miR-146a-3p targeted RELB to regulate cytokine secretion in endothelial cells of atherosclerotic mice
2020-07-23ZhongWeiWuShengJiZhaoChunFuLiChaoQuanLiu
Zhong-Wei Wu, Sheng-Ji Zhao, Chun-Fu Li, Chao-Quan Liu
Department of Cardiology, Hainan West Central Hospital, Danzhou City, Hainan Province, Hainan Danzhou 571700
Keywords:
ABSTRACT
1. Introduction
Atherosclerosis is a chronic disease of the arterial wall, which involves innate and adaptive immune inflammation mechanisms [1-3]. Inflammation is important at all stages of atherosclerosis. It involves the formation of early fat streaks. When the endothelium is activated and expresses chemokines and adhesion molecules, it leads to the recruitment and infiltration of monocytes / lymphocytes under the endothelium. When activated cells within the plaque secrete matrix proteases, degrade extracellular matrix proteins and weaken the fibrous cap, leading to rupture and thrombosis, it can also cause adverse clinical vascular events. Cells involved in the process of atherosclerosis are secreted and activated by soluble factors called cytokines. The cytokines are mainly activated by the NF-κB pathway [4]. RELB Proto-Oncogene (RELB) is a subunit of the multi-effect transcription factor NF-κB, which is present in almost all cell types and is involved in many biological processes, such as inflammation, immunity, differentiation, cell growth, and tumorigenesis and apoptosis. However, how RELB is dysregulated in patients with atherosclerosis is still unknown. In addition, miR-146a-3p is reduced in the serum of patients with atherosclerosis [5]. Based on this, this study explored whether the abnormal expression of miR-146a-3p is related to RELB dysregulation by establishing atherosclerotic mice, and whether it can regulate the cytokine secretion of endothelial cells in atherosclerotic mice, which aims to provide a theoretical basis for the drug development plan for atherosclerosis.
2.Materials and methods
2.1 Materials
RELB, GAPDH antibodies were purchased from cst company (Cat. No. 10544, 5174). DMEM / High Glucose medium was purchased from Qingdao Haosai Technology Co., Ltd. (Catalog No. CA0004-500ML), and AusGeneX Super Fetal Bovine Serum was purchased from Shanghai Xuanling Biotechnology Co., Ltd. (Catalog No. FBS500-S). The pMir-Glo miRNA functional research vector was purchased from Shanghai Haiji Haoge Biotechnology Co., Ltd. (Catalog No. HH-LUC-016). PBS was purchased from Biotechnology (Shanghai) Co., Ltd. (Catalog No. E607008). Dual luciferase reporter gene kit was purchased from Beijing Baierdi Biotechnology Co., Ltd. (Catalog No. SLDL-100) The miRNA transfection reagent was purchased from Shanghai Yanhui Biotechnology Co., Ltd. (Catalog No. 301704). The qPCR primers for miR-146a-3p, miR-146a-3p mimics, and miR-146a-3p inhibitor were synthesized by Beijing Qingke, which was referred to Xiang W et al. for sequence [6]. 20 healthy female ApoE-/-mice, weighing about 20 g at the time of purchase, about 27 g at the time of experiment, SPF grade, were purchased from Changzhou Cavens Experimental Animal Co., Ltd. (License number: SCXK (Su) 2016-0010, Certificate number: 201715828). 14 healthy male C57 mice, weighing about 20 g at the time of purchase, about 27 g at the time of the experiment, SPF grade, were purchased from Shanghai Slake Experimental Animal Co., Ltd. (License number: SCXK (Shanghai) 2013-0018, Certificate number: 0294246). The drinking water was sterilized second-grade ultrapure water, and the quality of the drinking water complied with the provisions of the National Standard "Sanitary Standards for Drinking Water" (GB5749-2006) of the People's Republic of China. The license number for the laboratory animal house is SYXK (Zhejiang) 2015-0008, and the feeding environment was as follows: temperature range of 20 ~ 25 ℃, relative humidity range of 40 ~ 70%. All mice were adapted to the animal room environment for one week before the test. GCSF, GMCSF, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 p40, IL-12 p70, IL-13, IL- 17, IFN-γ, MCP-1, RANTES, TNFα ELISA kits were purchased from Shanghai Qiaoyu Biotechnology Co., Ltd. (Catalog No. QN-PS0040, QY-BM11154, QY-x0605P, QY-BM10220, QNPS0051, QN -PS0050, QY-BM10211, QY-BM10205, QY-BM10156, QY-BM10178, QY-BM10175, QY-BM10172, QN-PS0179, QYBM10168, QY-Q11142, QY-BM11185, QY-BM10200). The MCP-5 ELISA kit was purchased from Shanghai Hengfei Biotechnology Co., Ltd. (Catalog No. SEC075Mu-1).
2.2 Construction of atherosclerosis mouse model and primary culture of endothelial cells
20 healthy female ApoE-/-mice and 14 healthy male C57 mice were established using high-fat diet formula to establish mouse atherosclerosis model. After a week of adaptive feeding, mice are fed high-fat formula feed for modeling. High-fat diet were purchased from Nantong Trophy Feed Technology Co., Ltd. (Batch number: TP26303); High-fat diet formula (unit: g / kg) was as follows:: casein 192.5, corn starch 138.5, sucrose 337.1, corn oil 10.5, anhydrous cream 199.3, Cellulose 60.3, minerals 42.2, vitamin 15.1, L-cystine 2.9, cholesterol 1.5, TBHQ 0.042. The scheme of primary culture of vascular endothelial cells was refer to Liu Yongjun [7].
2.3 Oil Red O staining
The frozen slices were dried at room temperature, washed with PBS, and placed in a fat stain (diluted oil red stain) in a sealed container for 15min; Then separated with 60% ethanol, washed with water; stained with hematoxylin for 30s, rinsed with water to blue; Glycerin PBS mounting.
2.4 HE staining
Frozen slices were dried at room temperature, washed with PBS, dipped the slices in hematoxylin stain for 5 minutes at room temperature, and washed with tap water for 1 min; dipped the slices in 1% hydrochloric acid alcohol solution for a few seconds, tapped the tissues to turn blue; dipped the slices in eosin Medium staining for 3-5min, just rinse off the floating color on the slide with tap water. Dehydration, transparency, sealing: 80% ethanol 0.5min, 95% ethanol I 0.5min, 95% ethanol II 0.5min, absolute ethanol I 0.5min, absolute ethanol II 0.5min, xylene I transparent 3min, xylene II transparent for 3 minutes, and sealed with neutral gum after removal; Staining results: the nucleus was blue or purple-blue, the cytoplasm is pink, and the red blood cells were bright red.
2.5 RNA extraction and real-time fluorescence quantitative PCR
Take an appropriate amount of tissue sample in a 1.5 mL centrifuge tube, add 2-3 grinding steel balls, then add 1 mL Trizol, and place it in a tissue grinder at 30 Hz for 120 s to grind the tissue sufficiently, and transfer the tissue fluid to a new 1.5 mL centrifuge tube. The total RNA of the sample was extracted using the Trizol-spin column method. The first strand of cDNA synthesized with the RNA was extracted by RT kit. Real time fluorescent quantitative PCR was carried out with mir-146a-3p specific primers.
2.6 Dual Luciferase Reporting System
The 3 ′ UTR of RELB was cloned into the pMir-Glo plasmid, and the UG of the 3 ′ UTR of RELB was cloned into the pMir-Glo plasmid. A total of 5 × 104 HELA cells per well were seeded in 24-well plates. After culturing for 24 hours, cells were transfected with a mixture containing 200ng/ml dual luciferase reporter plasmid and 40nM miR-146a-3p mimics. The luciferase activity was measured by a microplate reader 24 hours after transfection using a luciferase report detection kit. All transfections were independently repeated at least three times.
2.7 Western Blot
Add the electrophoresis buffer to the electrophoresis tank, turn on the power, and keep electrophorese at 80V constant voltage until the bromophenol blue runs through the concentrated gel layer. The time is about 30 minutes. The separation gel electrophoresis voltage was 120V. When bromophenol blue migrates to the lower edge of the separation gel, turn off the power and stop the electrophoresis. First soak the PVDF membrane in methanol for about 30 s, then soak in the transfer buffer for 10 min, and then put it into the electrotransfer buffer with filter paper and gel. Put the sponge, filter paper, gel, PVDF membrane, filter paper, and sponge in order according to the sandwich method, carefully remove all air bubbles, the PVDF membrane is located on the anode side, the gel is located on the cathode side, and inserted into the electrophoresis tank, and poured into the transfer membrane buffer solution with 200mA steady current transfer. According to the molecular weight of the transferred protein, the gel running speed is about 1 min/kd. Immerse the PVDF membrane in the blocking solution containing 5% skimmed milk powder, shake slowly on a shaker, and block at room temperature for 1 hour. Remove the blocked PVDF membrane, immerse it in 1 × TBST buffer, and wash slowly on a shaker for 5 min. Then transfer to the incubation box containing the primary antibody (NF-KB dilution ratio of 1: 1000) and incubate at 4 ° C overnight. Remove the PVDF membrane from the incubator, immerse it in 1 × TBST buffer, and wash slowly on a shaker 3 times for 10 min each time. The PVDF membrane was transferred to an antibody incubation box containing secondary antibodies (Goat anti-Rabbit IgG and Goat anti-Mouse IgG antibody diluted 1: 5000), and incubated on a shaker at room temperature for 1 hour. The PVDF membrane was immersed in 1 × TBST buffer and washed slowly on a shaker 3 times for 10 min each time. Put the PVDF membrane on the freshkeeping membrane, mix an appropriate amount of liquid A and B in the medium volume of the ECL kit, mix it and add it to the surface of the membrane, move it into the gel imaging analyzer, and develop it in the chemical photosensitive mode.
2.8 Detection of cytokine indicators
The indicators of GCSF, GM-CSF, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 p40, IL-12 p70 , IL-13, IL-17, IFN-γ, MCP-1, MCP-5, RANTES, and TNFα were detected by ELISA. The culture supernatant of mouse endothelial cells was collected as an analyte. The microtiter plate was fully coated with the antigen/analyte solution in the coating buffer. Cover the plate and incubate at 4 °C overnight. Wash the plate three times with 300ul of washing buffer. Add blocking buffer to the plate, and incubate at 37 °C for 1 hour. Prepare the analyte-antibody mixture for samples and standards. Add the analyte and standard mixture to the selected wells, and incubate at 37 °C for 1 hour. Wash three times with 300ul of washing buffer. Add enzyme-conjugated secondary antibody to each well, and incubate at 37 °C for 1 hour. Wash three times with 300ul of washing buffer. Add substrate solution to selected wells, and incubate at room temperature until the desired color change is observed. Add reaction stop solution and read the absorbance value.
2.9 Statistical analysis
All data were expressed as mean ± standard deviation and analyzed using SPSS 23 software. Differences between groups were analyzed by student ’s t test. P <0.05 was considered to indicate a statistically significant difference.
3. Results
3.1 Establishment of atherosclerosis mouse model.
Through oil red O staining and HE staining experiments, fat staining was red and cell nuclei were blue. It was found that under HE staining microscope, there were plaques in the blood vessel cavity (as shown by black arrows), as shown in Figure 1 and Figure 2. Therefore, the ApoE-/-mouse atherosclerosis model was successfully constructed.

Fig.1 Oil red O stained ApoE-/-mouse vascular cavity

Figure 2 HE staining of ApoE-/-mouse vascular cavity
3.2 The expression level of miR-146a-3p in vascular endothelial tissue of atherosclerotic mice was reduced.
Compared with the control group, the expression of miR-146a-3p in the vascular endothelial tissue of atherosclerotic mice was reduced (P <0.05), as shown in Figure 3.
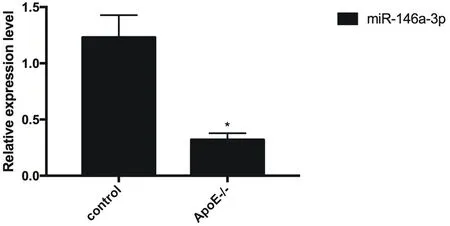
Figure 3 miR-146a-3p expression decreased in vascular endothelial tissue of atherosclerotic mice
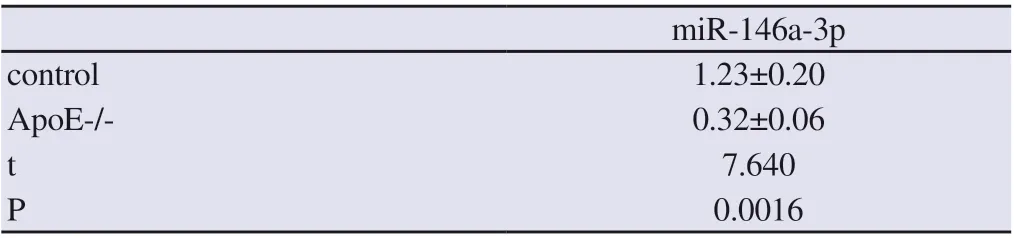
Table 1 Expression of miR-146a-3p in vascular endothelial tissue of atherosclerotic mice
3.3 miR-146a-3p targeted RELB
Through the analysis of the Targetscan online tool, it was found that miR-146a-3p highly matched the 3′UTR of the subunit RELB of the NF-kB complex, and the matching region was located at 367-373 of 3′UTR, as shown in Figure 4. AG mutation was performed on the matched region, and it was found that miR-146a-3pp targeted the 3′UTR of RELB (P <0.05), as shown in Figure 5.

Figure 4 miR-146a-3p was highly matched with RELB's 3'UTR

Figure 5 miR-146a-3p targeted RELB

Table 2 miR-146a-3p targeted RELB
3.4 Cytokine secretion from endothelial cells in atherosclerotic mice after overexpression of miR-146a-3p
After transfection of miR-146a-3p mimics in vascular endothelial cells of atherosclerotic mice, the level of miR-146a-3p was detected by real-time fluorescence quantitative PCR, and it was found that the level of miR-146a-3p was increased (P <0.05). At the same time, by immunoblotting, it was found that the expression level of RELB subunit of NF-kB complex decreased, and the level of GCSF, GMCSF, IL-2, IL-3, IL-4 , IL-5, IL-6, IL-9, IL-10, IL-12 p40, IL-12 p70, IL-13, IL-17, IFN-γ, MCP-1, MCP-5, RANTES, and TNFα cytokines secreted by vascular endothelial cells of atherosclerotic mice was decreased (P <0.05), as shown in Figure 6, Figure 7 and Table 4.

Figure 6 Overexpression of miR-146a-3p
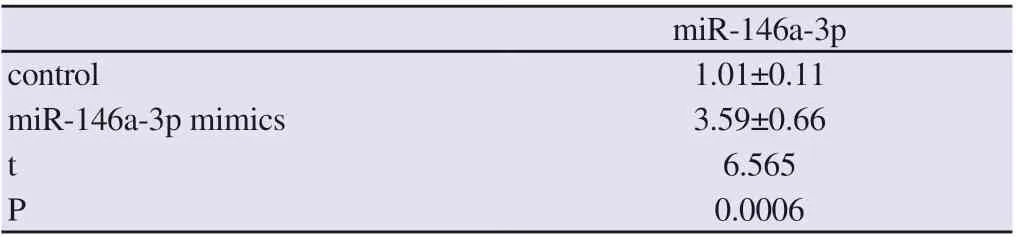
Table 3 Overexpression of miR-146a-3p
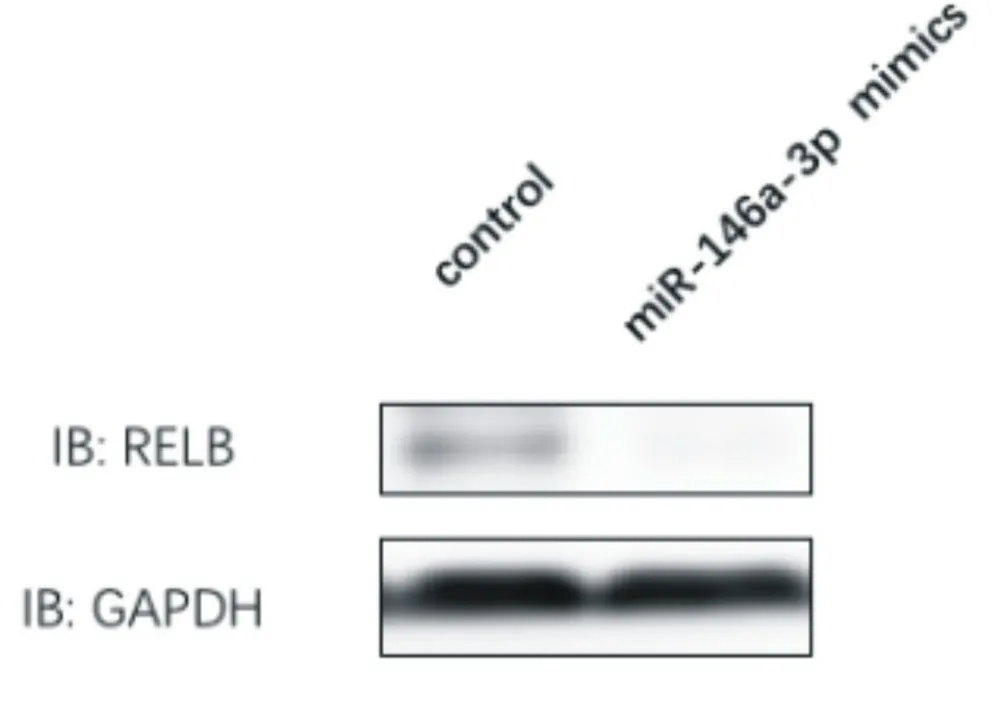
Figure 7 Expression level of RELB subunit of NF-kB complex after overexpression of miR-146a-3p

Table 4 Cytokine secretion levels of vascular endothelial cells in atherosclerotic mice after overexpression of miR-146a-3p
3.5 Cytokine secretion from endothelial cells in atherosclerotic mice after knocking down miR-146a-3p
After transfection of miR-146a-3p inhibitor in vascular endothelial cells of atherosclerotic mice, the level of miR-146a-3p was detected by real-time fluorescent quantitative PCR, and it was found that the level of miR-146a-3p decreased (P <0.05). At the same time, the expression level of the RELB subunit of the NF-kB complex was increased by immunoblotting, and the level of GCSF, GM-CSF, IL-2, IL-3, IL-4 , IL-5, IL-6, IL-9, IL-10, IL-12 p40, IL-12 p70, IL-13, IL-17, IFN-γ, MCP-1, MCP-5, RANTES, and TNFα cytokines secreted by vascular endothelial cells in atherosclerotic mice (P <0.05) were increased, as shown in Figure 8, Figure 9 and Table 6.

Figure 8 miR-146a-3p was knocked down

Table 5 miR-146a-3p was knocked down

Figure 9 The expression level of RELB subunit of NF-kB complex after knocking down miR-146a-3p.
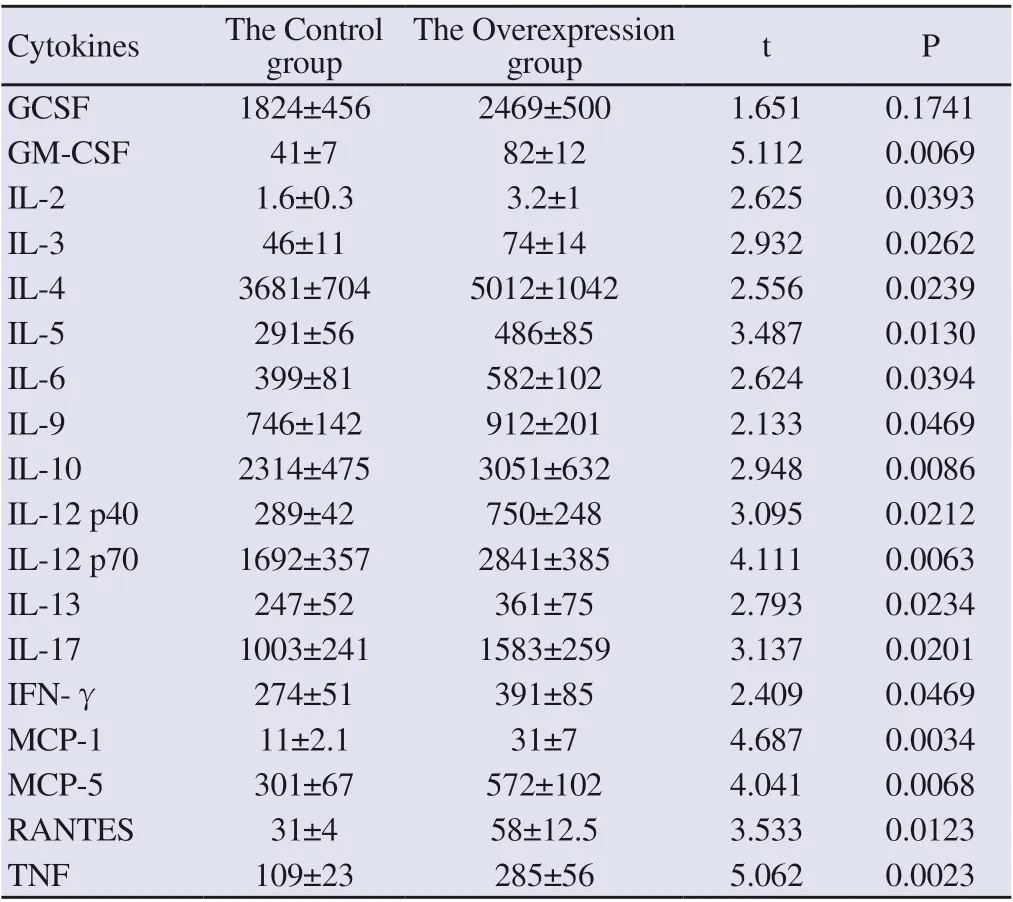
Table 6 Cytokine secretion levels of vascular endothelial cells in mice with atherosclerosis after knocking down miR-146a-3p
4. Discussion
The World Health Organization estimates that one-third of global deaths are related to cardiovascular diseases, such as myocardial infarction and stroke [8]. Atherosclerosis is the main cause of cardiovascular disease-related events and the associated morbidity and mortality of the disease [9, 10]. Atherosclerosis is a chronic inflammatory disease of large and middle arteries that can be triggered by several risk factors, including a diet rich in saturated fat, lack of exercise and smoking. The progression of atherosclerosis is affected by innate and adaptive immune system responses, which are regulated by various cytokines.
Cytokines can affect all stages of atherosclerosis development, from the initial recruitment of circulating monocytes and other immune cells, to mature plaque formation and stability [11]. The use of a mouse model of atherosclerosis has greatly improved our understanding of the role of cytokines and their signaling during disease progression. Usually wild-type mice do not develop atherosclerosis, however, ApoE knockout mice (ApoE-/-) can develop atherosclerotic lesions while feeding standard food [12]. Plaque formation in these mice can be accelerated by feeding a high-fat diet. In this study, a mouse model of atherosclerosis was successfully established through a high-fat formula feeding program.MicroRNA expression and function can be highly tissue-specific [13]. In this study, it was found that the expression level of miR-146a-3p in vascular endothelial tissue of atherosclerotic mice was lower than that of the control group (P <0.05). Also, miR-146a-3p targeted the 367-373 region of 3'UTR of RELB. RELB is a subunit of NFκB, which is a multi-effect transcription factor that can regulate the secretion of downstream cytokines. Gysler SM et al. [14] found that miR-146a-3p can regulate IL-8 secretion through TLR4 and TLR8 in human trophoblasts. It is suggested that miR-146a-3p is a molecule closely related to cytokines, and may regulate the secretion of cytokines in various tissues.
Cytokines are a series of small proteins involved in cell signaling pathways and can be divided into several categories, including interferon (IFN), colony stimulating factor (CSF), interleukin (IL), chemokine, and transforming growth factor (TGF) and tumor necrosis factor (TNF) [15]. In addition, they can help atherosclerotic plaque development (promoting atherosclerosis) or attenuate plaque formation (anti-atherosclerosis) [16]. In this study, it was found that after overexpression of miR-520a-3p, the expression level of RELB decreased and the levels of cytokines GCSF, GMCSF, IL-2, IL-3, IL-4 , IL-5, IL-6, IL-9, IL-10, IL-12 p40, IL-12 p70, IL-13, IL-17, IFN-γ, MCP-1, MCP-5, RANTES, and TNFα secreted by endothelial cells in atherosclerotic mice dropped (P <0.05); After knocking down miR-520a-3p, the expression level of RELB increased, and the levels of cytokines GCSF, GM-CSF, IL-2, IL-3, IL-4, IL-5, IL -6, IL-9, IL-10, IL-12 p40, IL-12 p70, IL-13, IL-17, IFN-γ, MCP-1, MCP-5, RANTES, and TNFα secreted by endothelial cells in atherosclerotic mice increased (P <0.05 ). Because of their key role in influencing the inflammatory response during atherosclerosis, targeting mir-146a-3p represents a promising therapeutic strategy to reduce the development of the disease by inhibiting those that increase atherosclerosis and by promoting those that delay plaque formation.
In summary, miR-146a-3p inhibits the cytokine secretion of vascular endothelial cells in atherosclerotic mice by targeting RELB, a key molecule in the NF-kB signaling pathway.
杂志排行
Journal of Hainan Medical College的其它文章
- Network pharmacological study of Qingfei Paidu Decoction intervening on cytokine storm mechanism of COVID-19
- Study on the Potential Mechanism of Drug Pair "Honeysuckle-Astragalus" on COVID-19 based on Network Pharmacology
- Observation on clinical application effect of ankle rehabilitation robot
- Meta-analysis of the relationship between post-stroke depression and the risk of mortality
- Meta-analysis of clinical efficacy of combined traditional Chinese and western medicine in the treatment of granulomatous mastitis
- The effect of plasma uric acid on oxidative stress in ankylosing spondylitis by Keap1-Nrf2 signaling pathway
