The influence of vitamin D3 on airway inflammation and osteopontin expression in cough variant asthma rats
2020-07-23JianWeiGuYunLiYunFengYangYaoDanZhangJuHuaLiu
Jian-Wei Gu, Yun Li, Yun-Feng Yang, Yao-Dan Zhang,2, Ju-Hua Liu,2✉
1. Affiliated Hospital of North Sichuan Medical College
2. Hospital of Chengdu University of Traditional Chinese Medicine
Keywords:
ABSTRACT
1. Introduction
Cough variant asthma (CVA) was considered as a special type of asthma, characteristic with chronic cough (1). In Europe and America, CVA accounts for 14% - 29% of chronic cough. In China, CVA accounts for 32.6% (3). Osteopontin (OPN), a secretory cytokine with regulation immune balance function, was expressed in bronchial epithelial cells and alveolar macrophages under physiological conditions, and were highly expressed in iinterstitial macrophages, T-lymphocytes and vascular endothelial cells When stimulated by inflammation (4). It is confirmed that OPN positively related with airway inflammatory (5, 6), so OPN can be used as a target to reduce airway inflammation and airway hyperresponsiveness.
Vitamin D is a fat soluble vitamin and steroid hormone, which is related to calcium and phosphorus metabolism. In recent years, a large number of studies have confirmed that vitamin D has immunomodulatory function, which is closely related to the pathogenesis and progress of asthma. Low level vitamin D is an independent risk factor for acute exacerbation of asthma (7). Oral vitamin D can reduce airway inflammatory response and improve lung function by regulating cellular immune imbalance (8), but its specific molecular mechanism is not clear. Some studies have shown that vitamin D can regulate OPN expression in the model of unilateral ureteral obstruction (9). Whether vitamin D have influence on OPN expression in CVA model has not been reported. Therefore, the aim of the present study was to investigate the influence of vitamin D on airway inflammation and OPN expression in CVA rats, further provide theoretical for clinical use of vitamin D to intervene CVA.
2. Method
2.1 Experimental animals
A total of 30 healthy male SPF grade SD rats were enrolled in the study, with body weight 200-250g. Purchased from the experimental animal center of North Sichuan Medical College, license No: scxk (Sichuan) 2008-18, fed in the animal laboratory of North Sichuan Medical College under quiet environment with standard feed and tap water. The daily time is 12 hours alternate. After one week of adaptive feeding, the rats were randomly divided into three groups: blank group, model group and treatment group, each group with 10 rats.
2.2 Main reagents and instruments
Ovalbumin (OVA), sigma company (USA); aluminum hydroxide bottle, Beijing Bioson Biotechnology Co., Ltd. (China); PAS staining kit, Beijing Zhongshan Jinqiao Co., Ltd. (China); Rabbit anti mouse OPN antibody, Wuhan Sanying company (China); biotin Goat anti rabbit IgG, Abcam company (USA); ba400digital three eye camera microscope, Macaudi Industrial Group Co., Ltd. (China); Image Pro Plus 6.0 image analysis software, media Cybernetics (USA); small animal lung drug delivery and lung function measurement system espira, EMMS (UK), active vitamin D3 (Shanghai Xinyi)
2.3 Animal model and success criteria
1) Basic sensitization: model group and treatment group were intraperitoneally injected with 1% OVA + 10mg of aluminum hydroxide 1ml per rat at 0 and 7 days, the blank group were treated with 1ml normal saline; 2) Local excitation stage: model group and treatment group were inhaled 1%OVA suspension for 30min from the 15th day, with frequency of once a day and lasted for 21 days. The control group was replaced by normal saline. After 21 days of stimulation, CVA model were succeed if the following symptoms appeared: restlessness, sneezing, wheezing, rapid breathing and other symptoms; 3)Drug intervention lasted for 14days: starting from 21th day, the treatment group received vitamin D3 gavage 30 minutes before each stimulation. According to the literature (10), active vitamin D3 was dissolved in corn oil, 100 ng / ml, and 1 ml corn oil was replaced in model group and blank group. One rat died in the treatment group because of airway bleeding during gavage.
2.4 Measure parameters
1) Airway hyperresponsiveness: rats were anesthetized by intraperitoneal injection of 6% chloral hydrate (0.6ml / kg) and intubated after tracheotomy. Inhale 10 UL acetylcholine (ACh) (0, 3.125 mg / ml, 6.25 mg / ml, 12.5 mg / ml, 25 mg / ml mg / ml) in sequence according to concentration gradient, record RL value, take ACh concentration as abscissa, RL as ordinate, draw reaction curve.
2) Alveolar lavage fluid (BALF) was collected to measure inflammatory cells: 5 ml normal saline was used to lavage the lungs three times by air tube catheter, and BALF was recovered. After centrifugation, the cells were precipitated and treated, the cells number were counted under the microscope, and classified into four types according to Rayleigh Giemsa; Macrophages, lymphocytes, neutrophils, eosinophils. Observe the gross pathological changes of all tissues at 40 visual fields. Select several different visual randomly under 400 visual fields, count 200 cells by classification, and calculate the percentage of various inflammatory cells.
3) Observation lung histomorphology change: the middle lobe of the left lung were put in 10% formaldehyde after the rats were killed. Leica-2016 rotary microtome (Germany) was used for sections, with a thickness of 4-5um, Each specimen was cut into three sections, and the lung tissue was stained with hematoxylin eosin (HE) to observe the morphological changes of the lung tissue, goblet cells of the lung tissue were observed with PAS staining. The digital three eye camera system was used to observe the images of the sections. The gross lesions of all the tissues were observed at 40 visual fields at first, then 200 or 400 visual fields were used to observe specific lesions. Each field was observed in three visual fields. The average cell density was calculated as the average cell density in each visual field.
4) Detection of OPN protein expression in lung tissue by immunohistochemistry: paraffin fixed tissue was dehydrated by automatic dehydrator, embedded, and sectioned as follows: dewaxing, antigen repair, blocking endogenous peroxidase, blocking, dropping OPN antibody, adding second antibody and DAB Solution, microscope controlled color development, hematoxylin re staining, gradient ethanol dehydration and drying, diphenyl transparent, neutral gum sealing, and finally observed under light microscope. Three fields of vision are selected for each slice, and image acquisition is carried out by digital three eye camera microscope system. Three fields of vision are taken to acquire 400 times of microscopic image, image pro plus 6.0 analysis system were used to measures the IOD and area of the collected image, calculates the average optical density value (MD), the OPN expression was calculated by the average MD of the three images.
2.5 Statistical analysis
SPSS 22.0 was used for statistical analysis. Results were expressed as mean ± standard deviation, the independent sample t test were used to comparison difference between groups, One way ANOVA analysis were used to comparison is performed to comparison difference between groups above three and LSD were selected for pairwise comparison, person correlation analysis were used to analysis the correlation between two variables. The statistics results were significant with P < 0.05.
3.Results
3.1 Airway hyperresponsiveness
The airway resistance of rats after inhaling ACh was measured to evaluate the airway hyperresponsiveness. The lung resistance of model group and treatment group was slightly higher than that of normal rats at baseline, but the difference was not statistically significant (P > 0.05). After inhaling ACh, the airway resistance in each group increased gradually. When the concentration of ACh was 3.125-12.5mg/ml, the airway resistance in the treatment group was lower than that in the model group but higher than that in the blank group (P < 0.05). When the concentration of ACh was 25mg / ml, the airway resistance in the treatment group had no significant difference compared with that in the model group (P > 0.05), which was significantly higher than that in the blank group (P < 0.01). (shown in Figure 1 and table 1)
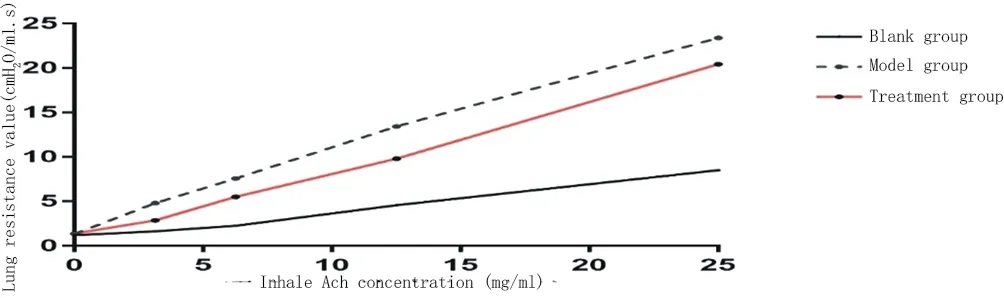
Figure 1:Pulmonary resistance curve in each group after inhaling acetylcholine

Table1 Pulmonary resistance value in each group after inhaling acetylcholine (cmH2O/ml.s)
3.2 Classification of inflammatory cells in BALF
The percentage of macrophages, lymphocytes, neutrophils and eosinophils in BALF of the model group and the treatment group were all increased compared with the blank group ( with all P < 0.05). In treatment group, the percentage of all types of inflammatory cells were all decreased compared with the model group except macrophages (P < 0.05) (shown in Table 2).

Table2 Classification of inflammatory cells in BALF of each group(%)
3.3 Morphological changes of lung tissue and count of goblet cells in each group
HE staining showed that the branches of bronchi in the blank group were normal, only a small amount of inflammatory cells infiltrated, the airway wall was thin, and the thickness was uniform. In the model group, a large number of inflammatory cells infiltrated around the bronchus, pulmonary interstitium and alveoli (indicated by the blue arrow), mucus secretion increased in the bronchi, alveoli were filled with inflammatory cells. Inflammatory cell infiltration was suppressed by Vitamin D in the treatment group (shown in Figure 2).

Figure 2:HE staining of each group(inflammatory cells was indicated by the blue arrow)
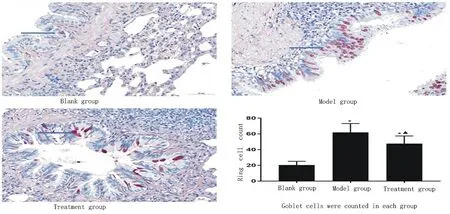
Figure 3:goblet cells in each group(*Compared with black group P<0.05; ▲compared with model group P<0.05).
3.4 Expression of OPN in lung tissue in each group
OPN positive cells were yellow or brownish yellow, mainly distributed in nucleus and cytoplasm (indicated by blue arrow). OPN protein content in model group and treatment group were increased compared with the blank group, (0.229 ± 0.018 vs 0.261 ± 0.015, t = -4.26, P < 0.01; 0.229 ± 0.018 vs 0.245 ± 0.003, t = -2.51, P = 0.02),furthermore, OPN protein content was suppressed by Vitamin D in the treatment group (t = -3.20, P = 0.01) (Show in Figure 3).

Figure 4: Expression of OPN in lung tissue of each group
3.5 Relationship between OPN and inflammatory cells
The percentage of macrophages, lymphocytes, neutrophils and eosinophils in BALF were positively correlated with OPN content in lung tissue (P < 0.05).

Figure 5:Relationship between OPN and inflammatory cells (r: correlation index)
4. Discussion
CVA is special asthma phenotype, characteristic with chronic cough, with clinical symptoms usually lasted for 8 weeks or longer. It has some common pathophysiological characteristics with typical asthma, such as airway hyperresponsiveness and chronic airway inflammation (1). It has been confirmed that CVA patients have airway hyperresponsiveness after inhalation of acetylcholine, whose inflammatory factors in sputum, such as IL-5, IL-8 and TNF-ɑ showed no significant difference conpared with typical asthma (11). In this experiment, ovalbumin was used to induce CVA rat model. The airway resistance value of the model group was significantly higher than that of the blank group after inhaling ACh of different concentrations. In addition, the number of inflammatory cells in BALF and the morphological examination of airway all indicated that the model group had airway inflammatory cell infiltration, so the CVA rat model in this experiment were successful.
Vitamin D is a steroid hormone, its role of regulation immune has become a hotspot in recent years (12). Studies have shown that serum vitamin D levels positively related to the severity of asthma and the response to treatment drugs. For example, serum vitamin D levels are positively related to the quality of life of patients with asthma. Low level vitamin D is an independent risk factor for acute exacerbation of asthma (7). Oral vitamin D can reduce airway inflammation and improve lung function by regulating the level of IL-17 / IL-10 (8). Vitamin D supplementation can reduce the inflammatory response and delay the airway remodeling of asthmatic rats by inhibiting the activity of Wnt5a / β - Catenin pathway (10). In animal experiments, vitamin D3 of 100ng / ml can significantly reduce airway inflammation, but 50 ng/ ml has no such effect (10). In this experiment, 100ng / ml vitamin D3 can significantly reduce the airway hyperresponsiveness and airway inflammatory cell infiltration in CVA rats, and reduce airway goblet cell proliferation, which is consistent with the common research in the same field. On the other hand, we further confirmed that its mechanism may be related to the regulation of OPN expression in lung tissue.
The serum level of OPN in asthmatic patients increased significantly in the acute attack stage, and was still higher than that in normal people even in the remission stage (13, 14). Lung biopsy showed that the expression of OPN in epithelial cells and inflammatory cells in the upper and lower bronchial was higher in asthmatic patients than that in normal people (15). In addition. In the ovalbumin induced asthma model, the number of bronchial epithelial cells and alveolar inflammatory cells expressing OPN were increased, and the OPN content in lung tissue homogenate also increased compared with normal rats. After blocking OPN with OPN specific antibody, the airway hyperresponsiveness of rats decreased, and the levels of inflammatory factors such as IL-4, IL-13, IL-10 and IL-12 in bronchoalveolar lavage fluid also decreased and even approached normal levels (15). In the model of OPN knockout rats, the airway hyperresponsiveness and mucus secretion induced by OVA sensitization were less than that of the normal rats (5). In this experiment, we confirmed that OPN expression in lung tissue of CVA rats was higher than that of normal rats. Active vitamin D3 treatment could reduce OPN protein expression in lung tissue of CVA rats. We further confirmed that OPN content in lung tissue was positively correlated with inflammatory cell count in BALF.
In conclusion, vitamin D3 can reduce airway hyperresponsiveness and inflammatory cells in CVA rats, which may be related to the regulation of OPN expression. However, the specific effect of vitamin D on airway inflammation and lung function is not completely consistent with the clinical results. The reason may be related to the different stages of disease development and the different levels of serum vitamin D. for example, the clinical effect of vitamin D supplementation on reducing the acute attack of asthma only appears in the patients whose serum vitamin D level is less than 30 ng / ml, and the improvement of lung function also occurs Only in patients with FEV1% below 80%, Therefore, the specific application of vitamin D in clinical needs more laboratory and clinical research.
杂志排行
Journal of Hainan Medical College的其它文章
- Network pharmacological study of Qingfei Paidu Decoction intervening on cytokine storm mechanism of COVID-19
- Study on the Potential Mechanism of Drug Pair "Honeysuckle-Astragalus" on COVID-19 based on Network Pharmacology
- Observation on clinical application effect of ankle rehabilitation robot
- Meta-analysis of the relationship between post-stroke depression and the risk of mortality
- Meta-analysis of clinical efficacy of combined traditional Chinese and western medicine in the treatment of granulomatous mastitis
- The effect of plasma uric acid on oxidative stress in ankylosing spondylitis by Keap1-Nrf2 signaling pathway
