Establishment of RT-LAMP Assay for Infectious Pancreatic Necrosis Virus
2020-07-15YangYaoXuLimingZhaoJingzhuangRenGuangmingLuTongyanandYinHaifu
Yang Yao , Xu Li-ming, Zhao Jing-zhuang, Ren Guang-ming, Lu Tong-yan, and Yin Hai-fu
1 Colllege of Animal Sciences and Technology, Northeast Agricultural University, Harbin 150030, China
2 Heilongjiang Fishery Research Institute, Chinese Academy of Fishery Sciences, Harbin 150070, China
Abstract: The purpose of this study was to establish a method for the rapid detection of infectious pancreatic necrosis virus (IPNV,Jasper serotype) using reverse transcription loop-mediated isothermal ampli fication (RT-LAMP). Four groups of speci fic primers were designed, according to the genome sequence of a Chinese IPNV isolate ChRtm213. The results showed that primer set B2 had the best ampli fication effect. When the final concentration of Mg2+ was 6 mmol · L-1, dNTPs were 1 mmol · L-1 and betaine was 0.4 mol · L-1,the reaction could be completed in a 63℃ water bath within 60 min. This RT-LAMP assay for the detection of IPNV had no crossreactivity with infectious hematopoietic necrosis virus, viral hemorrhagic septicemia virus, grass carp reovirus and spring viremia of carp virus. The detection limit was 3.2×10-12 ng · μL-1. The sensitivity of this method was 10-fold higher than that of a previously published RT-LAMP assay for detecting the Spajarup (Sp) serotype of IPNV. This method, aimed at detecting IPNV isolates that were currently prevalent in China, possessed the characteristics of strong speci ficity, high sensitivity and direct interpretation by the naked eyes. The IPNV RT-LAMP was successfully applied to determine the clinical samples, which indicated the IPNV RT-LAMP assay was suitable for the rapid and large-scale detections of IPNV in China.
Key words: infectious pancreatic necrosis virus, loop-mediated isothermal ampli fication, serotype, visual detection
Introduction
Infectious pancreatic necrosis virus (IPNV) is the pathogen responsible for infectious pancreatic necrosis of salmon and trout. IPNV belongs to the familyBirnaviridaeand is the archetypical species of the genusAquabirnavirus, whose members are naked and harbor a two-segmented, double-stranded RNA genome comprising fragments A and B (Dobos,1995). The genome fragment A is 3 099 bp long comprising two overlapping open reading frames(ORFs): the larger ORF encodes a 106 ku polyprotein(NH2-pVP2-NS-VP3-COOH) that is processed by viral proteases and packaged to form VP2 protein precursor (pVP2), NS protein (VP4 protein) and VP3;the smaller ORF encodes VP5, a 15 ku non-structural polypeptide.
The genome fragment B is 2 789 bp long and encodes a virion-associated RNA-dependent RNA polymerase (RdRp) VP1, which is capable of inducing the synthesis of viral RNAin vitro(Yao and Vakharia,1998; Doboset al., 1979). VP2 is an important structural protein containing major viral antigenic determinants associated with toxicity (Santiet al., 2003).The role of VP3 is linked to virus assembly and genome packaging (Pedersenet al., 2007). IPNV infection is highly contagious, with mortality rates up to 90%. Fish that survive carries the virus for their whole lives, causing a huge threat to the salmon and trout farming industries (Bieringet al., 2005; Roberts and Pearson, 2010).
In the 1950s, IPNV was first reported in a freshwater brook trout (Salvelinus fontinalis) facility in North America (Woodet al., 1955). In 1960, Wolfet al.isolated the first IPNV in America. In the 1980s,the first outbreak of IPNV in a rainbow trout fish farm in China resulted in the large-scale death of rainbow trout (Niu and Zhao, 1988), but IPNV was not isolated by cell line in China until 1989 (Jianget al., 1989). Recently, IPNV has been detected in many rainbow trout farms in China (Jiet al., 2017;Zhuet al., 2017). However, there are no effective drugs for the prevention and the control of IPNV in China. Therefore, it is important to monitor IPNV effectively. One complication in the detection of IPNV is that this virus has up to 10 serotypes and genome sequences differ considerably among different serotype strains (Hill and Way, 1955; Bainet al.,2010), indicating that specific detection methods should be established according to the most prevalent IPNV strains. In the 1980s, the most prevalent IPNV strains in China belonged to Spaiarup (Sp) serotype(Tong and Hetrick, 1989; Jianget al., 1990), whereas recent studies have found that currently circulating IPNV strains in China belong to Jasper serotype (Liuet al., 2017; Zhuet al., 2017), indicating that the previous prevalent IPNV strains have transformed into Jasper IPNV strains in China. It is reasonable to establish an efficient detection method for prevalent Jasper IPNV strains.
Previously published IPNV detection technologies mainly include immunological detection methods, such as enzyme-linked immunosorbent assay (Tong and Hetrick, 1989), indirect immunocolour assay (Wang,2012), molecular biological detection methods, such as real-time fluorescent quantitative RT-PCR (Xuet al., 2011) and quantitative triple real-time reverse transcription PCR (Hofereret al., 2017). The immunological detection methods require antibodies against different serotypes of IPNV, which can be difficult and time-consuming to obtain, and the molecular biological detection methods require specialized equipments and operators, limiting on-site or largescale detections of pathogens. However, RT-LAMP detection method has low requirement on specialized equipment, which makes it more appropriate for largescale detections of pathogens on field.
The loop-mediated isothermal amplification(LAMP) technique is a nucleic acid isothermal amplification technique developed by Notomiet al(2000). The detection can be completed in an hour at constant temperature, and the sensitivity is higher than that of ordinary PCR (Nagamineet al., 2001).The results of RT-LAMP can be detected not only by agarose gel electrophoresis, but also directly using colour detection reagent SYBR Green I stain for visual inspection (Soliman and Elmatbouli, 2006). Due to the high sensitivity and visual detection (Nakamuraet al., 2007; Notomiet al., 2000), RT-LAMP technology has been widely applied to the detection of aquatic pathogens, such asCyprinid herpesvirus2 (CyHV-2)(Zhanget al., 2014),Salmonid alphavirus(Liuet al.,2018) andIridovirus(Caipanget al., 2004).
To establish an RT-LAMP assay for prevalent IPNV in China, four sets of specific primers were designed, according to the genome sequence of IPNV ChRtm213 and the IPNV RT-LAMP detection method was established and optimized.
Materials and Methods
Cells and virus strains
Chinook salmon embryo cells (CHSE-214) were gifted by Professor Zeng Lingbing of the Fish Disease Teaching Laboratory of the Chinese Fishery Science Institute at Yangzi River Fisheries Institute. IPNV strain ChRtm213 (Jiet al., 2017), infectious hematopoietic necrosis virus (IHNV) strain Sn1203 (Xuet al.,2014), spring viremia of carp virus (SVCV) (Jiet al.,2017) and grass carp reovirus (GCRV) were from lab stocks. Viral hemorrhagic septicemia virus (VHSV)(ATCC: VR-1387) were purchased from China Center for Type Culture Collection (CCTCC).
Primer design
Primer sets targeting the genome IPNV strain ChRtm213 (GenBank accession numbers: KX23491 and KX23490) were designed using the online software Primer Explorer V5 (https:/primer explorer.jp/lampV5). To obtain the best primer sets, genome sequences of IPNV, including VP2, VP3 and B chain,were used as templates for primer design. Each set comprised six primers: external positive primer (F3),external reverse primer (B3), internal forward primer(FIP), backward internal primer (BIP), loop primer(LooF) and reverse loop primer (LooB). Detailed information about the primers is listed in Table 1.The published primers for the RT-LAMP detection of IPNV NVI-015 (Sp serotype) (Santiet al., 2003)designed by Solimanet al. (2009) were also synthesized and shown in Table 1.
RNA extraction
CHSE-214 cells infected with IPNV ChRtm213 were collected, when a cytopathic effect was observed on over 70% of the cells. The cell culture suspension was freeze-thawed three times before RNA extraction.IPNV was purified by centrifugation, according to previous studies (Hsu and Leong, 1985; Changet al.,1978). The steps were as the Trizol reagent instructions(TaKaRa, USA). RNA from CHSE-214 cells was utilized as a negative control. The extracted RNA was stored at -80℃ or used directly for RT-LAMP.
Selection of the best primers for RT-LAMP
The 25 μL reaction mixture for RT-LAMP comprised 40 pmol each of the inner primers FIB and BIP,10 pmol each of the outer primers F3 and B3, 20 pmol each of the loop primers (Loop-F and Loop-B),1.0 mmol · L-1each of dNTPs (TaKaRa, USA), 2.5 μL of 10×isothermal ampli fication buffer (containing a final concentration of 2 mmol · L-1MgSO4) (New England BioLabs, Beijing), 1 μL of 100 mmol · L-1MgSO4(New England BioLabs, Beijing), 0.4 mol · L-1betaine (Sigma,USA), 10 U AMV reverse transcriptase (Promega,USA) and 8 UBst2.0 DNA polymerase (New England BioLabs, Beijing) and the final concentration of the system RNA sample was 3.2×10-1ng · μL-1.
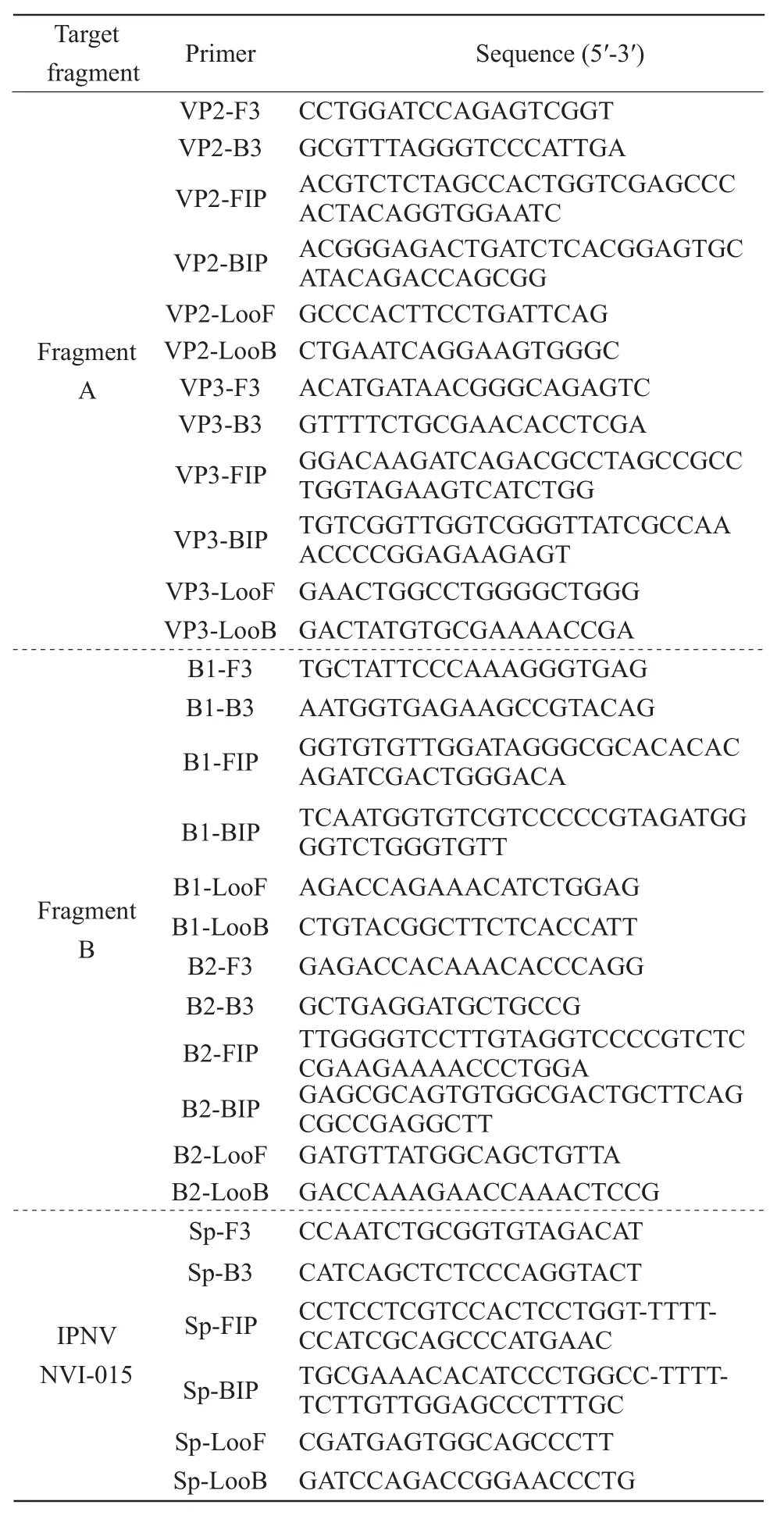
Table 1 Sequences of RT-LAMP primers used to amplify IPNV strain ChRtm213
DEPC water was added to a final reaction mixture volume of 25 μL. The test was carried out in the biosafety cabinet (Beijing Donglian Harbin Instrument Manufacturing Co., Ltd). The mixture was incubated at 63℃ for 1 h in a water incubator and then heated at 80℃ for 2 min to stop the reaction. The test was carried out in a biosafety cabinet. After the reaction, 5 μL of the ampli fication products was analyzed by 2% agarose gel electrophoresis and visualized by the addition of 5 μL of 100 diluted SYBR Green I (Invitrogen, USA). After gently shaking, the result was judged by observing a color change of the reaction solution.
Optimization of RT-LAMP detection reaction mixture
Optimized RT-LAMP primers were used to analyze the detection mixture and study the influence of various factors of the amplification by RT-LAMP.The main factors in the reaction mixture were tested at various concentrations and included Mg2+(final concentrations of 2, 4, 6, 8 and 10 mmol · L-1), betaine( final concentrations of 0.0, 0.4, 0.8 and 1.2 mol · L-1),dNTPs ( final concentrations of 0.5, 1.0 and 1.5 mmol · L-1)andBst2.0 DNA polymerase. The reaction was carried out at temperatures of 63℃, 65℃ and 67℃.At the end of the reaction, both visual detection and gel electrophoresis were carried out to analyze the in fl uence of these variables. Positive samples showed green, while negative samples showed reddish-brown.RNA from normal CHSE-214 cells was used as a negative control. The electrophoretic bands of positive samples were ladder-like patterns of bands, while not any bands could be observed from negative samples.The same results were determined in all the independent triple repeated experiments.
Speci ficity of RT-LAMP
Most rainbow trouts could be infected with IPNV,IHNV and VHSV. Fish that was infected with these three viruses had similar clinical symptoms, with mortality rates up to 90%. Therefore, this study selected IHNV, VHSV, SVCV and GCRV for speci ficity analyses. The RNAs of IPNV, IHNV, VHSV, SVCV and GCRV were used as templates to analyze the speci ficity of the RT-LAMP assay for IPNV detection. RNA from CHSE-214 cells was used as a negative control.
Sensitivity of RT-LAMP assay compared with a published IPNV RT-LAMP assay
IPNV genomic RNA was subjected to 10-fold dilution and used as template for sensitivity analysis of the established IPNV RT-LAMP assay. To compare the sensitivity of the reaction mixtures, the optimal primers and Sp were used for comparison (Table 1).
IPNV-RT-LAMP on clinical samples
The ability of RT-LAMP to detect IPNV in clinical specimens was assessed by testing salmon spleen samples from different fish farms. The spleen samples were lab stocks and previously subjected to viral detection in our lab. The samples were named as No.1-7, and No.1 and No.4 were IPNV negative. RNA was extracted from the spleen samples by using Trizol reagent.
Results
Optimization of primers
Four sets of RT-LAMP primers were designed for use in the RT-LAMP assay (Table 1). RT-LAMP products ampli fied with the primer sets VP2, VP3 and B2 showed green when SYBR Green I was added.Whereas, RT-LAMP products amplified with primer set B1 showed reddish-brown, therefore, represented the negative control. Following gel electrophoresis,no specific ladder-like banding pattern was observed with the products ampli fied by primer set B1 or primer set VP2, whereas a ladder-like pattern of bands was observed with the products amplified by primer sets B2 and VP3. Furthermore, the ladder-like pattern of bands for the products amplified by B2 primers showed earlier was clearer, more speci fic and showed earlier than that of products ampli fied by VP3 primers.These results indicated that primer sets B2 and VP3 both functioned well in the IPNV RT-LAMP assay and the amplification efficiency of primer set B2 appeared to be optimal and was therefore chosen for all the subsequent experiments (Fig. 1).

Fig. 1 RT-LAMP assay performed using different primer sets
Optimization of IPNV RT-LAMP reaction mixture
The concentrations of the main components in the ampli fication reaction mixture, including Mg2+, betaine and dNTPs, were optimized using primer set B2 for the detection of IPNV by the RT-LAMP assay. The results of Mg2+concentration analysis showed when the absolute concentration of Mg2+was 4-8 mmol · L-1,the amplified products showed green and a ladderlike pattern of bands was observed following SYBR Green I addition and gel electrophoresis, respectively.However, when the final concentration of Mg2+was 2 mmol · L-1, the products appeared reddish-brown and no ladder-like pattern of bands was observed by gel electrophoresis (Fig. 2). The results showed that the optimal concentration of Mg2+was 4-8 mmol · L-1.
The results of betaine concentration optimization analysis showed when the final concentration of betaine was 0.0-1.2 mol · L-1, all of the amplified products showed green after the addition of SYBR Green I and a clear ladder-like pattern of bands was observed by gel electrophoresis (Fig. 3). Therefore, the final concentration of betaine of 0.0-1.2 mol · L-1was selected for this assay.
The dNTPs concentration optimization analysis showed when the final concentration of dNTPs was 0.5-1.5 mmol · L-1, the ampli fication products presented green following the addition of SYBR Green I and gel electrophoresis analysis showed a ladder-like pattern of bands (Fig. 4). The final concentration of dNTPs of 0.5-1.5 mmol · L-1was optimal for this assay.
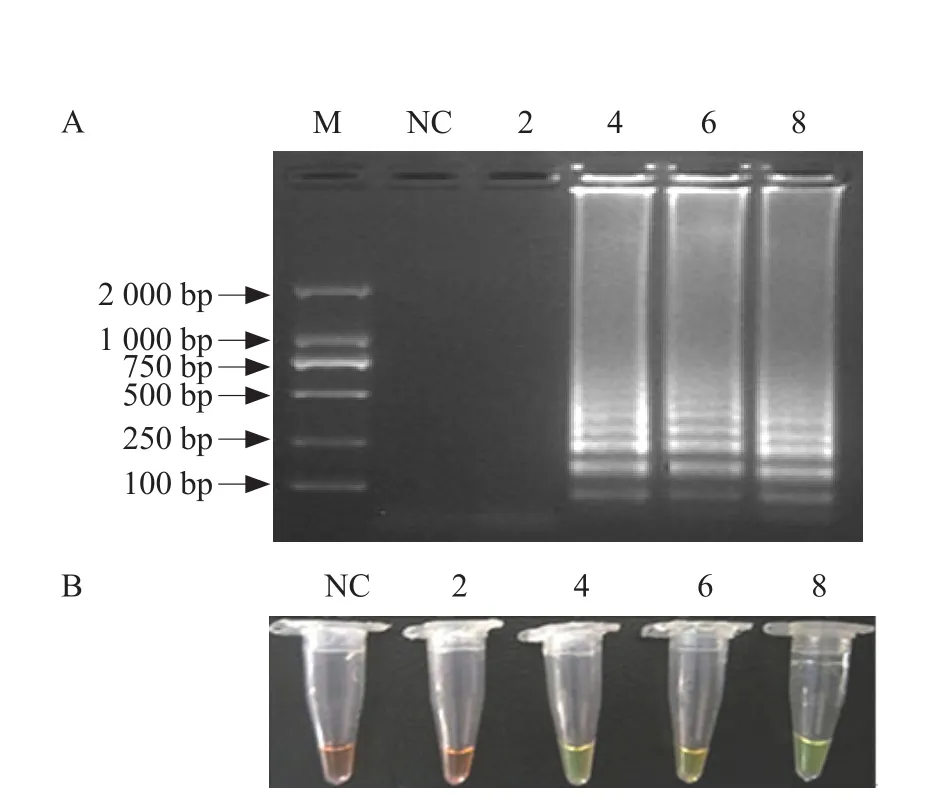
Fig. 2 Optimization of Mg2+ concentration in IPNV RTLAMP assay
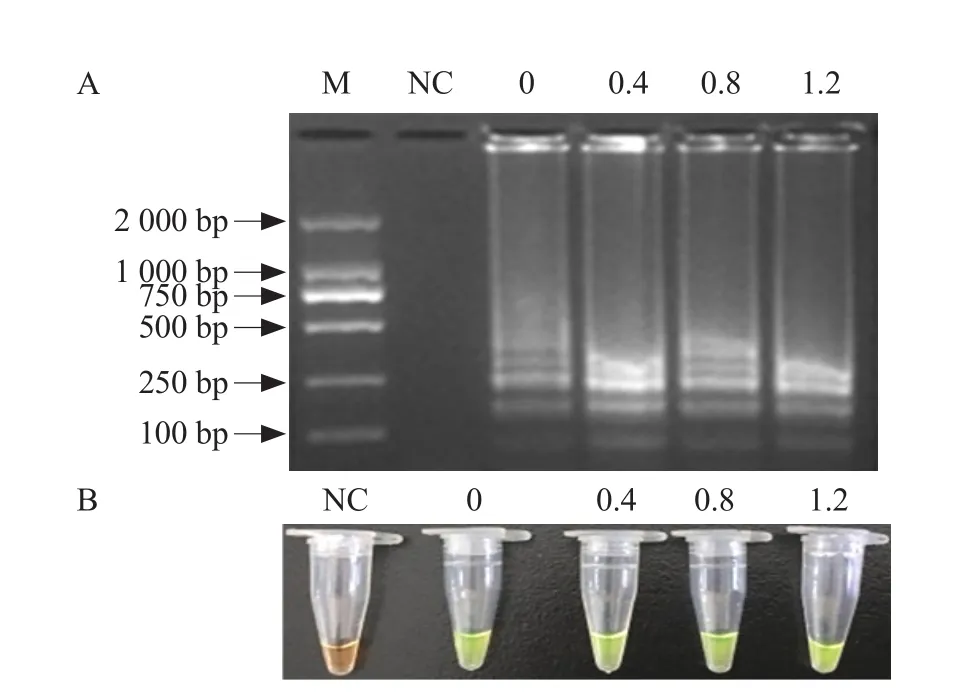
Fig. 3 Optimization of betaine concentration in IPNV RTLAMP assay
Optimization of reaction temperature
To detect the optimal reaction temperature, amplification was performed at 63℃-67℃ using primer set B2, Mg2+concentration of 6 mmol · L-1, betaine concentration of 0.4 mol · L-1and dNTPs at concentration of 1.0 mmol · L-1. The results showed when SYBR Green I was added to the amplified products, all of them showed green and specific ladder-like banding patterns were observed following gel electrophoresis.However, the green and ladder-like pattern of the products amplified at 63℃ was slightly clearer than that of products amplified at 65℃ and 67℃. An optimal reaction temperature of 63℃ was therefore selected for this assay (Fig. 5).
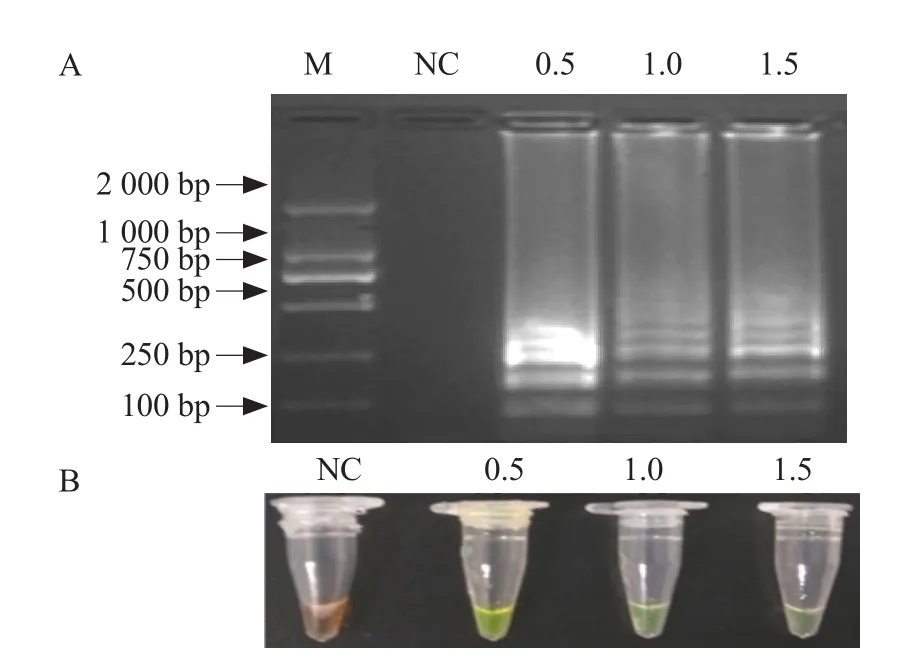
Fig. 4 Optimization of dNTPs concentration in IPNV RTLAMP assay
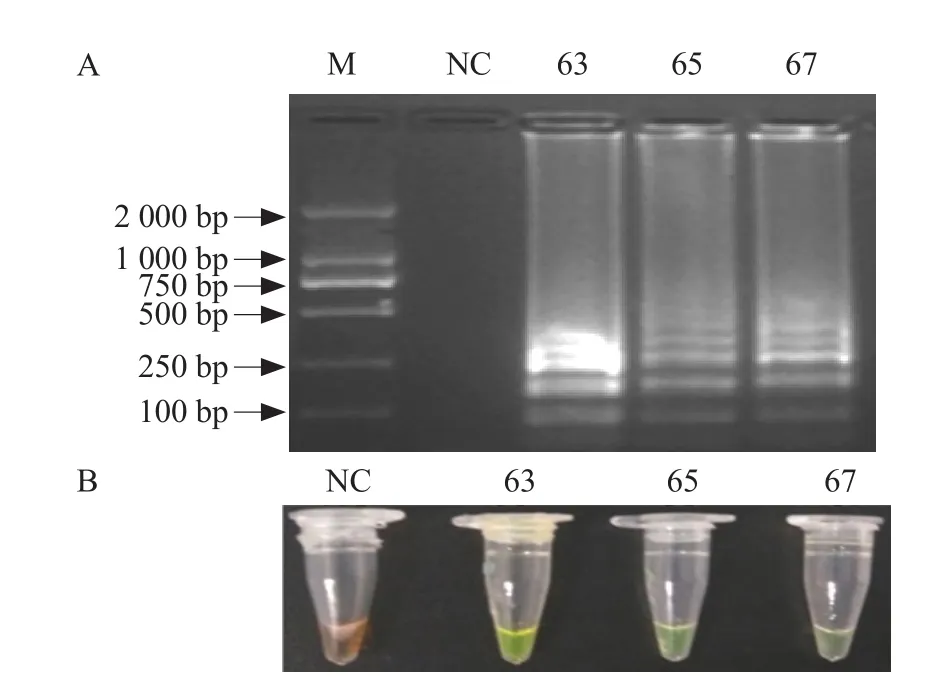
Fig. 5 Optimization of reaction temperature for IPNV RTLAMP assay
RT-LAMP speci fic detection
The amplified products obtained using IPNV as a template showed green and a ladder-like pattern of bands was observed following gel electrophoresis. By contrast, the amplification products obtained using SVCV, GCRV, VHSV and IHNV as templates were reddish-brown and no ladder-like pattern of bands was observed following gel electrophoresis. These results indicated that the RT-LAMP assay had no crossreactivity with IHNV and VHSV, therefore, displayed high speci ficity (Fig. 6).
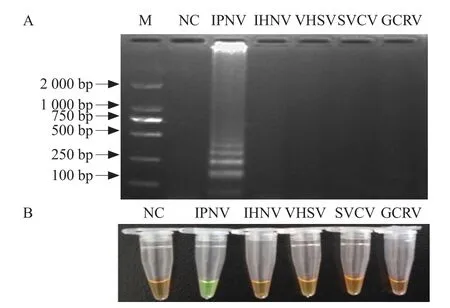
Fig. 6 Speci ficity of IPNV detection by RT-LAMP assay
Sensitivity of RT-LAMP assay
IPNV RNA was 10-fold diluted to determine the sensitivity of the RT-LAMP assay using the optimized primers and conditions. A previously published primer set Sp and assay conditions were used for comparison. Using primer set B2, the amplified products showed green and a ladder-like banding pattern was observed following the addition of SYBR Green I and gel electrophoresis, respectively, when the IPNV RNA template was not less than 0.0008 fg. At the same concentration of IPNV RNA, neither green nor ladder-like bands were observed using primer set Sp, but green and dispersed bands were observed,when IPNV RNA was not less than 0.008 fg. Therefore,the minimum detection limit of primer set B2 was 3.2×10-12ng · μL-1, while the minimum detection limit of primer set Sp was 3.2×10-11ng · μL-1. These results indicated that the RT-LAMP assay established in this study was more sensitive than the previously published RT-LAMP assay (Fig. 7).

Fig. 7 Sensitivity of IPNV detection by RT-LAMP assay
IPNV-RT-LAMP analysis on clinical samples
The RT-LAMP assay was used to detect seven clinical spleen samples from different fish farms in China. No. 2, 3, 5, 6 and 7 turned green after addition of SYBR Green I, but No. 1 and No. 4 were still brown. Ladder-like bands' results were the same as before. The results indicated that samples No. 2, 3, 5, 6 and 7 were IPNV positive and No. 1 and No. 4 were IPNV negative (Fig. 8), which were accordant with records of these clinical samples in our lab.
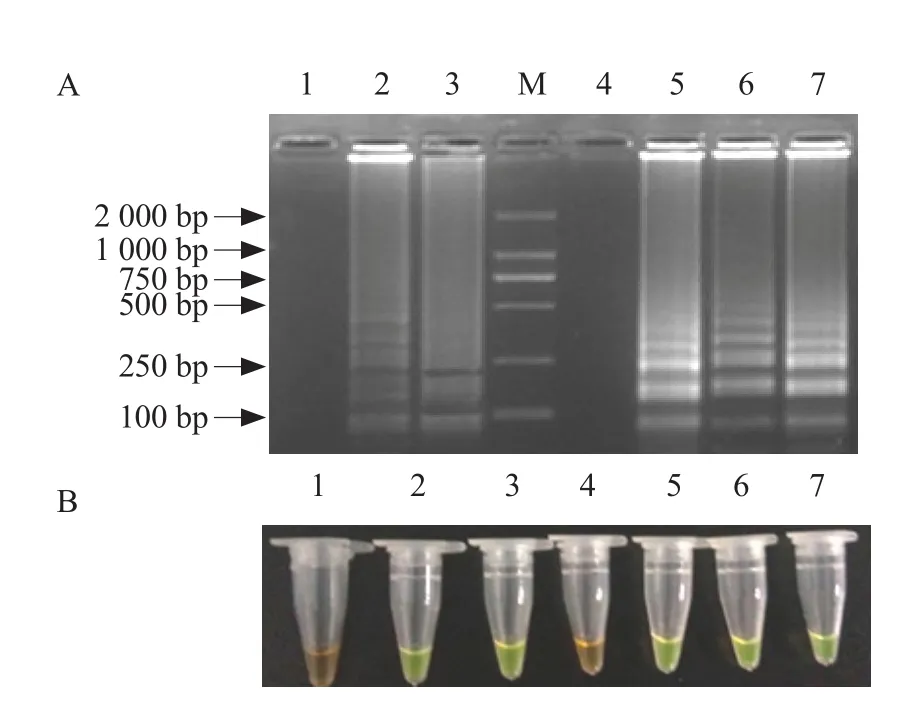
Fig. 8 RT-LAMP detection method application
Discussion
IPNV was one of the infectious pathogens that threatened salmonid fish worldwide (Liuet al., 2017).IPNVs could be divided into 10 serotypes and six genogroups (1-6) (Blakeet al., 2001). Variations at the nucleotide and amino acid level existed among different serotype strains. Previous studies showed that Sp serotype IPNV was prevalent in China in 1980s (Tong and Hetrick, 1989; Jianget al., 1989).However, recent studies showed that the prevalent IPNV in China has transformed into Jasper serotype(Jiet al., 2017; Zhuet al., 2017). For better monitor of the current IPNV in China, an RT-LAMP assay was established using a currently prevalent Chinese IPNV strain ChRtm213 of Jasper serotype in this study.Optimization of the primers, other reaction components and the reaction mixture was carried out, along with specificity and sensitivity analyses, to validate this rapid detection method for IPNV strains in China.
Screening of the four primer sets revealed that one primer set allowed for more stable and efficient ampli fication, indicating that the primer sequence was a key factor affecting the detection efficiency of the RT-LAMP assay. It had been reported in the literature that Mg2+was required for the activity ofBstDNA polymerase and ensured the speci ficity of the product and free Mg2+enhanced visualization of the results(Xuet al., 2011). In this study, the concentration of Mg2+in the reaction mixture was optimized. The results showed that the assay worked well, when the concentration of Mg2+was 4-8 mmol · L-1, which was accordant with a previous study for detecting GCRV by RT-LAMP (Zhanget al., 2013).
The dNTPs concentration played an important role in efficient DNA amplification. In this study, an optimized concentration of dNTPs of 0.5-1.5 mmol · L-1was determined, which was accordant with a previous study for detecting IHNV with RT-LAMP reported by Liuet al(2014).
Betaine could promote the destruction of DNA secondary structure by reducing the stability of the helix structure of DNA, and excessive amounts of betaine could inhibit RT-LAMP (Zhang and Mei,2002). Xiong (2011) reported that the optimal concentration of betaine was 0.6 mol · L-1in a RT-LAMP assay for the detection ofScylla serratareovirus. In this study, the concentration of betaine was optimized and optimal levels of detection were obtained, when the concentration of betaine was within the range of 0.0-1.2 mol · L-1.
Specificity analysis showed that the established IPNV RT-LAMP detection method lacked crossreactivity with the RNA extracted from fish pathogenic viruses IHNV, VHSV, SVCV and GCRV, confirming the high speci ficity of the developed assay. To assess the sensitivity of the RT-LAMP assay, comparisons were made with a published IPNV RT-LAMP method(Solimanet al., 2009) using IPNV ChRtm213 RNA as a template. The results showed that the IPNV detection method established in this study was more suitable for the detection of Chinese IPNV and the sensitivity was as high as 3.2×10-12ng · μL-1. The IPNV RT-LAMP detection method established in this experiment was successfully applied to clinical determination, which indicated that the IPNV RT-LAMP was suitable for large-scale detections.
Conclusions
In this study, RT-LAMP technology was applied to establish a rapid, sensitive and highly specific IPNV detection method. Using Mg2+concentration of 6 mmol · L-1, dNTPs at 1 mmol · L-1, betaine at 0.4 mol · L-1, primer set B2 and 63℃ water bath, the reaction could be completed within 1 h. The detection limit of this method was 3.2×10-12ng · μL-1, which was 10-fold higher than that of a previously published RT-LAMP assay for IPNV detection. Furthermore,the assay showed no cross-reactivity with four other salmonid viruses IHNV, VHSV, SVCV and GCRV.The IPNV RT-LAMP was successfully applied to determine clinical samples.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Regeneration Function Analysis of GmESR1 in Transgenic Soybean
- Effect of Salt Stress on Nitrogen Assimilation of Functional Leaves and Root System of Rice in Cold Region
- Effects of Straw Returning with Different Tillage Patterns on Corn Yield and Nitrogen Utilization
- Effects of Rare Earth Lanthanum and Cerium on Antioxidant Enzyme Activities in Soybean Leaves
- Regulating Effect of Exogenous Silicon on Soil Fertility in Paddy Fields
- Uptake of B, Co and Ni by Plants from Oil Contaminated Soil Capped with Peat
