromagnetic oxychlorides: MSb2O3(OH)Cl (M=Mn, Fe, Co) with 2D spin-dimer structures., 2016, 45(45): 18183–18189.
[16] WANG W H, REN X. Flux growth of high-quality CoFe2O4single crystals and their characterization., 2006, 289(2): 605–608.
[17] LI J, FANG L, LUO H.Li4WO5: a temperature stable low-firing microwave dielectric ceramic with rock salt structure., 2016, 36(1): 243–246.
[18] SHELDRICK G M, SCHNEIDER T R. SHELXL: High-resolution Refinement. London: Academic Press, 1997, 277: 319–343.
[19] SPEK A. Single-crystal structure validation with the program PLATON., 2003, 36: 7–13.
[20] CHARKIN D O, LIGHTFOOT P. Synthesis of novel lead– molybdenum and lead–tungsten oxyhalides with the pinalite structure, Pb3MoO5Cl2and Pb3WO5Br2., 2006, 91(11/12): 1918–1921.
[21] OKADA H M K, MARUMO F, IWAI S. The crystal structure of K2W3O10., 1976, B32: 1522–1525.
[22] TAMADON F, SEPPELT K. The elusive halides VCl5, MoCl6, and ReCl6., 2013, 52(2): 767–769.
[23] GROH M F, MUELLER U. AHMED E.Substitution of conventional high-temperature syntheses of inorganic compounds by near-room-temperature syntheses in ionic liquids., 2013, 68(10): 1108–1122.
[24] CHEN Y, ZHANG Y, FENG S. Hydrothermal synthesis and properties of pigments Chinese purple BaCuSi2O6and dark blue BaCu2Si2O7., 2014, 105: 167–173.
[25] LUTZ HEINZ D, SCHNEIDER M. The crystal structure of Li2MnCl4., 1990, 45(11): 1543–1547.
[26] LUTZ HEINZ D, WUSSOW K, KUSKE P. Ionic conductivity, structural, IR and Raman spectroscopic data of olivine, Sr2PbO4, and Na2CuF4type lithium and sodium chlorides Li2ZnCl4and Na2MCl4(M=Mg, Ti, Cr, Mn, Co, Zn, Cd)., 1987, 42: 1379–1386.
[27] WEISSER M, TRAGL S, MEYER H J. Crystal structure of lithium hexachlorotungstate(V), LiWCl6., 2008, 223(1): 5–6.
[28] LIANG Z, TANG K, SHAO Q,Synthesis, crystal structure, and photocatalytic activity of a new two-layer Ruddlesden–Popper phase, Li2CaTa2O7., 2008, 181(4): 964–970.
助熔剂法合成钨氧氯化合物Li23CuW10O40Cl5
李淑芳, 赵爽, 李满荣
(中山大学 化学学院, 生物无机和合成化学教育部重点实验室, 广州 510275)
由于不同阴离子之间的电负性、离子半径、极化率和氧化态之间的差异, 混合阴离子化合物可以产生不同于单一类型阴离子的新特性。混合阴离子金属材料在电子、湿度探测器、气体传感器、太阳能电池电极等领域有着广泛的应用前景。助熔剂方法是一种广泛应用于混合离子晶体生长的方法, 它以适当的金属盐作为助熔剂, 在较温和的条件下进行复分解反应。助熔剂法在混合阴离子化合物的合成中具有重要意义。钨氧氯化合物Li23CuW10O40Cl5单晶以高质量的Li4WO5为前驱体, 以CuCl2为助熔剂通过两步法合成。通过X射线单晶衍射确定其晶体结构。结果表明, Li23CuW10O40Cl5结晶属于P63/mcm空间群, 晶胞参数分别为=1.02846(3) nm,=1.98768(9) nm,=1.82076(11) nm3,=2。单胞中分别包含五个晶体学独立的Li原子, 两个W原子, 一个Cu原子, 两个Cl原子以及五个O原子。结构中, W(1)原子和一个Cl原子及五个O原子相连接, 形成畸变八面体, 而W(2)原子与四个O原子相连接形成四面体, Cu原子与六个O原子相连形成八面体。因此, Li23CuW10O40Cl5的晶体结构主要由[CuO6]和[W(1)O5Cl]八面体以及[W(2)O4]四面体构成。助熔剂法合成钨氧氯化合物Li23CuW10O40Cl5对今后探索新型的混合阴离子化合物具有重要意义。
钨氧氯化合物; CuCl2助熔剂; 晶体结构; X射线衍射
TQ174
A
date:2019-11-25;
date: 2020-01-16
National Natural Science Foundation of China (2180153)
LI Shufang (1989–), female, PhD. E-mail: lishufang@mail.sysu.edu.cn
李淑芳(1989–), 女, 博士. E-mail: lishufang@mail.sysu.edu.cn
LI Manrong, professor. E-mail: limanrong@mail.sysu.edu.cn
李满荣, 教授. E-mail: limanrong@mail.sysu.edu.cn
1000-324X(2020)07-0834-05
10.15541/jim20190598
Li2CO3(Macklin, 99.99%), WO3(Aladdin, 99.99%), and CuCl2(Macklin, 98%).
