Prevalence and antimicrobial susceptibility patterns of bacteria in ICU patients with lower respiratory tract infection: A cross-sectional study
2020-07-23BirasenBeheraKundanKumarSahuPriyadarsiniBhoiJatindraNathMohanty
Birasen Behera, Kundan Kumar Sahu✉, Priyadarsini Bhoi, Jatindra Nath Mohanty
1Department of Microbiology, IMS and SUM Hospital, Siksha “O” Anusandhan University (Deemed to be), K8, Kalinga Nagar, Bhubaneswar-751003, Odisha,India
2Medical Research Laboratory, IMS and SUM Hospital, Siksha “O” Anusandhan University (Deemed to be), K8, Kalinga Nagar, Bhubaneswar-751003,Odisha, India
ABSTRACT Objective: To investigate the prevalence of isolated organisms in patients with lower respiratory tract infections and the antibiotic susceptibilities at a tertiary care center.Methods: In this observational and cross-sectional analysis, 114 patients admitted in the intensive care unit were enrolled. The endotracheal aspirates and bronchoalveolar lavage were collected.The bacteria were isolated and identified, and finally, antimicrobial sensitive pattern of the isolated bacteria was examined.Results: The prevalence of infection was 72.72% in male patients and 27.28% in females. The predominant bacteria were Klebsiella pneumoniae (37.50%) followed by Acinetobacter spp. (36.36%),Pseudomonas aeruginosa (7.95%), Escherichia coli (6.81%),Proteus mirabilis (2.27%), atypical Escherichia coli (1.13%),Enterococcus spp. (1.13%), Elizabethkingia meningoseptica(1.13%), Staphylococcus aureus (1.13%), Proteus vulgaris (1.13%),Citrobacter freundii (1.13%), and Citrobacter koseri (1.13%). High resistance to cephalosporins (82.18%) was demonstrated in all Gram-negative bacteria. Bacteria showed susceptibility to colistin(88.75%) followed by tigecycline (83.11%), gentamycin (36.18%),and amikacin (49.23%).Conclusions: As the most frequent respiratory organisms,Klebsiella pneumoniae and Acinetobacter spp. have increased resistance to cephalosporins and susceptibility to colistin followed by tigecycline.
KEYWORDS: Antimicrobial susceptibility; Lower respiratory tract infection; Klebsiella pneumonia; Cephalosporins
1. Introduction
Lower respiratory tract infection (LRTI) is common in an intensive care unit (ICU), with increased from 10% to 25%, and mortality from 22% to 71%. Antibiotic resistance is a crucial public health issue. The antibiotic-resistant strains of bacteria are the major problem during infection control, especially for these places where considerable resources and costs are unavailable[1,2]. Recent reports have also described antimicrobial-resistant organisms as “nightmare”bacteria that result in excessive deaths and disastrous spending[3].The impact of antimicrobial-resistant organisms is more severe in low and medium-income countries[4]. Highly resistant strains of Gram-negative bacilli (GNB) continue to spread rapidly in hospitals causing therapeutic problems in many parts of the world,especially for developing countries because isolation facilities are not enough to admit all the patients with infections due to resistant organisms[5,6].
Recent surveillance information from the national nosocomial infection surveillance system of the Centers for Disease Control of USA showed hospital-acquired pneumonia (HAP) or commonly known as ‘nosocomial pneumonia’ is the most typical infection within the ICUs[7,8]. Nosocomial bacteria are multi-drug resistant that are hard to eradicate by available antibiotics. In hospitalized patients both extensive and non-specific use of broad-spectrum antibiotics have led to the spread and development of multi-drugresistant strains that produce extended-spectrum beta-lactamase(ESBL)[9]. Intubated patients with mechanical ventilation are more prone to HAP. If we consider the severity of other cases in litreture study, we find that HAP cases varies from 9%-78%. HAP is the most typical nosocomial infection labeled as ventilator-associated pneumonia (VAP) among mechanically ventilated patients in ICU. Several studies found that mortality rate of VAP ranged from 24% to 80%. For the colonization and causation of VAP, various organisms are implicated. It is possible that different organisms enter into the trachea through different paths, which makes it difficult to distinguish the existence of organisms in the trachea as colonizers or pathogens[10,11]. The patterns of infectious bacteria and antimicrobial susceptibility tests vary from country to country, as well as hospital to hospital and even among ICUs within the hospital. The studies on the susceptibility patterns of locally prevalent organisms are scare.So this study assessed the prevalence of microbes causing LRTIs and their antimicrobial susceptibility pattern of ICU patients.
2. Materials and methods
2.1. Ethical approval
This study was undersigned by the Institutional Ethical Committee of IMS and SUM Hospital (Ref no/DMR/IMS-SH/SOA/16075),Bhubaneswar, and individual patient consent also were obtained.
2.2. Participants
The present study was conducted in the Department of Microbiology, IMS & SUM Hospital from January 2019 to June 2019. During this period, 114 patients admitted to the ICU of the hospital with multiple clinical entities were included in the study.Pediatrics patients and patients diagnosed as LRTIs at the time of admission were excluded from the study.
2.3. Isolation and identification of bacteria
The endotracheal aspirates and bronchoalveolar lavage were collected using a 22’ 14F suction catheter fitted with a mucus extractor. The catheter was introduced gently approximately up to a distance of 25-26 cm. Then gently aspiration without installation of saline was done, and subsequently the catheter was taken out from ET tube. Then 3-4 mL of 0.9% sterile saline was injected into the mucus collector to flush the contents, and the samples were immediately sent to the microbiology laboratory for processing within an hour. The samples were first stirred and homogenized. The direct examination was done to determine the presence of pus cell and bacteria in the form of Gram stain and Ziehl-Neelsen stain as per the protocol.
Simultaneously semi-quantitative cultures by the calibrated loop method using a nichrome wire loops holding 0.01 mL fluid were done on media like sheep blood agar, Mac Conkey agar, and chocolate agar using standard techniques. The plates were incubated at 37 ℃ under an aerobic atmosphere except for the blood agar and chocolate agar plates which were incubated in a candle jar at 37 ℃. The plates were checked at 24 h and 48 h of incubation for any growth. Plates without growth after 5 d were discarded. After observation of the colony morphology, Gram staining was done from isolated colonies, and identification was done using available biochemical reactions. After the completion of identification,antibiotic susceptibility testing was done on Muller Hinton agar by the Kirby-Bauer’s disc diffusion method. In addition, antibiotic susceptibility testing was also done with automated method by Vitek-Ⅱ in some cases.
2.4. Biochemical identification test
Necessary biochemical tests were done for the identification of bacteria. The following tests were performed as per the requirements:catalase test, slide coagulase test, tube coagulase test, oxidase nitrate,motility reduction test, indole test, methyl red test, Voges-Proskauer test, citrate utilization test, urease production test, triple sugar iron test, and mannitol motility test.
2.5. Antibiotic sensitivity patterns
Both manually and automated methods such as Vitek-Ⅱ were used for susceptibility testing of identical bacteria.
2.5.1. Detection of methicillin-resistant Staphylococcus
The test was done on a Muller Hinton agar with cefoxitin a disc in our laboratory. If zone size > or =22 mm was considered positive and zone size < or =21 mm was negative.
2.5.2. ESBL test
Ceftazidime 30 μg and ceftazidime+clavulanic acid (30 μg+10 μg)disc were put on a Muller Hinton agar plate culture of inoculum equal to 0.5 Mc Farland opacity standard for land turbidity standard.An increase in zone size of ceftazidime+clavulanic acid by > or =5 mm component to ceftazidime disc alone is considered as ESBL positive.
2.5.3. Detection of metallobeta lactamase by combined disc diffusion method
A lawn culture of test isolate (0.5 Mc Farland opacity standard) was done on MHA plate. Two 10 microgram imipenem discs were placed on inoculated plates. To one of the imipenem discs, 10 μL of 0.5M EDTA solution was added. After overnight incubation, if the zone of inhibition of imipenem + EDTA discs compared to imipenem alone is >7mm, then the test was considered positive.
3. Results
Among the 114 specimens, 88 samples showed growth, whereas 26 samples showed no growth or contamination with saliva. Out of 88 samples, all are found bacterial isolates but no fungal isolates were found.
Out of the 88 patients, 64 patients (72.72%) are males and 24(27.28%) patients are female. Besides, 25 patients (28.40%) were in the age group of 50-60 years, followed by 60-70 years (22.72%)and 70-80 years (13.63%).
Among the total 88 bacterial isolates, the most isolates were the GNB (98.9%). The most prevalent GNB wasKlebsiella pneumoniae(K. pneumoniae) (37.50%) followed byAcinetobacterspp. (36.36%),
Pseudomonas aeruginosa(7.95%),Escherichia coli(6.81%),Proteus mirabilis(2.27%), atypicalEscherichia coli(1.13%),Enterococcusspp. (1.13%),Elizabethkingia meningoseptica(1.13%),Proteus vulgaris(1.13%),Citrobacter freundii(1.13%), andCitrobacter koseri(1.13%) only in a single case Gram-positive coccusi.e.Staphylococcus aureus(S. aureus) was isolated
A high rate of resistance to cephalosporins (82.18%) was demonstrated by all GNB. The susceptibility rate was 88.75% for colistin followed by tigecycline (83.11%. Susceptibility rate of gentamycin was 36.18% whereas amikacin was 49.23% (Figure 1).
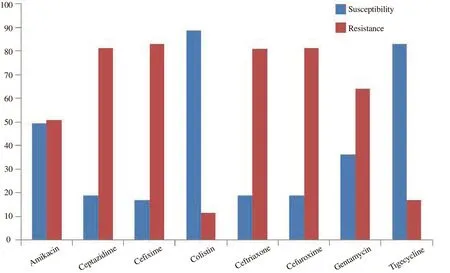
Figure 1. Graphic representation of antibiotic susceptibility pattern of isolated bacteria (%).
4. Discussion
This study was to find out the bacteriological profile of LRTI specimens in ICU patients and their antimicrobial susceptibility pattern against antibiotics. Both ventilator and non-VAP patients were included for the study and their incidence, isolation, and antimicrobial resistance pattern were determined.
The national nosocomial infection surveillance of the Centers for Disease Control of USA reported 60% of nosocomial pneumonia due to aerobic GNB. However, in our study, we found the predominant organism was GNB except for Gram-positive bacteria (S. aureus)in one single case. The results are similar to studies of Goelet al., Kumariet al., Barsantiet al., who found that the incidences of GNB isolates were 92.2%, 93.0% and 97.4%,respectively[1,2,12]. In our study,K. pneumoniaewas the most common GNB isolates (37.5%) followed byAcinetobacterspp.(36.3%). A total of 22.8% of the specimens were sterile on culture,and it may be attributed to previous antibiotic therapy or nonrepresentative specimens.
There was an overall prevalence of GNB among LRTI isolates withK. pneumoniae, non-fermenting GNB, andP. aeruginosabecause of the general isolates, that found as conjointly affirmed from the studies created by Veenaet al.[2]. For ICU patients,pneumonia is a repeating obstacle, as it is regularly polymicrobial with mostly multi-drug-resistant GNB such asK. pneumoniae,Acinetobacter, andP. aeruginosa. Antibiotic resistance is now a common problem in developed and developing countries. We have detected a very high rate of resistance to cephalosporins among the varied Gram-negative isolates (82.18%). Homogenous surveys were conducted by numerous reporters together with Goelet al.and Kumariet al.[1,2].
The high rate of resistance to third-generation cephalosporins may be due to the extensive applications, under or overdosage,incomplete and irregular treatment. Another reason may be that our study conducted at tertiary care hospital where the patients showing high resistance to cephalosporins accounted for the majority of the admissions. Besides, around 66% of resistance to carbapenem is a matter of concern. In other studies carbapenem resistance is lower compared to that of our study. This discovery manifests that carbapenem ought to be sagaciously employed in oxygenated patients[13,14].
In our study, antimicrobial susceptibility to colistin was found to be highest (88.75%) followed by tigecycline (83.11%).The resistance rate of some GNB to aminoglycoside, such as gentamycin is higher than to amikacin, which has been well identified in several hospitals. In our research amikacin susceptibility rate was higher (49.23%) compared to gentamycin(36.18%). Aminoglycoside resistant strains are extra usual at places with poor penetration of drugs. To be concluded, multi-drugresistantKlebsiella,Acinetobacter, andPseudomonasare the most usual etiological agents of LRTIs in ICU[15]. There is an alarmingly high rate of resistance to cephalosporins, beta-lactamase inhibitors,and carbapenems. So a definitive bacteriological diagnosis and susceptibility testing will be helpful in effective management of LRTI and prevention of antimicrobial resistance. Optimization of antimicrobial therapy is important for ICU patients as antimicrobial utilization is crucially higher. Censoriously ill and the aged sufferers are at higher risk of GNB-LRT infections, and all the multi-resistant microorganisms are frequently found in high-risk areas such as ICU.
5. Conclusions
The incidence of infection was clearly in excess in male patients.Nowadays, antibody resistance of bacteria to antibiotics in ICU is posing a problem in the clinic. Moreover, cross-infection inside ICU gives rise to notoriously resistant strains, such asK.pneumoniaeandAcinetobacterspp. Formulating local antibiotic policies guided by the nature of bacterial isolation and their antibiotic susceptibility patterns may help the clinicians to offer more effective treatment. Besides, a clinically diagnosed case of pneumonia is usually treated with an empiric antibiotic, before definitive sensitivity reports are available.
Conflict of interest statement
The authors report no conflict of interest.
Authors’ contributions
B.B. and K.K.S.: Design of the study, clinical evaluation,supervised the project, final approval of the final version; B.B., P.B.:Clinical evaluation, data collection, writing and drafting the article,final approval of the final version; K.K.S. and J.N.M.: Data analysis and interpretation, critical revision of the article, final approval of the final version.
杂志排行
Journal of Acute Disease的其它文章
- "Resuscitator": Golden medtech proposal introducing a new era for CPR
- Incidence of Enterobius vermicularis in acute appendicitis: A systematic review and meta-analysis
- Effect of 8-week and 12-week triple therapy (doxycycline, rifampicin,and gentamicin) on brucellosis: A comparative study
- Open reduction and internal fixation for radial head fractures: A prospective observational study
- Cope’s sign and complete heart block secondary to acute cholecystitis: A case report
- Adult-onset Still's disease: A case report
