Porcine NF-κB p65 Subunit: Molecular Characterization, Tissue Expression and Transcriptional Profile in Porcine Epidemic Diarrhea Virus-infected IPEC-J2 Cells
2020-07-15LiuHaixinWangHongweiCaoLiyanDanteZarlengaGeXuyingZhangYueYinXuetingZhangRuiliRenYudongHuangXiaodanandLiGuangxing
Liu Hai-xin, Wang Hong-wei, Cao Li-yan, Dante S Zarlenga, Ge Xu-ying, Zhang Yue, Yin Xue-ting, Zhang Rui-li, Ren Yu-dong, Huang Xiao-dan*, and Li Guang-xing*
1 College of Veterinary Medicine, Heilongjiang Key Laboratory for Laboratory Animals and Comparative Medicine, Northeast Agricultural University,Harbin 150030, China
2 Harbin Veterinary Research institute, Chinese Academy of Agricultural Science, Harbin 150040, China
3 Animal Parasitic Diseases Laboratory, Agricultural Research Service, United States Department of Agriculture, Building 1180, BARC-East,Beltsville, MD, 20705, USA
4 College of Electrical and Information Technology, Northeast Agricultural University, Harbin 150030, China
Abstract: The p65 protein is a functional subunit of NF-κB family and exhibits a crucial role in host immune and in fl ammatory responses, apoptosis and tumor proliferation if improperly-regulated. Given its ubiquitous association with nearly all the animal cells and its pleotropic functions, the gene encoding NF-κB p65 subunit was cloned and sequenced from porcine kidney (PK-15) cells. The gene was 1 662 bp in length, encoded a 553-amino acid protein and contained the prototypical NF-κB functional domains. Real-time quantitative RT-PCR and Western blot were used to characterize the transcription and expression levels of the p65 in different pig tissues. The results indicated that the p65 gene and protein were both broadly expressed in pig tissues, but most highly expressed in the intestine-associated lymph nodes and the lungs. To localize the recombinant protein in intestinal porcine epithelial cells (IPEC-J2),the gene was subcloned into the vector pEGFP (pEGFP-p65). Using fl uorescence microscopy, the protein was found con fined to the cytoplasm in normal cells; however, during porcine epidemic diarrhea virus (PEDV) infection, mRNA and protein expression were significantly up-regulated and the protein exhibited an overt tendency for nuclear translocalization consistent with a regulatory role in antiviral innate immunity.
Key words: porcine NF-κB p65, tissue expression, bioinformatic analysis, porcine epidemic diarrhea virus
Introduction
The nuclear transcription factor, NF-κB, is a ubiquitous protein among animal cells and exhibits a plethora of functions including, but not limited to host immune and in fl ammatory responses, apoptosis and tumor cell proliferation (Grinberg-Bleyeret al., 2018; Grinberg-Bleyeret al., 2017; Zhanget al., 2017). In mammals,there are five members of NF-κB family: (RelA), RelB,c-Rel, NF-κB1 (p50/p105) and NF-κB2 (p52/p100)(Hayden and Ghosh, 2008; Baud and Karin, 2009). All the NF-κB members have a N-terminal Rel homology domain (RHD), which interacts with DNA, IκB and other Rel proteins. In addition, the p65 subunit,RelB and c-Rel possess a C-terminal transcriptional activation domain (TAD) (Zhanget al., 2017). In most cells, NF-κB exists as both homo- and hetero-dimers where the classical hetero-dimer consists of p50 and p65 subunits. It is generally accepted that activation of NF-κB is under strict control by the inhibitory molecule of IκB (Ghoshet al., 1998). In resting cells,NF-κB is sequestered and retained in the cytoplasm as a complex with IκB, this masks the nuclear localization sequence (NLS) of the p65 subunit which restricts its translocation to the nucleus (Hayden and Ghosh, 2008).When stimulated with signal molecules, such as tumor necrosis factor-α(TNF-α) and interleukin-1 (IL-1), IκB is phosphorylated by IκB kinase (IKK) complex. The Lys residues of phosphorylated IκB are ubiquitinated which is followed by degradation and dissociation from NF-κB and which in turn leads to the activation of NF-κB (Silverman and Maniatis, 2001). Activation of NF-κB by external stimuli plays a critical role in positively regulating NF-κB target genes related to immune responses and inflammation (Liuet al., 2015; Pahl,1999). In viral infections, activation of NF-κB exhibits pronounced function in both innate and adaptive immune responses. Many coronaviruses undergo immune evasion or induce proinflammatory cytokines by modulating NF-κB activation. For instance,transmissible gastroenteritis virus (TGEV) infection activates NF-κB signaling pathways and induces related cytokines (IL-6, IL-8 and TNF-α) that elicit inflammatory and antiviral immune responses (Dinget al., 2017).
The p65 protein, which is a functionally active subunit of NF-κB, is the only ubiquitously expressed TAD-containing NF-κB protein in mammalian cells and is important in a multitude of pathways. NF-κB is involved in inflammatory bowel diseaseassociated diarrhea. The binding of TNF to the p65 activates NF-κB which reduces DRA expression by interfering with DRA promotor binding thereby providing a viable target for therapeutic intervention in inflammatory-bowel disease (Kumaret al., 2017).It has been shown previously that NF-kB family,particularly, c-Rel and the p65, is crucial for the development, maintenance and function of regulatory T cells (Tregs) (Grinberg-Bleyeret al., 2018; Ruanet al., 2009). Reports on the p65 in dog, cat and bovine have been published (Ishikawaet al., 2015;Doleschallet al., 2006); however, little information is available on the porcine p65. To this end, the porcinep65 gene was cloned from PK-15 cells and relevant bioinformatic analyses were performed,including real-time quantitative RT-PCR (qRT-PCR)and Western blot to assess mRNA and protein expression levels, respectively, in pig tissues. The effects of virus inducted changes in the p65 were also investigated by examining alterations in expression patterns of the p65 in intestinal porcine epithelial(IPEC-J2) cells infected with porcine epidemic diarrhea virus (PEDV).
Materials and Methods
Cells, cell culture and virus
IPEC-J2 cells were cultured in Dulbecco's modified eagle medium/nutrient mixture F-12 (DMEM-F12;Gibco, USA) supplemented with 8% fetal bovine serum(FBS; CellMax, China), 1×insulin-transferrin-selenium(ScienCell, USA), 100 U · mL-1penicillin-streptomycin solution at 37℃ in a humidified atmosphere of 5%CO2. PEDV strain CV777 was typically maintained in the laboratory and propagated in IPEC-J2 cells.
Extraction of the total RNA and cloning of porcine NF-κB p65 gene
The total RNA was isolated from PK-15 cells using TRIzol reagent (Invitrogen, USA) and used for cDNA(PrimeScriptTM, TaKaRa, Japan) perp manufacture suggestions. PCR ampli fication of the porcine p65 was performed using the hyperfusion™ high- fidelity PCR Kit (Apexbio, USA) and forward (p65-F: 5'-ATGGAC GACCTCTTCCCCCTCATC-3') and reverse (p65-R: 5'-TTAGGAGCTGATCTGACTCAGAAGGG-3') primers(GenBank Accession Number, NM_001114281.1).Ampli fication conditions were as the followings: 98℃for 2 min, 35 cycles of 95℃ for 15 s, 58℃ for 15 s and 72℃ for 1 min, followed by a 10 min final extension at 72℃. The PCR product was recovered from a 1.5%agarose gel then cloned into pClone007 blunt (Tsingke,China). At last, three PCR-positive colonies were picked and sent to Tsingke Company for sequencing.
Bioinformatics analysis
The predicted amino acid sequence of the p65 was generated using NCBI server (https://www.ncbi.nlm.nih.gov/orffinder/) and ExPASy software (https://web.expasy.org/translate/). The sequence was aligned with reference p65 sequences from other species (Table 1)using the ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/). Phylogenetic trees were constructed using ClustalX 2.0 and MEGA 6.0 and neighbor-joining(NJ) methods. Basic physicochemical properties of the p65 protein, including molecular weight (MW)and isoelectric point (pI), were determined using ProtParam (https://web.expasy.org/protparam/).The presence of a putative signal peptide and transmembrane domain were assessed using SignalP 4.1(http://www.cbs.dtu.dk/services/SignalP/) and TMpred(http://www.ch.embnet.org/software/TMPRED_form.html), respectively. Conserved domains were identi fied using the SMART tool (http://smart.embl-heidelberg.de) and subcellular localization was predicted using PSORT II (https://psort.hgc.jp/form2.html) and NetNES (http://www.cbs.dtu.dk/services/NetNES/).Phosphorylation sites were performed using NetPhosK(http://www.cbs.dtu.dk/services/NetPhos/).
qRT-PCR analysis of porcine p65 mRNA expression in different tissues of pigs
Twelve different porcine tissues were examined for the p65 mRNA levels by qRT-PCR using FastStart Universal SYBR Green Master (ROX) (Roche,Switzerland) and a Roche LightCycler 480 RT-PCR machine (Applied Science, Basel, Switzerland). The total RNA was isolated from heart, liver, spleen, lung,kidney, duodenum, jejunum, ileum, submandibular lymph nodes (SLN), duodenal lymph nodes (DLN),jejuna lymph nodes (JLN) and iliac lymph nodes(ILN). The forward and reverse porcine p65 primers were 5'-AGTACCCTGAGGCTATAACTCG-3' and 5'- TGAGAAGTCCATGTCCGCAAT-3', respectively.The forward and reverse primers forβ-actin housekeeping gene were 5'-CCGGGACCTGACCGACTACCTC-3' and 5'-TGGCCATCTCCTGCTCGAAGTC-3', respectively.Relative expression of the p65 mRNA was normalized toβ-actin using the 2−ΔΔCTmethod. All the reactions were conducted in triplicate and independent experiments.
Western blot analysis
Concentrations of proteins extracted from different tissues, cell extracts and nuclear extracts were determined using a BCA kit (Beyotime, China).Fourty μg of proteins were separated on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis(SDS-PAGE) gels then electro-transferred to 0.45 μm nitrocellulose membranes (Millipore, Canada) as described. Membranes were blocked for 1 h at 37℃ in TBST (1 mol · L-1Tris-HCl, 5 mol · L-1NaCl and 10%Tween-20) containing 5% BSA, then incubated at 4℃ with rabbit anti-p65 monoclonal antibody(1 : 1 000) (MAbs; CST, USA) and mouse anti-β-actin MAbs (control; 1 : 1 000) (Beyotime, China) overnight followed by goat anti-rabbit or anti-mouse IgG-HRP secondary antibodies (1 : 3 000) for 1 h. After washing,target proteins were visualized and photographed using an enhanced chemiluminescence (ECL) detection system (Clinx Science Instruments, China).
Plasmid construction, transfection and immuno- fl uorescence assay
The cloned DNA fragment encoding the fulllength porcine p65 was re-amplified with primers containingHindⅢ andKpnⅠenzyme sites, respectively, then cloned into the pEGFP-N1 vector which generated a N-terminal, GFP-tagged recombinant protein. IPEC-J2 cells were seeded onto 24-well plates at a concentration of 1×105cells · well-1and cultured to 80% con fl uence. The cells were transfected with the pEGFP-p65 plasmid using Lipofectamine 2000 (Invitrogen, USA). After 18 h, the cells were infected with PEDV at 1.0 MOI. Thirtysix hours post transfection, cells were fixed with 4%formaldehyde for 30 min, permeabilization with 0.1%Triton X-100 for 15 min at room temperature, then counterstained for 5 min with 0.01% 4, 6-diamidino-2-phenylindole (DAPI) (Sigma, USA). The cells were washed three times with phosphate-buffered saline(PBS) and the fluorescent images examined under Nikon ECLIPSE Ti-S Inverted Microscope System(Nikon, Japan).
Luciferase reporter assays
IPEC-J2 cells were seeded into 24-well plates for cotransfected with 0.5 μg pNFκB-Luc and 0.1 μg phRLTK plasmids using Lipofectamine 2000 reagent, then cells were infected with PEDV or mock infected. The stimulation of polyinosinicpolycytidylic acid [poly(I:C)] was performed 12 h prior to cell harvest. The luciferase activities were detected 30 h post transfection using dual-luciferase reporter assay system(Promega) and the phRL-TK vector was used as an internal control in this experiment.
Nuclear protein extraction in PEDV-infected IPEC-J2 cells
Protein extraction from IPEC-J2 cells infected with PEDV was performed using NE-PER™ nuclear and cytoplasmic extraction reagents (Thermo,USA) following manufacturer's protocol. Briefly,IPEC-J2 cells were infected with PEDV (1.0 MOI).At 6, 12, 18, 24 and 36 h post infection (PI), cells were trypsinized (0.25% trypsin-EDTA solution),centrifuged at 500×g for 5 min, then washed with PBS and pelleted in a 1.5 mL tube. The cells were air dried,suspended in ice-cold cytoplasmic extraction reagent(CER I) to vortex vigorously for 15 s, then incubated for 10 min on ice. Pre-chilled CER II was added and the cells were vortexed for 10 s and centrifuged at 16 000×g for 5 min. Cell pellets were suspended in cold nuclear extraction reagent (NER), incubated for 40 min, then centrifuged at 16 000×g for 10 min. The resulting supernatant (nuclear extract) was transferred to a clean pre-chilled tube and stored at -80℃ for later use.
Statistical analysis
All the statistical analyses were performed with SPSS 11.0 and Gs'raphPad Prism 7 software (San Diego,CA). Students' test was used to analyze the experiment data from at least three separate experiment.p<0.05 or less represented the difference was significant(*p<0.05; **p<0.01.)
Results
Cloning and sequence analysis of porcine NF-κB p65 gene
Sequencing results indicated that the ORF of the porcine p65 was 1 662 bp in length and encoded a 553-amino acid protein (Fig. 1A). Three mutations 283 T→C, 1 378 A→G and 1 456 A→G were observed when compared to the genome-derivedSus scrofa p65 gene. Thep65 gene isolated from PK-15 cells had nucleotide homology of 99.8% 99.6%, 99.0% and 97.8%, respectively compared to those of domestic pigs, Banna miniature pig (Sus scrofabreed Banna mini pig inbred line), common warthog (Phacochoerus africanus) and babirusa (Babyrousa babyrussa). The analysis indicated that the isolatedp65 gene was 100% congruent within the IPT domain, the ankyrin protein binding site and the DNA binding site, which suggested that the non-synonymous base changes would have no effect on the corresponding biological function of the protein.
Bioinformatics analysis of porcine NF-κB p65 gene
Analysis of the primary structure using the SMART architecture research program showed that the porcine p65 contained a RHD proximal to N terminus(21-186 aa), an immunoglobulin-plexin-transcription(IPT) domain (193-289 aa) and a low complexity region (379-427 aa). The results of the ProtParam analysis indicated that the porcine p65 protein had a molecular mass of 60.4 ku, a predicted pI of 5.54 and an instability index of 50.96 which was consistent with it being an unstable acidic protein with a net negative charge [65 negatively charged (Asp+Glu)and 55 positively charged (Arg+Lys) amino acid residues]. Analyses from SignalP 4.1 and TMpred did not support the presence of a signal peptide or transmembrane region (Hayden and Ghosh, 2008) (Fig. 2).NetPhosK analysis indicated multiple, putative phosphorylation sites including protein kinase A (PKA)and protein kinase C (PKC). The SWISS-MODEL software was used to establish the homology modeling and predict the tertiary structure of the porcine p65 protein. The three non-synonymous mutations of the p65 protein from PK-15 cells did not significantly affect its tertiary structure, highly similar folds and helices were found in both tertiary structures of the isolated p65 and pig (Sus scrofa)p65.

Fig. 1 Identi fication of p65 gene ampli fication and recombinant plasmid construction

Fig. 2 Bioinformatic analysis of porcine p65 protein
Homology and phylogenetic analysis of NF-κB p65 protein
Searching in NCBI database by BLASTX showed that the created p65 (PK-15 cells) displayed a highly amino acid sequence similarity compared with domestic and wild pigs and other mammals (eg., Babirusa: 98%,Bactrian camel: 96%), the p65 protein was highly similar among different species, indicating that NF-κB p65 was well conserved over the course of evolution.Previous studies indicated that the selective pressure acting on RHD in the p65 protein was higher than on TAD and resulted in more variations in TAD based on its lower amino acid identity (Doleschallet al., 2007).To better understand the evolutionary relationship of the porcine p65 protein, phylogenetic trees were constructed based on the amino acid sequences from 22 different vertebrates using MEGA 6.0. As shown in Fig. 3, the p65 proteins derived from a recent common ancestor and could be divided into two branches,and all the porcine p65 proteins were in the same group. The p65 proteins from the same animal species preferentially clustered together, suggesting that thep65 gene was conserved during the evolution.
Subcellular localization of porcine NF-κB p65 protein
To investigate subcellular localization of the p65 protein, motif prediction algorithms were used to identify key protein domains that defined cellular predilection sites. Results showed the highest proclivity for the nucleus (56.5%) with substantially less tendency to reside in the cytoplasm (17.4%), cytoskeleton (13.0%), mitochondria (8.7%) or golgi apparatus (4.3%). This was supported by the fact that the p65 protein harbored a nuclear localization sequence(NLS) (Birbachet al., 2004) and a pat4 motif (amino acids 301 to 304), which consisted of a continuous stretch of four basic amino acids (arginine or lysine) and might function in protein localization. A pat7 sequence,which started with a proline and followed within three residues by a segment containing three basic residues(Zhenget al., 2004), was also identi fied.
Tissue expression patterns of porcine NF-κB p65
qRT-PCR was used to assess the p65 mRNA expression levels in various pig tissues. The data showed that the p65 transcripts presented in all the examined tissues (Fig.4A) with the highest levels occurring in SLN, JLN and lung, moderate levels in DLN, duodenum, spleen, liver,ILN, jejunum, ileum and kidney, and the lowest levels in the heart. Similar results were obtained from all the four pigs. Antibody binding to the p65 protein was studied to support or refute the RT-PCR data. Results showed that the p65 protein was most prevalent in liver and JLN (Fig. 4B), followed by DLN, ILN, SLN,lung, jejunum, duodenum, spleen and heart. The lowest levels of antibody binding were observed in the kidney and ileum. Surprisingly, the p65 protein expression(Fig. 4C) did not coincide with tissue-based mRNA expression levels (Fig. 4A) particularly in the livers.This discrepancy might related to the microRNAs and epigenetic regulation (Bustinet al., 2009); however, it was likely associated with its cellular function and with subcellular translocation of the protein. Because the protein was pleotropic and mobile, localized production might not coincide with predilection sites following synthesis as one might prediction. This was supported in part by preferential migration to the nucleus following viral infection.

Fig. 3 Phylogenetic tree of p65 proteins taken to represent evolutionary history
Nuclear translocation of NF-κB p65 in PEDV-infected IPEC-J2 cells
To corroborate subcellular localization predictions and the effect of PEDV infection on subcellular localization, thep65 gene was subcloned into the expression vector pEGFP-N1 and used to transfect IPEC-J2 cells. Immuno-fluorescence assays in normal cells did not support the predictive characters of the programs which localized the p65 protein predominantly to the nucleus. The pEGFP-p65 protein exhibited diffuse cytoplasmic localization and was observed in lesser amounts in the nucleus in unstimulated IPEC-J2 cells (Fig. 5A).

Fig. 4 Expression patterns of p65 gene in different tissues of pigs
These results were validated in different cell lines, such as ST and PK-15 cells (data not shown).The immuno-fluorescence assay demonstrated the localization of the p65 protein in resting cells, which was consistent with previous reports (Hayden and Ghosh, 2008). The reason why the p65 protein was different from the predicted results of the online software was that the p65 protein bound to inhibitory protein IκB and presented in an inactive state in the cytoplasm where IκB could combine with the homologous domain (RHD) of the p65 through its ankyrin repeated domain (Zhanget al., 2017).Stimulation, such as proinflammatory cytokine and growth factors, could activate the inactive NF-κB/IκB complex, then the activated IκB underwent degradationviaubiquitination and released the p65 into the nucleus to exert its related cellular activation functions (Bonizzi and Karin, 2004). At this time,the subcellular localization of the active p65 was consistent with the predicted results, mainly located in the nucleus.
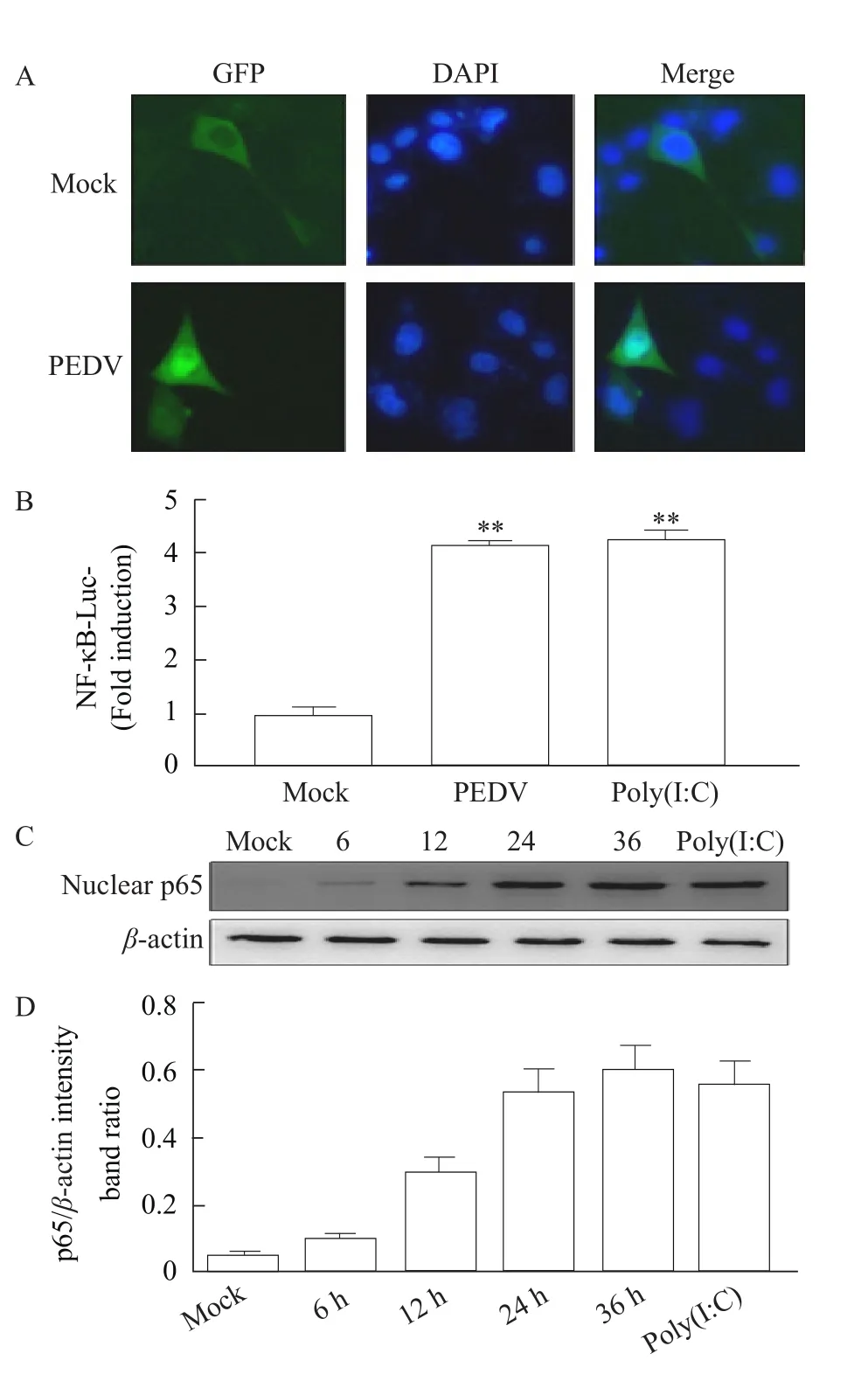
Fig. 5 Increased production and nuclear translocalization of NF-κB p65 in PEDV-infected IPEC J2 cells
In consequence, obvious nuclear translocalization of the p65-GFP was observed in PEDV-infected IPEC-J2 cells. To further investigate the relationship between PEDV infection and NF-κB p65 expression,the research first co-transfected IPEC-J2 cells with pNF-κB-Luc plasmid and pRL-TK plasmid and lysed the cells for detection of NF-κB luciferase reporter plasmid activity 36 h after transfection. As shown in Fig. 5B, both PEDV and poly (I:C) could significantly induce activation of NF-κB compared with mock group. The expression of the nuclear p65 at different time points after PEDV infection was also studied.As shown by Western blot analysis (Fig. 5 C and D),PEDV infection resulted in the translocation of NF-κB p65 from the cytoplasm to nucleus in IPEC-J2 cells. Nuclear accumulation of the p65 protein began to increase at 12 h PI and was significantly increased at 24 and 36 h PI. As shown in Fig. 5A, nuclear translocalization of the p65-GFP was observed in PEDV-infected IPEC-J2 cells; however, a small amount of proteins was observed in the cytoplasm of mock cells. The results indicated that PEDV infection activated and increased the production of NF-κB p65 protein in a time-dependent manner and promoted nuclear translocation in IPEC-J2 cells.
Discussion
The NF-κB family, as a ubiquitous transcription factor,was capable of binding to the promoter regions of many genes to initiate transcription of genes (Zhanget al., 2017). Therefore, NF-κB, which was widely distributed in various tissue cells, played an important role in regulating various physiological processes, such as immune response and inflammatory response. For example, NF-κB inhibited the endogenous apoptotic pathway through regulating the target genesBcl-2 andBcl-Xl and could also inhibit the exogenous apoptotic pathway by up-regulating the expression of c-IAP1/2(cellular inhibitor of apoptosis proteins 1/2) and FLIP(FLICE-like inhibitory protein). In addition, NF-κB could inhibit apoptosis indirectly by regulating the activity of the p53 (Lee and Sancar, 2011).
Since nuclear translocation of the p65 subunit was a necessary process in activation of NF-κB, the results suggested that PEDV infection could regulate NF-κB signaling pathway by stimulating nuclear translocation of the p65 protein and promoting the expression of NF-κB-mediated proinflammatory cytokines in IPEC-J2 cells. These results were consistent with the previous report showing that PEDV infection induced NF-kB activation in intestinal epithelial cells (IECs)(Caoet al., 2015). However, with the evolution of viral immune evasion mechanisms, some viruses could cause immunosuppression by down-regulating NF-κB in host cells. For example, the avian leukosis virus subgroup J (ALV-J) blocked the phosphorylation of IκBα on Ser32/36 amino acid residues which decreased the expression of NF-κB p65, increased host immunosuppression and enhanced susceptibility to secondary infection (Linet al., 2018). In like manner,African swine fever virus (ASFV) negatively regulated NF-κB signaling (Wanget al., 2018) which could affect evasion of the host immune system.
The p65 subunit had been widely studied and discussed as the most important functional subunit of NF-κB family. In the classical pathway of NF-κB activation, the p65/p50 heterodimer was translocated into the nucleus and bound to κB site on the target gene promoter/enhancer, which could rapidly induce transcription of multiple target genes and various inflammatory factors production (Chatterjeeet al.,2016). Therefore, NF-κB was known as a switch that controlled the early gene expression. In recent years, with the deepening of researches, it had been found that the p65 was not only a central regulator of innate immunity, but also associated with various pathological processes in the body. Activation of the p65 promoted the expression of pro-inflammatory factors to induce chronic in fl ammation, which in turn mediated the formation of cancer through NF-κB signaling pathway.
Conclusions
In conclusion, this study successfully isolated thep65 gene from the porcine kidney cells (PK-15 cells) and constructed the recombinant plasmid pEGFP-p65.By constructing the p65 protein genetic phylogenetic tree of different species, this study found that the newly isolated porcine p65 protein from PK-15 cells was on the same branch of the p65 protein from pigs of other breeds and was genetically conservative.This study also examined the distributions of the p65 at mRNA and protein levels in piglets by qRT-PCR and Western blot. This experiment also illustrated the association of coronavirus infection with NF-κB activation. This study had important guiding significance for future researches on the p65 related aspects.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Regeneration Function Analysis of GmESR1 in Transgenic Soybean
- Effect of Salt Stress on Nitrogen Assimilation of Functional Leaves and Root System of Rice in Cold Region
- Effects of Straw Returning with Different Tillage Patterns on Corn Yield and Nitrogen Utilization
- Effects of Rare Earth Lanthanum and Cerium on Antioxidant Enzyme Activities in Soybean Leaves
- Regulating Effect of Exogenous Silicon on Soil Fertility in Paddy Fields
- Uptake of B, Co and Ni by Plants from Oil Contaminated Soil Capped with Peat
