ZnCuAl-LDH/Bi2MoO6 Nanocomposites with Improved Visible Light-Driven Photocatalytic Degradation
2020-07-23GuiyunYuFengxianHuWeiweiChengZitongHanChaoLiuYongDai
Guiyun Yu , Fengxian Hu , Weiwei Cheng , Zitong Han , Chao Liu ,*, Yong Dai ,*
1 School of Chemistry & Chemical Engineering, Yancheng Institute of Technology, Yancheng 224051, Jiangsu Province,P.R.China.
2 School of Materials Science and Engineering, Yancheng Institute of Technology, Yancheng 224051, Jiangsu Province, P.R.China.
Abstract:In this study, pure Bi2MoO6 was synthesized via a solvothermal method.A ZnCuAl-layered double hydroxide (LDH)/Bi2MoO6 (denoted as LDH/Bi2MoO6)nanocomposite was synthesized via a steady-state coprecipitation route using Bi2MoO6 as the matric material.LDH was deposited on the surface of Bi2MoO6 with a close contact interface.The specific surface area of the resulting LDH/Bi2MoO6 composite increased up to 19.1 m2∙g-1 owing to the stacking arrangement between LDH and the Bi2MoO6 nanosheets, resulting in the generation of a large number of reactive sites.In addition, the light absorption region of the LDH/Bi2MoO6 composite was larger than those of pure LDH and Bi2MoO6 because of the formation of a heterojunction structure and the possible quantum size effect.The photocatalytic performance of the as-prepared samples was evaluated by carrying out the degradation of rhodamine B (RhB) using them under visible light irradiation.Compared to pure LDH and Bi2MoO6, the LDH/Bi2MoO6 nanocomposite exhibited enhanced photocatalytic activity for the degradation of RhB.With an increase in the LDH content,the photocatalytic activity of the LDH/Bi2MoO6 composite first increased and then decreased.Although the addition of an optimum amount of LDH was beneficial for the generation of electron-hole pairs, excessive LDH on the surface of Bi2MoO6 decreased the visible light absorption ability of both the components, thus reducing photocatalytic activity of the composite.This indicates that an appropriate LDH:Bi2MoO6 molar ratio is necessary for obtaining LDH/Bi2MoO6 composites with excellent photocatalytic activity.Furthermore, the LDH/Bi2MoO6 composite showed high photocatalytic stability and reusability.The structure of the LDH/Bi2MoO6 composite remained almost unchanged even after four photodegradation cycles.The enhanced photocatalytic performance of the composite can be attributed to the combined effect of its heterojunction structure and high specific surface area, which are beneficial for effective separation of photogenerated charge carriers and the availability of a large number of active sites for photocatalysis.It was found that ·OH and were the main reactive species, while e- and h+ contributed little to the photodegradation process.The generation, transfer, and separation of photoinduced electrons and holes in the composites were investigated by transient photocurrent responses,electrochemical impedance spectroscopy Nyquist plots, and photoluminescence measurements.The results showed that the heterojunction structure of the composites played a key role in enhancing their photocatalytic activity.A possible photodegradation mechanism was proposed for the composite.This study will provide a facile approach for the preparation of LDH- and/or Bi2MoO6-based nanocomposites.The LDH/Bi2MoO6 nanocomposite prepared in this study showed huge potential to be used as a visible-light photocatalyst for degrading environmental pollutants.
Key Words:Layered double hydroxide;Bi2MoO6;Nanocomposite;Active site ;Visible-light photodegradation;Heterojunction
1 Introduction
In recent years, the large amount of toxic waste water was discharged into the environment due to the great development of industrialization, which has a serious impact on water resources.In addition, the increasing demand of water and the declining of available fresh water were more serious problems in water resources.The purification and recycling of industrial waste water has become an important research field1,2.Therefore,there is an urgent issue to develop an environmental, efficient and stable visible-light-response semiconductor photocatalyst to deal with industrial wastewater3,4.
Bismuth molybdate is a direct transition n-type semiconductor material with a narrow forbidden band width (Eg< 3.2 eV) and thus a potential visible light absorption capacity5.Bismuth molybdate has three typical crystal structures,i.e.α-Bi2Mo3O12,β-Bi2Mo2O9and γ-Bi2MoO66.Among these three crystal structures, γ-Bi2MoO6is the only layered Aurivillius structure with a structural stability at low temperatures7.However, the photocatalytic effect of the single Bi2MoO6was not ideal,because of the low visible light absorption efficiency and the high recombination rate of electron holes8,9.The hetero-junction semiconductor photocatalysis tends to have beneficial photocatalytic activity10.Hence, the researchers have attempted to prepare many high-performance photocatalyst materials by combining bismuth molybdate with other semiconductor materials, such as BiOI/Bi2MoO611, Bi2O2CO3/Bi2MoO612,MoS2/Bi2MoO613and CdS/Bi2MoO614.
Layered composite hydroxides (LDHs), with a brucite regular octahedral structure, consist of a positively charged host laminate and interlayered anions as a guest.The general formula iswhere M2+represents a divalent cation, M3+a trivalent cation, and An-interlayered anion, respectively15,16.Because LDHs have large specific surface area, positively charged host layers, host-guest interactions and tunable interlayer space17,18, they showed a great advantage in the field of adsorption19,20, bio/medical fields21,22and environmental friendly functional additives23,24.At present, some reports about hydrotalcite-based semiconductor photocatalysts have been widely studied, such as ZnCr-LDH25,ZnFe-LDH26, g-C3N4/NiFe-LDH27, BiOCl/NiFe-LDH28and BiVO4/LDH29.However, there are few discussions about new composites of hydrotalcite and bismuth molybdate, and the visible-light-driven photocatalytic performance of LDH-based catalysts is still not satisfactory for practical applications.
In this work, we have synthesized LDH/Bi2MoO6nanocomposite by using a steady state co-precipitation method.The photocatalytic performance of the obtained sample was investigated in detail through the degradation of RhB solution under visible light irradiation.Subsequently, the active species and the corresponding photocatalytic mechanism were explored.
2 Experimental
2.1 Materials
Aluminum nitrate nonahydrate (Al(NO3)3∙9H2O, AR, 99.0%),zinc nitrate hexahydrate (Zn(NO3)2∙6H2O, AR, 98.0%) and copper nitrate hexahydrate (Cu(NO3)2∙6H2O, AR, 99.0%) were purchased from Shanghai Aladdin Biochemical Technology Co.,Ltd.Bismuth nitrate pentahydrate (Bi(NO3)3∙5H2O, AR, 99.0%),sodium molybdate dehydrate (Na2MoO4∙2H2O, AR,99.0%),sodium hydroxide (NaOH, AR), ethylene glycol ((CH2OH)2,AR, 99.7%) and absolute ethanol (CH3CH2OH, AR, 99.7%) were purchased from Shanghai Sinopharm Chemical Reagent Co.,Ltd.All chemicals were directly used without further purification.
2.2 Preparation of photocatalysts
Pure Bi2MoO6was synthesized by a solvothermal method30.In a typical synthetic process, a desired amount of Bi(NO3)3∙5H2O (1 mmol) and Na2MoO4∙2H2O (0.5 mmol) were respectively added into the mixed solution of ethylene glycol (20 mL) and absolute ethanol (40 mL) under the vigorous stirring to form a clear solution.Then, NaOH (2 mol∙L-1) solution was dropwise added into the above solution to adjust pH value to 5.The resulted mixture was heated at 160 °C for 12 h using a stainless steel Teflon-lined autoclave.The target precipitate was washed by distilled water and absolute ethanol, and then dried at 60 °C overnight.
The synthetic process of LDH/Bi2MoO6nanocomposite is shown in Scheme 1.1 mmol of Bi2MoO6was well dispersed in distilled water (40 mL) with a ultrasonic treatment for 1 h, and then 1 mmol of Zn(NO3)2∙6H2O, 0.5 mmol of Al(NO3)3∙9H2O and 0.5 mmol Cu(NO3)2∙6H2O were added into the above suspension, respectively.The pH value of the precursor solution was also adjusted to 9 using NaOH (2 mol∙L-1) solution with a continuous stirring.After 10 h, the resultant LDH/Bi2MoO6nanocomposite (denoted as LDH/Bi2MoO6) was collected,washed and dried at 60 °C for 12 h.Other LDH/Bi2MoO6-xphotocatalysts were also prepared by the same procedure, wherexrepresented the molar ratio (1 : 2, 1 : 3, 2 : 1 and 3 : 1) between LDH and Bi2MoO6.For comparison, ZnCuAl-LDH (denoted as LDH) was also prepared by a similar process without the addition of Bi2MoO6.
2.3 Characterization
The X-ray powder diffraction (XRD) patterns of the prepared materials were obtained on a Shimadzu XRD-6000 diffractometer using CuKαradiation.The FT-IR spectra were recorded on a NICOLET IS-10 spectrometer.The morphology was observed through scanning electron microscopy (FEI QUANTA 200) and transmission electron microscopy (TEM)(JEM-2100, an acceleration voltage of 200 kV).The Brunauer Emmett Teller (BET) specific surface areas were measured with N2adsorption/desorption isotherms recorded on a Beckman Coulter SA 3100 instrument at 77 K.X-ray photoelectron spectra (XPS) was performed on a photoelectron spectrometer(Thermoelectric Inc., ESCALAB 250Xi) using AlKαradiation.The U V-Visible diffuse reflectance spectra were measured by a Shimadzu UV-3600 spectrophotometer using BaSO4as a reference.The photoluminescence (PL) spectra were detected by a JASCO FP 6500 fluorescence spectrometer at an excitation wavelength of 325 nm.
The electrochemical measurements were carried out on an electrochemical workstation (CHI-660D, Shanghai Chenhua)with a standard three electrode cell.The photocurrent was measured under a visible light irradiation at a bias voltage of 0.5 V using a 0.5 mol∙L-1Na2SO4electrolyte solution.The electrochemical impedance spectroscopy (EIS) was performed at an open circuit potential of 5 mV in the frequency range of 0.01 Hz-1000 kHz using a mixed solution of KCl (0.1 mol∙L-1),K3[Fe(CN)6] (5 mmol∙L-1), and K4[Fe(CN)6] (5 mmol∙L-1) as an electrolyte solution.
2.4 Photocatalytic evaluation
For each photocatalytic experiment, 0.1 g of photocatalyst was dispersed into RhB solution (5 mg∙L-1, 200 mL) with a stirring for 30 min in dark.Then, the above mixed suspension solution was irradiated under visible light (300 W Xenon lamp,λ≥ 420 nm).During the irradiation, the certain volume of solution (~5 mL) was taken out for the further analysis by a UVVis spectrophotometer at 554 nm.
2.5 Active species trapping
To identify the role of the active species, 10 mmol∙L-1of different scavengers, including isopropanol (IPA),benzoquinone (BQ), sodium oxalate (Na2C2O4) and potassium dichromate (K2Cr2O7), were added into the RhB aqueous solution, respectively, These scavengers are related to ·OH,·O2-, h+and e-, respectively3,31.Then, the remaining testing process was the same to the above photocatalytic processes.
3 Results and discussion
The crystal structures of LDH, Bi2MoO6and LDH/Bi2MoO6were measured by means of XRD patterns.As shown in Fig.1a,some characteristic peaks at 10.60°, 21.11°, and 34.14° can be respectively indexed to (003), (006) and (101/012) planes of the regular layered structure of LDH, indicating the formation of the octahedron phase of LDH.For pure Bi2MoO6, some diffraction peaks at 10.81°, 28.29°, 32.58° and 33.19° are related to (020),(131), (200/002) and (060) planes, respectively.After combining LDH with Bi2MoO6, the characteristic peaks of Bi2MoO6are maintained in LDH/Bi2MoO6composite although their intensities become weak to a certain extent, indicating that the introduction of LDH does not affect the layered structure of Bi2MoO6.Additionally, the characteristic peaks of LDH are not visible in LDH/Bi2MoO6composite owing to the formation of an overlapping peak and/or a relatively low mass ratio of LDH.
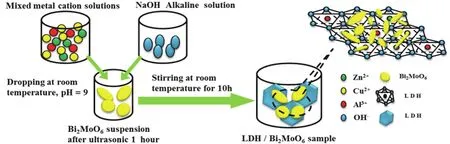
Scheme 1 A schematic illustration for preparing LDH/Bi2MoO6 nanocomposite.

Fig.1 (a) XRD patterns, (b) FT-IR spectra, (c) N2 adsorption-desorption isotherms, and (d) UV-visible DRS spectra of LDH,Bi2MoO6 and LDH/Bi2MoO6 (Inset is the (αhv)n/2 versus photo energy (hv) of the as-prepared samples.).
The local structures of the as-prepared samples were measured using FT-IR spectra (Fig.1b).The bulk LDH shows the absorption bands at 1381, 1636 and 3528 cm-1, attributed to thestretching vibration, the deformation vibration of H―O―H, and tensile vibration of ―OH, respectively32,33.For pure Bi2MoO6, the absorption peaks at 1000-400 cm-1are assigned to lattice vibrations of M―O34,35, and peaks at 3524 and 1637 are corresponded to ―OH vibration.The peak at 1380 cm-1is assigned tostretching vibration, which is caused by nitrates from raw materials.Peak around 1050 cm-1is associated with the adsorbed CO2on Bi2MoO6.After combining LDH with Bi2MoO6, the obtained LDH/Bi2MoO6shows three characteristic absorption peaks (842, 797 and 737 cm-1) owing to the stretching vibration of Mo-O bond, and other peaks (575 and 448 cm-1) due to the telescopic vibration of Bi―O bond36-38.Compared with pure Bi2MoO6and LDH, the absorption characteristic peaks of LDH/Bi2MoO6show a slight shift due to the interaction between LDH and Bi2MoO6.
Fig.1c shows nitrogen adsorption-desorption isotherms of LDH, Bi2MoO6 and LDH/Bi2MoO6.Pure LDH shows typical IV isotherms related to capillary condensation in mesoporous region39, and hysteresis loop at low relative pressuredue to the formation of slit-shaped pores.Both Bi2MoO6and LDH/Bi2MoO6show typical II isotherms40.In addition, the specific surface areas of LDH and Bi2MoO6are calculated to be 5.5 and 15.0 m2∙g-1, respectively.After combining LDH with Bi2MoO6, the specific surface area of the resulted LDH/Bi2MoO6is increased to 19.1 m2∙g-1, which is attributed to the stacking arrangement between LDH and Bi2MoO6nanosheets.The larger specific surface area means more reactive sites exposed to enhance the visible-light-driven photocatalysis.
The UV-visible diffuse reflectance spectra (DRS) of the asprepared samples are shown in Fig.1d.The absorption edges of LDH, Bi2MoO6and LDH/Bi2MoO6can be estimated to be 425,475 and 520 nm, respectively41, showing that LDH/Bi2MoO6can be excited to produce more charge carriers than pure Bi2MoO6 and LDH.The absorption band between 600 and 1000 nm in LDH is attributed to Cu2+2Eg →2T2g (D)d-dtransitions of Cu2+, which is a distorted six-fold coordination with octahedral arrangement of Cu2+due to Jahn-Teller effect occurring in LDH framework42,43.Furthermore, the absorption edge of LDH/Bi2MoO6nanocomposite exhibits an obvious red-shift to a longer wavelength (520 nm), which is attributed to the synergistic effect between LDH and Bi2MoO6.
The band gap value can be calculated based on the following equation:αhv = A(hv - Eg)n/2, whereα, h, v, Eg,are the absorption coefficient, Planck’s constant, optical frequency and band gap, respectively44-46.The inset of Fig 1d shows the plots of (αhv)2/nvshvfor LDH, Bi2MoO6 and LDH/Bi2MoO6.For direct transition semiconductors of LDH and Bi2MoO6,nequals 1.The band gap energy (Eg= 2.40 eV) of LDH/Bi2MoO6is smaller than that of Bi2MoO6(Eg= 2.70 eV) and LDH (Eg= 2.95 eV), indicating that LDH adjusts the forbidden band structure of Bi2MoO6, leading to the enhanced light absorption ability of the resulted nanocomposite.In addition, the calculated bandgap value of LDH/Bi2MoO6 is smaller than that of pure LDH and Bi2MoO6due to the formation of heterojunction structure (see below) and the possible quantum size effect47,48.The LDH/Bi2MoO6composite with a larger optical-response range is expected to exhibit a better photocatalytic activity than its pure counterparts.
The morphology and microstructure of Bi2MoO6,LDH/Bi2MoO6and LDH were investigated by SEM images.It can be clearly observed that bulk Bi2MoO6(Fig.2a, b) shows a sheet-like structure with a thickness of 30-40 nm.As shown in Fig.2c, d, pure LDH is consisted of many regularly stacked nanosheets with a thickness of 10-15 nm.After combining LDH with Bi2MoO6, the resulted sample of LDH/Bi2MoO6composite(Fig.2e, f) exhibits an agglomeration, which is mainly due to the deposition of LDH on the surface of Bi2MoO6by a self-assembly process through the electrostatic interaction.
As LDH and Bi2MoO6both have a layered structure, such a 2D-2D close contact may lead to the formation of heterojunction between two components.The formed heterojunction between LDH and Bi2MoO6is beneficial for the effective separation of photo-excited electron/holes pairs, leading to the improved photocatalytic activity.
To further reveal the morphology and microstructure in the resulted LDH/Bi2MoO6nanocomposite, TEM images were taken and are displayed in Fig.3.The overlapping layers can be clearly observed with the dark black-like rectangular areas for Bi2MoO6and greyish areas for LDH (Fig.3a, b).The enlarged HRTEM images of LDH/Bi2MoO6are shown in Fig.3c, d.Three sets of inter-planar spacing values of 0.26, 0.35 and 0.38 nm can be observed due to the (101) plane of LDH, and (121) and (111)planes of Bi2MoO6,respectively.It further confirms the formation of a heterojunction structure between LDH and Bi2MoO6.The SAED image of LDH/Bi2MoO6(Fig.3e) also provide a direct evidence for the presence of both LDH ((101)plane) and Bi2MoO6((131), (040) and (021) planes).As shown in Fig.3f, EDS spectrum of LDH/Bi2MoO6nanocomposite consists of Bi, Mo, O, Zn, Cu and Al elements, indirectly illustrating the coexistence of both LDH and Bi2MoO6.Furthermore, STEM-EDS elemental mapping images of LDH/Bi2MoO6are shown in Fig.4.It is clearly visible that Bi2MoO6is combined with LDH nanosheets, leading to the formation of heterojunction structure.

Fig.2 SEM images of (a, b) Bi2MoO6, (c, d) LDH, and (e, f) LDH/Bi2MoO6 at different resolutions.
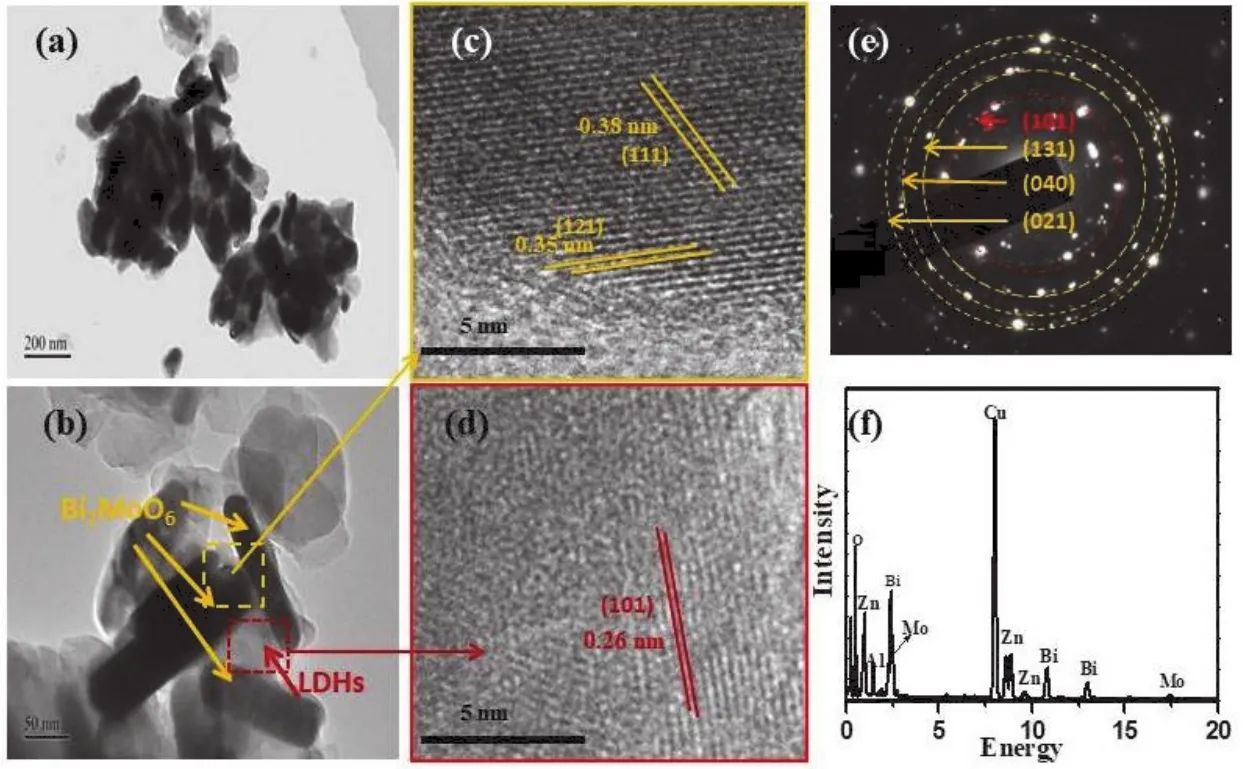
Fig.3 (a, b) TEM images, (c, d) HRTEM images, (e) SAED image and (f) EDS spectrum of LDH/Bi2MoO6.
The positions of valence band and chemical states in LDH,Bi2MoO6and LDH/Bi2MoO6were studied by XPS technique.Fig.5a shows two Zn 2pXPS peaks at 1022.2 eV (Zn 2p3/2) and 1045.1 eV (Zn 2p1/2) for pure LDH.By comparison, these two peaks at 1022.7 and 1045.7 eV can be also observed for LDH/Bi2MoO6, with a slight position change due to the interaction between LDH and Bi2MoO6.The introduction of Bi2MoO6may greatly affect the chemical environment of Zn2+,leading to the disappearance of Zn2+satellite peak in LDH/Bi2MoO6.The Cu 2pspectrum of pure LDH (Fig.5b)shows four signals at approximate 935.1 eV (Cu 2p3/2), 943.4 eV(Cu 2p3/2), 954.8 eV (Cu 2p1/2), and 962.8 eV (Cu 2p1/2)49.As shown in Fig.5c, two signals at 74.2 and 77.1 eV are attributed to the Al 2p3/2orbitals and 2p3/2satellite peak of Al3+in LDH34.After combining with Bi2MoO6, Cu 2pand Al 2pspectra of LDH/Bi2MoO6are almost the same as that of LDH.
As shown in Fig.5d, three peaks at 529.9, 531.9 and 534.5 eV are related to M―O, ―OH and H2O in O 1sspectrum of LDH.The pure Bi2MoO6shows two peaks at binding energies of 530.2 and 531.0 eV due to Bi―O and Mo―O, respectively31.For LDH/Bi2MoO6, peaks at 529.9, 530.7, 531.7 and 533.0 eV are clearly visible, corresponding to Bi―O, Mo―O, ―OH and H2O, respectively.The O 1sXPS spectrum of LDH/Bi2MoO6is shifted positively, revealing the electron transfer between LDH and Bi2MoO6.In Fig.5e, the Bi 4fspectrum of Bi2MoO6shows two peaks of Bi3+at binding energies of 159.3 and 164.6 eV,being slightly higher than those of LDH/Bi2MoO6.As shown in Fig.5f, Mo 3dspectrum of Bi2MoO6shows two signals at 232.6 eV (Mo 3d5/2) and 235.8 eV (Mo 3d3/2) due to the presence of Mo6+in MoO68,50.For LDH/Bi2MoO6, additionally, two new satellite peaks at 231.9 and 235.0 eV are visible, further confirming the presence of interaction between LDH and Bi2MoO6.

Fig.4 HRTEM image, STEM-EDS elemental mapping spectra, and the distribution of Zn, Cu, Al, Bi, Mo and O elements on LDH/Bi2MoO6.
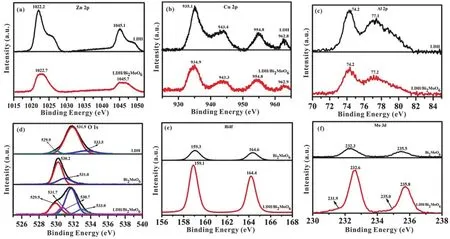
Fig.5 XPS spectra of (a) Zn 2p, (b) Cu 2p, (c) Al 2p, (d) O 1s, (e) Bi 4f and (f) Mo 3d of the as-prepared samples.
Therefore, the changes in binding energies for Zn 2p, Cu 2p,Al 2p, Bi 4f, Mo 3dand O 1sXPS spectra are closely related to the space structure, valence and the surrounding chemical environment.Meanwhile, it indicates that there is a strong interfacial chemical interaction between Bi2MoO6and LDH,which further confirms the formation of heterojunction between two components.
The photocatalytic performance was evaluated using RhB aqueous solution as an organic contamination under visible light.As displayed in Fig.6a, no obvious degradation is observed for RhB aqueous solution in the blank test.Only 2.5% of the RhB concentration was reduced within 60 min for pure LDH due to the surfaced adsorption effect, while 30% for pure Bi2MoO6.After combining LDH with Bi2MoO6, the resulted LDH/Bi2MoO6nanocomposites presents a greatly increased photocatalytic activity with 80% RhB degraded within 60 min.As shown in Fig.S1 (see Supporting Information), as the added amount of LDH is increased, the photocatalytic activity of LDH/Bi2MoO6 composites is firstly increased with a highest value for LDH/Bi2MoO6and then decreased.Although the proper amount of LDH is beneficial for the generation of electron-hole pairs, an excessive amount of LDH on the surface of Bi2MoO6will cause the decreased visible light absorption ability for both components and then reduced photocatalytic activity.It indicates that an appropriate mole ratio between LDH and Bi2MoO6is necessary for achieving an excellent photocatalytic activity.
As shown in Fig.6b, isopropanol (·OH scavenger),benzoquinone (scavenger), sodium oxalate (h+scavenger)and potassium dichromate (e-scavenger) were employed to investigate the role of the active species.The residual amounts of RhB are 75%, 85%, 40%, and 45%, respectively, indicating that ·OH andare the main reactive species, while e-and h+contribute a little in the degradation process.
In view of the practical application of the photocatalyst, the photocatalytic stability of LDH/Bi2MoO6 was also studied by recycling experiment (Fig.6c).After photocatalytic degradation of RhB, the sample was re-used for the cycling experiment.After recycling for four times, there was no significant decrease of the photocatalytic activity and the slightly reduced activity is mainly owing to the mass loss of photocatalyst during the centrifugation to collect the used catalyst for the next use.In addition, the XRD pattern of LDH/Bi2MoO6(Fig.6d) is almost unchanged before and after photocatalysis.These results indicate that LDH/Bi2MoO6nanocomposite has a good structural stability and recycling performance.
The electrochemical testing was employed to deeply explore the reasons for the enhanced photocatalytic activity.The transient photocurrent responses of LDH, Bi2MoO6and LDH/Bi2MoO6under visible light are shown in Fig.7a.The higher photocurrent density, the higher effective charge separation ability of photo-generated electron-hole pairs51.The pure Bi2MoO6 exhibits the lower photocurrent intensity.After combination with LDH, the photocurrent of LDH/Bi2MoO6 is almost twice that of pure Bi2MoO6, indicating that the construction of hetero-junction between LDH and Bi2MoO6can promote the effective separation of photo-generated electronhole pairs and accelerates the interfacial charge transfer.This is the main reason for the excellent photocatalytic performance of LDH/Bi2MoO6.
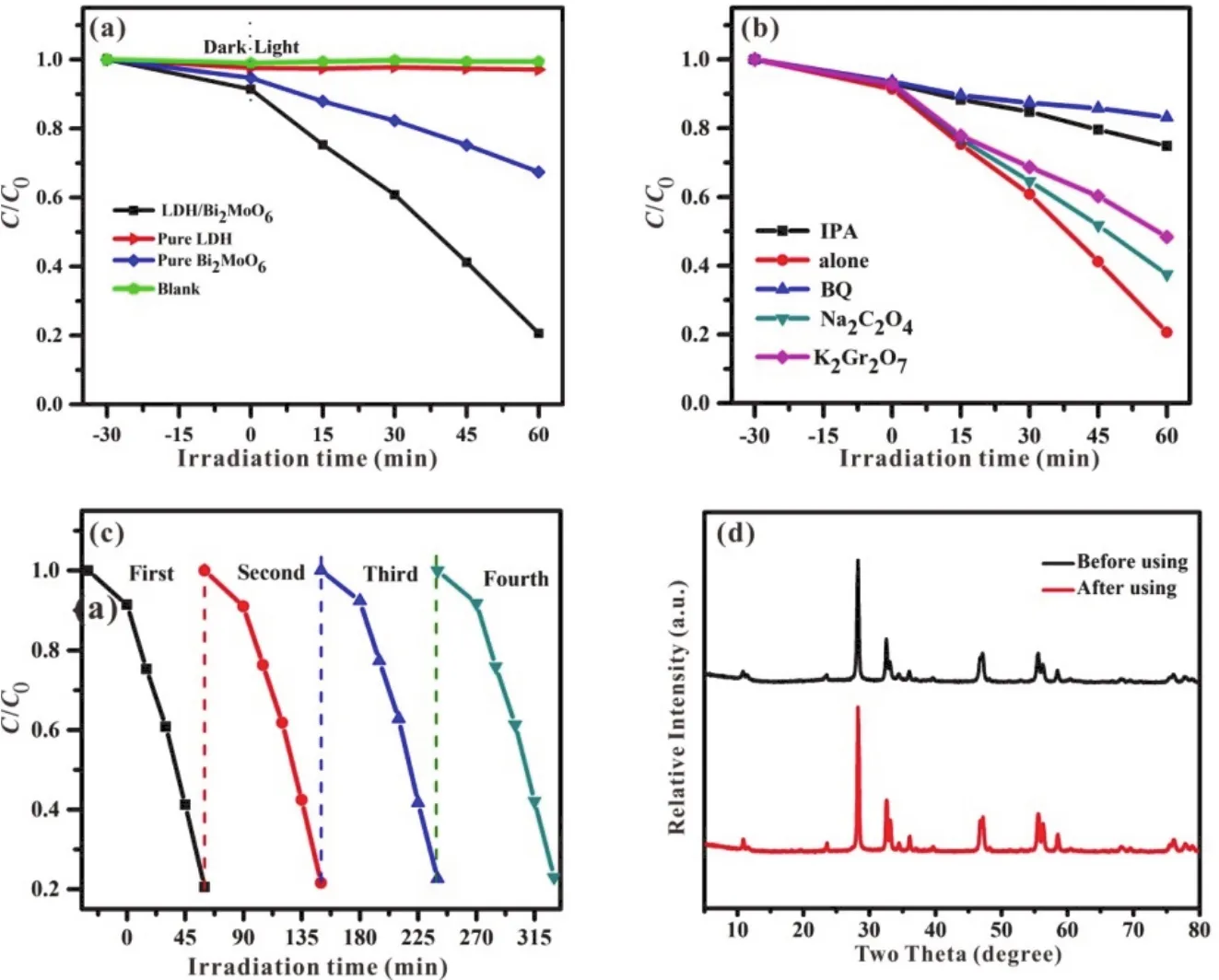
Fig.6 (a) Visible light photocatalytic degradation rate of RhB solution over different samples, (b) effects of different scavengers on the degradation efficiency of RhB over LDH/Bi2MoO6, (c) photodegradation of RhB over LDH/Bi2MoO6 for four successive cycles and(d) XRD patterns of LDH/Bi2MoO6 before and after photodegradation of RhB.
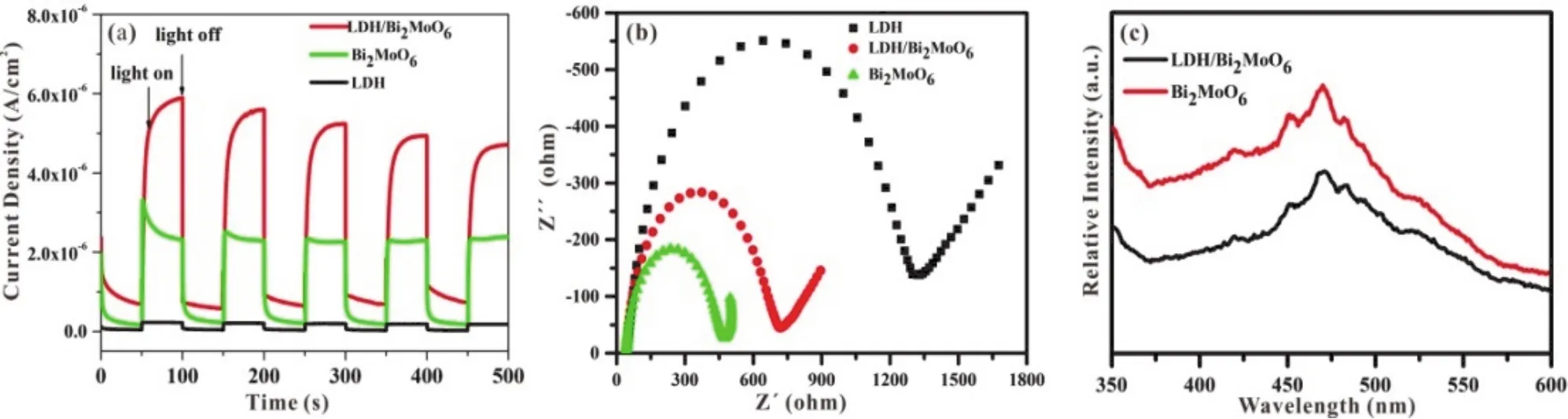
Fig.7 (a) Transient photocurrent response, (b) Nyquist plots of EIS for LDH, Bi2MoO6 and LDH/Bi2MoO6 thin film electrodes.(c) Photoluminescence (PL) spectra with an excitation wavelength of 325 nm for Bi2MoO6 and LDH/Bi2MoO6.
Fig.7b shows EIS Nyquist plots of the as-prepared samples to reveal the charge transfer process52.The resistance value(corresponding to the ordinate of the highest point of the arcs) of LDH, Bi2MoO6and LDH/Bi2MoO6is 550, 180 and 280 Ω,respectively.The magnitude of the resistance reflects a fairly reliable result.It intuitively reflects the fast electron transfer process of composite.Moreover, the arc radius of LDH/Bi2MoO6 is slightly larger than that of Bi2MoO6, indicating that the magnitude of the electron transport resistance is not a determinant of the photocatalytic performance.
The PL spectra of LDH/Bi2MoO6and Bi2MoO6are shown in Fig.7c.The PL intensity was positively related to the recombination rate of the photo-generated electron-hole pairs53.The lower peak intensity indicates the lower recombination rate of the electron-holes pairs54.The emission intensity of LDH/Bi2MoO6is obviously lower than that of Bi2MoO6,indicating that LDH/Bi2MoO6can effectively accelerate the transfer of photo-generated electron-hole pairs.Hence, it also indicates that the formed heterojunction can significantly reduce the recombination of electron-hole pairs.
In order to further clarify the reason for the enhancement of photocatalytic activity, the CB and VB values of LDH and Bi2MoO6were obtained according to the following formula55,56:
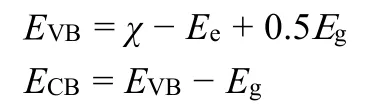
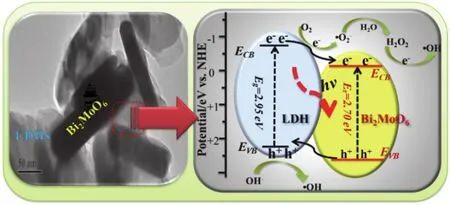
whereχ,EeandEgare the absolute electronegativity, the energy of free electrons on the hydrogen scale (~4.5 eV), and the band gap energy of the semiconductor, respectively.Theχvalues of LDH and Bi2MoO6are 5.25 and 5.54 eV8, respectively.Thus,the potentials of samples were calculated to be -0.68 eV (ECB)and +2.27 eV (EVB) for LDH, as well as -0.31 eV (ECB) and+2.39 eV (EVB) for Bi2MoO6.It can be seen that the forbidden band structure of LDH and Bi2MoO6can meet the requirements for forming the hetero-junctions.As theEVB= +2.39 eV of Bi2MoO6is more positive thanE(·OH/OH-= +1.99 eVvsNHE)57, the formed holes can directly react with OH-to produce ·OH radicals.TheECB= -0.68 eV of LDH is more negative thanE(eVvsNHE), the CB electrons allows the reduction of the dissolved O2intoradicals5.The proposed charge separation and transfer pathways under visiblelight irradiation in LDH/Bi2MoO6nanocomposite are shown in Scheme 2.

Scheme 2 A schematic illustration for the proposed charge separation and transfer pathways in LDH/Bi2MoO6 nanocomposite under visible-light irradiation.
4 Conclusions
In summary, we have successfully synthesized LDH/Bi2MoO6 nanocomposites by a steady-state co-precipitation route at room temperature, in which a 2D-2D close contact was achieved and hence a heterojunction structure was formed between two layered components.Compared with its pure constituents, the resulted LDH/Bi2MoO6nanocomposite exhibited a greatly increased visible-light driven photocatalytic activity in the degradation of RhB as well as a good stability.The combined effects of the heterojunction structure and the high specific surface area are respectively beneficial for the effective separation of photogenerated charge carriers and exposure of more active sites for photocatalysis, leading to the enhanced photocatalytic performance.The produced ·OH andare the main reactive species while e-and h+contribute a little in the photodegradation process.A possible mechanism for photocatalytic degradation was proposed based on the experimental results.This work provides a simple way for the preparation of LDH- and/or Bi2MoO6-based nanocomposites and the obtained LDH/Bi2MoO6 nanocomposite might find potential application as visible light photocatalysts in purifying the environment and alleviating resource shortages.
Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
