Asymmetric Hybrid Capacitor Based on NiCo2O4 Nanosheets Electrode
2020-07-23YongliTongMeizhenDaiLeiXingHengqiLiuWantingSunXiangWu
Yongli Tong, Meizhen Dai, Lei Xing, Hengqi Liu, Wanting Sun, Xiang Wu
School of Materials Science and Engineering, Shenyang University of Technology, Shenyang 110870, P.R.China.
Abstract:The looming global energy crisis and ever-increasing energy demands have catalyzed the development of renewable energy storage systems.In this regard, supercapacitors (SCs) have attracted widespread attention because of their advantageous attributes such as high power density, excellent cycle stability, and environmental friendliness.However,SCs exhibit low energy density and it is important to optimize electrode materials to improve the overall performance of these devices.Among the various electrode materials available, spinel nickel cobaltate (NiCo2O4) is particularly interesting because of its excellent theoretical capacitance.Based on the understanding that the performances of the electrode materials strongly depend on their morphologies and structures, in this study, we successfully synthesized NiCo2O4 nanosheets on Ni foam via a simple hydrothermal route followed by calcination.The structures and morphologies of the as-synthesized products were characterized by X-ray diffraction, scanning electron microscopy, and Brunauer-Emmett-Teller (BET) surface area analysis, and the results showed that they were uniformly distributed on the Ni foam support.The surface chemical states of the elements in the samples were identified by X-ray photoelectron spectroscopy.The as-synthesized NiCo2O4 products were then tested as cathode materials for supercapacitors in a traditional three-electrode system.The electrochemical performances of the NiCo2O4 electrode materials were studied and the area capacitance was found to be 1.26 C∙cm-2 at a current density of 1 mA∙cm-2.Furthermore, outstanding cycling stability with 97.6% retention of the initial discharge capacitance after 10000 cycles and excellent rate performance (67.5% capacitance retention with the current density from 1 to 14 mA∙cm-2) were achieved.It was found that the Ni foam supporting the NiCo2O4 nanosheets increased the conductivity of the electrode materials.However, it is worth noting that the contribution of nickel foam to the areal capacitance of the electrode materials was almost zero during the charge and discharge processes.To further investigate the practical application of the assynthesized NiCo2O4 nanosheets-based electrode, a device was assembled with the as-prepared samples as the positive electrode and active carbon (AC) as the negative electrode.The assembled supercapacitor showed energy densities of 0.14 and 0.09 Wh∙cm-3 at 1.56 and 4.5 W∙cm-3, respectively.Furthermore, it was able to maintain 95% of its initial specific capacitance after 10000 cycles.The excellent electrochemical performance of the NiCo2O4 nanosheets could be ascribed to their unique spatial structure composed of interconnected ultrathin nanosheets, which facilitated electron transportation and ion penetration, suggesting their potential applications as electrode materials for high performance supercapacitors.The present synthetic route can be extended to other ternary transition metal oxides/sulfides for future energy storage devices and systems.
Key Words:NiCo2O4 nanosheet;Electrochemical performance;Asymmetrical supercapacitor;Cathode material;Cycle stability
1 Introduction
In past few years, researchers have triggered tremendous efforts to design some emerging energy storage devices1-7.Among them, supercapacitors have attracted great attention as promising devices due to their high power density, ultralong cycle life and fast charge-discharge rates8-15.In general,capacitors can be divided into electric double layer capacitors(EDLCs) and pseudocapacitors based on charge storage mechanism.EDLCs arise from charge separation at the interface between electrodes and electrolyte, while capacitances of pseudocapacitors rely on reversible faradic reaction of active materials, which provide much higher capacitances than EDLCs.The performance of pseudocapacitors is highly dependent on the types and structures of electrode materials.Transition metal oxides have been studied as pseudocapacitor materials, such as Co3O4, NiO, SnO2, Fe2O3, etc.16-18.However, the poor electrical conductivities and slow ion diffusion rates restrain their applications as capacitor electrode materials.
Recent reports show that binary metal oxides possess superior electrochemical performances than single transition metal oxides, which present rich redox reactions and high electrical conductivities19-25.Meanwhile, spinel structured NiCo2O4nanomaterials have attracted tremendous interest26-28.In its structure, nickel element is distributed in octahedral sites and cobalt element occupies both tetrahedral and octahedral sites.Owing to its structural feature and synergetic effects, NiCo2O4electrode shows high electrochemical activities during redox reactions.Furthermore,p-type NiCo2O4electrode material with a direct bandgap of 2.1 eV shows high electrical conductivity which is convenient to electron transfer between cations.
Moreover, it is true that performance of NiCo2O4 electrode materials could be tremendously improved by constructing unique structures with high surface area29,30.In this work, we construct NiCo2O4nanosheets grown on nickel foam by a simple hydrothermal method.The as-prepared product as electrode materials shows an area capacitance of 1.26 C∙cm-2at current density of 1 mA∙cm-2and cycling stability with 97.6% retention of the initial discharge capacitance after 10000 cycles.An asassembled supercapacitor with the as-prepared samples as positive electrode shows an energy density of 0.14 and 0.09 Wh∙cm-3at 1.56 and 4.5 W∙cm-3, respectively.After 10000 cycles,it still maintains 95% of initial capacitance.
2 Experimental
All chemicals and reagents were of analytically grade and used directly without further purification.Typical procedure was shown as below.The synthesis of NiCo2O4 product was performed by dissolving 5 mmol·L-1Co(NO3)2·H2O (99%) and 2.5 mmol·L-1Ni(NO3)2·H2O (98%) in 60 mL deionized water,then 0.2 g NH4F (≥ 96.0%) and 1 g hexamethylenetetramine(HMT) (98%) was added into the above solution and stirred until complete dissolution.Subsequently, the mixture and a piece of cleaned nickel foam were transferred into 100 ml autoclave and kept 120 °C for 2 h.The obtained samples were treated with deionized water several times, then, dried in an oven at 60 °C for 12 h.Finally, the samples were calcined at 350 °C for 2 h.Average mass loading of the as-synthesized NiCo2O4nanosheets is 1.2 mg·cm-2.
The morphology of NiCo2O4samples was studied by scanning electron microscope (SEM, Hitachi-4800).XRD patterns were collected using a wide-angle X-ray diffractometer (XRD, 7000,Shimadzu) with CuKα radiation (λ= 0.1541 nm, 40 kV).BET surface area was calculated by pure nitrogen adsorption isotherm data at 77 K in a physisorption analyzer (ASAP2020 Micromeritics, Norcross, USA).X-ray photoelectron spectrum(XPS, PHI-5400, PE, USA) was tested through ESCALAB250 with using an AlKα sources.All the electrochemical performances of the samples were conducted on a CHI 660e electrochemical work station (Shanghai Chenhua Instrument Inc.).The as-synthesized NiCo2O4products were used as cathode material, platinum foil as counter electrode and Hg/HgO as reference one in a traditional three-electrode system for supercapacitors, 3 mol·L-1KOH aqueous solution was used as the electrolyte.
The asymmetric supercapacitors (ASC) were assembled with a piece of NKK paper as the separator between two pieces of electrodes face-to-face and solid-state polymer gel electrolyte(PVA/KOH) as electrolyte.In a typical procedure, the negative electrode is composed of acetylene black (20% (w, mass fraction)), active carbon (70% (w)) and polytetrafluoroethylene(PTFE, 10% (w)), and adding a bit of NMP as the solvent.The mixture was then coated onto Ni foam, and dried at 120 °C for 8 h.The electrolyte was prepared as follows: 4 g PVA was dissolved in 35 mL deionized water, stirring for 30 min at 80 °C.Then, 4 g KOH was dissolved in 5 mL deionized water and then added into above solution, stirring magnetically until the solution became clear.Before assembling, the electrodes were immersed in the electrolyte for 15 min.Then, they were assembled together.The loading mass of the positive and negative materials is matched precisely based on the charge balance mechanism (m+/m-= C-⋅V-/C+⋅V+).The loading mass of active carbon is 0.58 mg·cm-2.
The specific capacitance (C), energy density (E), and power density (P) were calculated from the discharge curves based on the following equations:

whereIis current density, Δtrefers discharge time, andVis applied potential window.mrepresents mass of Co2NiO4on nickel foam andsis the geometrical area of the electrode.
3 Results and discussion
The morphologies of as-prepared products are studied through SEM, as shown in Fig.1a and 1b.It is found that the as-prepared products present sheet-like structures and uniformly grow on Ni foam.Fig.1b shows the as-synthesized nanosheets possess average thickness of 20 nm and connect with each other.The crystal structures of the as-synthesized samples are studied through XRD (Fig.1c).The diffraction peaks located at 44.6°,51.9° and 76.6° could be ascribed to Ni foam.Those at 18.9°,31.3.°, 36.8°, 44.6°, 59.2° and 64.9° could be well indexed to the(111), (220), (311), (400), (511) and (440) crystal planes of NiCo2O4(PDF#20-0781).Specific surface area is tested by BET analysis, as shown in Fig.1d.It is found that NiCo2O4nanosheets present a specific surface area of 14.06 m2·g-1.XPS is used to further investigate surface chemical composition and chemical valence of NiCo2O4 sample.Fig.1e exhibits Co 2pemission spectrum, which could be well fitted with two spinorbit peaks.The fitting peaks at 779.5 and 794.8 eV are ascribed to Co3+, and binding energies at 781.6 and 796.4 eV might be ascribed to Co2+.The weak satellite peak shows that majority of Co exists in the form of Co3+.High-resolution Ni 2pspectra possess typical Ni 2p1/2 (796.5, 787.5 eV), Ni 2p3/2 (780.7, 773.5 eV) and two satellite peaks, as shown in Fig.1f.The satellite peak exhibits that most of Ni exists in Ni3+ion form31.

Fig.1 SEM images and structure characterization of as-prepared electrode materials.
Electrochemical performances of NiCo2O4products are measured in three electrode system with 3 mol·L-1KOH solution as electrolyte.Fig.2a presents CV curves of NiCo2O4 electrode at different scan rates.Redox peaks position is at about 0.24 and 0.42 V at 10 mV·s-1, which shifts with the increase of scan rate.In addition, the areas of CV curves increase with the increasing of scan rate, suggesting good interfacial kinetics and excellent charge/discharge performance.Figure 2b shows GCD curves of NiCo2O4 electrodes.The symmetrical GCD curves present excellent capacitive performance.The voltage plateaus are at about 0.32 and 0.41 V, respectively, which indicates the presence of redox reactions.It is in agreement with CV curves.In addition, it can be seen that NiCo2O4nanosheets exhibit an areal capacitance of 1.26 C·cm-2at a current density of 1 mA·cm-2.The areal capacitance can reach 0.85 C·cm-2with current density increasing to 14 mA·cm-2.The electrochemical impedance spectra (EIS) of the as-prepared samples are conducted at the frequency from 100 kHz to 0.01 Hz (Fig.2c).In low frequency region, the straight line shows diffusive resistance of electrolyte ions.The intersection with real axis shows bulk resistance (Rs) and the semicircle diameter suggests charge transfer resistance (Rct) in high frequency region32.Rsof NiCo2O4electrode is 1.07 Ω.To investigate the stability of the electrode materials, cycling stability test is conducted at a current density of 20 mA·cm-2, as revealed in Fig.2d.Because of the activated process of the electrode materials, their capacitance increase firstly.Up to about 2000 cycles, the capacitance reaches maximum and begin to decrease slowly due to the volume change of the charging and discharging process.It is observed that NiCo2O4 nanosheets electrode still maintains 97.6% of initial areal capacitance, revealing its excellent cycle stability.
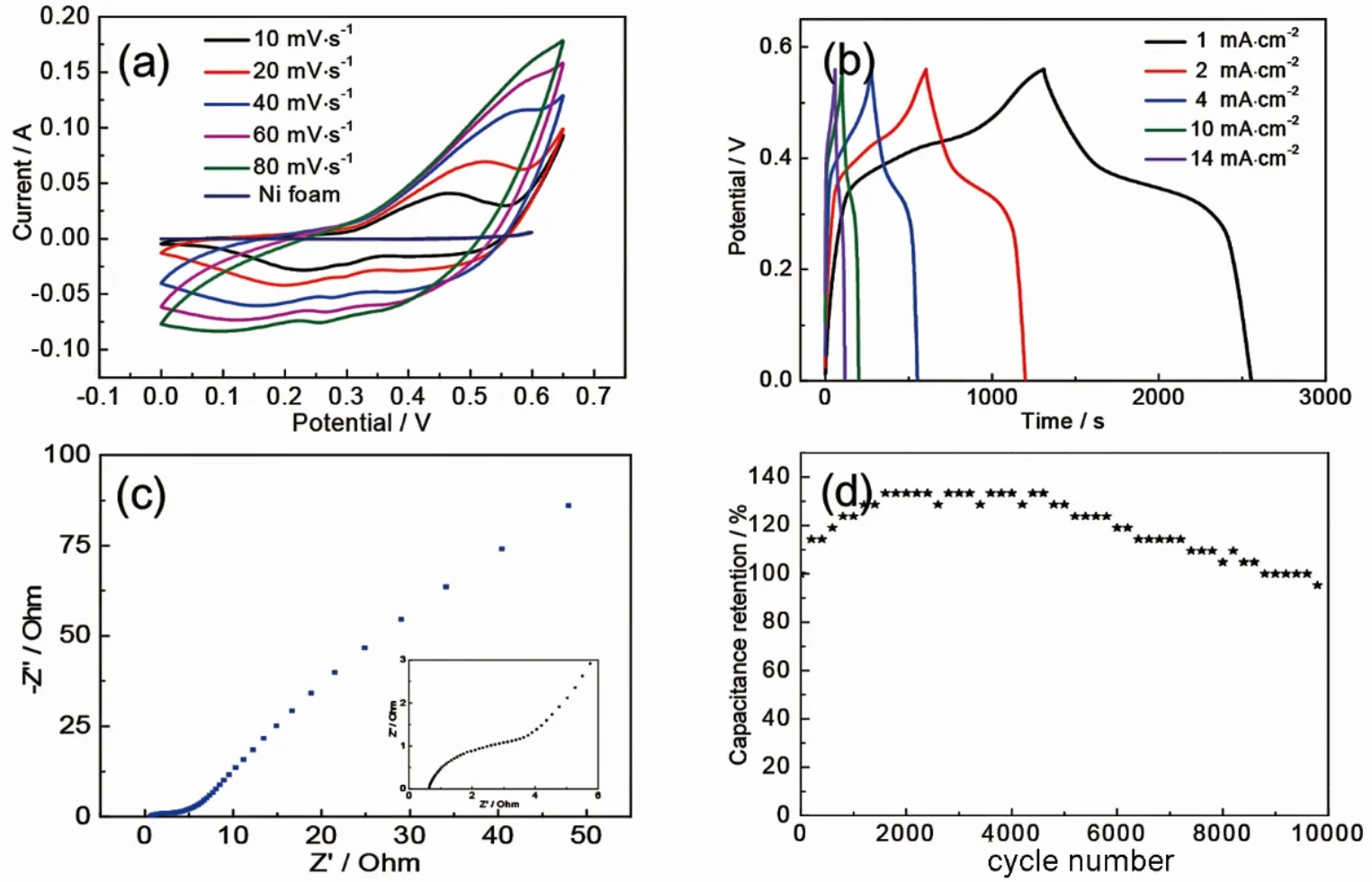
Fig.2 Electrochemical performance of the as-prepared electrodes.
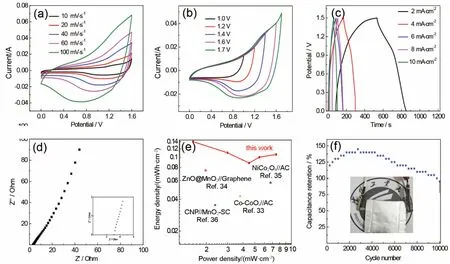
Fig.3 Electrochemical performances of device.
To further investigate practical application of NiCo2O4 nanosheets electrode, a device is assembled with the as-prepared samples as positive electrode and AC as negative electrode.Fig.3a presents CV curves of NiCo2O4//AC device with a voltage window of 0-1.6 V.It can be found that CV curves exhibit both electrical double-layer and Faradaic capacitive characteristic at various scan rates.Fig.3b shows CV curves at different potential windows with a scan rate of 50 mV·s-1, which the shape of CV curves maintain stably without polarization even when operating voltage reaches up to 1.6 V.However, when the voltage reaches 1.7 V, polarization occurs.GCD curves of the device are presented in Fig.3c.From the curves, areal specific capacitance can be calculated to 432, 353, 281.1, 326.9 and 338.5 mF·cm-2at current density of 2, 4, 6, 8 and 10 mA·cm-2, respectively.EIS of the device is measured with an open potential of 10 mV in Fig.3d.It can be obtained thatRs value of NiCo2O4//AC is 3.1 Ω, revealing the device present excellent electrochemical performance.Ragone plot of the device is shown in Fig.3e.The device exhibits an energy density of 0.14 and 0.09 Wh·cm-3at 1.56 and 4.5 W·cm-3, respectively, which is superior to the previous reports33-36.Cycling stability of the device is tested at a current density of 4 mA·cm-2(Fig.3f).The device possesses a capacitive retention of 95%, suggesting its excellent cycling stability.
4 Conclusions
NiCo2O4 nanosheets are prepared by a simple hydrothermal approach.The as-prepared product delivers high areal capacitance and maintains excellent retention of initial discharge capacitance after 10000 cycles.NiCo2O4//AC device still maintains 95% of the initial specific capacitance at 7.85 mF·cm-2.NiCo2O4 nanosheets electrode in this work can be used as a potential material for electrochemical supercapacitor.
