In Situ Determination of Iron Distribution and Oxidation State in Broiler Duodenal Wall by Synchrotron Radiation µ-XRF and µ-XANES
2020-07-15SunRuitaoLiuDasenZhangMeimeiBabarindeEmmanuelLiuHuaweiZhangPengandShiRubin
Sun Rui-tao, Liu Da-sen, , Zhang Mei-mei, Babarinde Emmanuel, Liu Hua-wei, Zhang Peng, and Shi Ru-bin
1 College of Animal Sciences and Technology, Northeast Agricultural University, Harbin 150030, China
2 College of Science, Northeast Agricultural University, Harbin 150030, China
3 College of Life Sciences, Northeast Agricultural University, Harbin 150030, China
Abstract: Micro-X-ray fl uorescence (μ-XRF) and micro-X-ray absorption near edge structure (μ-XANES) have not been widely used in animal nutrition. In situ determination of iron distribution and oxidation state in broiler duodenal wall was done by μ-XRF and μ-XANES techniques. Fifty newly hatched healthy (1-day-old) Arbor Acres commercial male broilers were used in this experiment.The chicks were fed a corn-soybean meal basal diet (96.00 mg of Fe/kg diet) from the 1st day to the 21st day, but were fed a semipuri fied diet (12.50 mg of Fe/kg diet) after 21 days to deplete the body Fe stores. On the 28th day, after an overnight fast, all the birds were randomly allotted to four different Fe sources [FeSO4, Fe-Gly (II), FeCl3 and Fe-Gly (Ⅲ)]. Three points five mL nutrient solution was injected to a broiler's duodenum by technology of in situ ligated intestinal loops and a perfusion group without Fe sources added to the media was designed to exclude the effect of endogenous Fe. Being incubated for 30 min, duodenal midpieces sections were collected for analyses by μ-XRF and μ-XANES techniques. It was found that Fe concentration for ferrous Fe sources [FeSO4 and Fe-Gly (Ⅱ)] was significantly higher than that for ferric Fe sources [FeCl3 and Fe-Gly (Ⅲ)] in the duodenal wall, moreover, Fe-Gly (Ⅱ)group samples had the largest amount of Fe. μ-XANES spectra of Fe for the four sources were basically the same in the duodenal wall,which indicated that despite the ferric iron supplied to duodenum, ferrous iron could also be absorbed into the duodenal wall.
Key words: µ-XRF, µ-XANES, Fe source, Fe concentration, duodenal wall
Introduction
Iron (Fe) is an essential element for poultry and it plays an important role in many digestive,physiological and biosynthetic processes within the body and some of proteins and enzymes in the animal proteome require iron for their structures or functions(Hareet al., 2015). Forms of added iron in diet include inorganic iron (FeSO4) and organic iron, in which iron is chelated with ligand, such as glucose and amino acid, moreover, iron amino acid is used popularly.Fenget al. (2009) indicated that supplementation with Fe-glycine (Fe-Gly) improves iron tissue storage and antioxidant enzyme activities. Additionally, a reduction in fecal Fe concentrations is found, when pigs are fed diets containing Fe as Fe-Gly compared to FeSO4. And the higher levels of chelated minerals in the diets can promote bone concentration and dressing percentage compared to the inorganic mineral sources(Ellenet al., 2012). Researches on organic minerals are undertaken actively, because the chelated minerals are more effectively absorbed into the intestines than inorganic oxide and sulfate (Wedekindet al.,1992; Aoyagi and Baker 1994); however, there isn't a clear statement about the absorption mechanism of the chelated minerals. To date, two hypotheses,namely the complete absorption hypothesis and the competitive absorption hypothesis, have existed to explain absorption of the chelated minerals (Ashmead,1993). The former hypothesis indicates that mineral and chelate are not separated and assimilated together into the blood through the intestinal wall, the later states that mineral and chelate in the digestive tract may avoid being combined with precipitant and inhibitor in the lumen, so that more mineral amino acid chelates arrive at absorption sites of intestinal wall than inorganic mineral elements, then they are hydrolyzed in the absorption point of intestinal brush border and assimilated into the blood circulation in the form of ions. However, no direct experimental evidences prove which of the two hypotheses better explains the absorption of organic mineral elements into the intestinal wall on account of the limits of the experimental techniques.
X-ray fluorescence (XRF) has the advantages of being multi-elemental, not destructive, fast and with good accuracy and precision characteristics (De Carvalhoet al., 2018). Two-dimensional imaging of element, including the quantity and distribution in an inhomogeneous sample with ppm detectability, is nobtained (Bertucciet al., 2016). X-ray absorption near-edge structure (XANES) is the part of the spectrum comprised the features just prior to the edge as well as the edge itself and the position of both the distinctive features and the edge itself is often used to identify oxidation states of elements (Young, 2014)and XANES is strongly sensitive to formal oxidation state and coordination chemistry (e.g., octahedral and tetrahedral coordination) of the absorbing atom.XANES is applied for determination of Fe3+/∑Fe ratios with its energy shift of the pre-edge peak as a quantitative measure of oxidation state (Zhanget al., 2018). Christensenet al. (2004) also reported that XANES may be used for determining selenium oxidation states in animal mineral supplements.Meanwhile, transition metal oxidation states based on XANES spectra reveals the presence of divalent,trivalent and/or mixed valence transition metals in the materials as high-spin and low-spin complexes (Adaket al., 2017). Nowadays, this technology has been used in human or animal cells and tissues to get information about the concentration of different elements and the oxidation states (Lisa and Qi, 2007; Polgáriet al.,2011).
Generally, synchrotron radiation facility may produce X-ray beam with brighter and tunable characters (Hareet al., 2015). Furthermore, X-ray microprobe may be obtained, which is called micro-X-ray. When micro-X-ray comes from synchrotron radiation (SR) facility, XRF and XANES are called SR micro-X-ray fl uorescence (SRμ-XRF) and SR micro-X-ray near edge absorption structure (SRμ-XANES),respectively. The techniques of SRμ-XRF and SRμ-XANES analyze sample within micron area and without any special sample preparation. However, SRμ-XRF and SRμ-XANES have been used rarely in animal nutrition researches, therefore, the objective of the present study was to determine iron distribution and valence in the duodenal wall of broiler using SRμ-XRF and SRμ-XANES techniques.
Materials and Methods
Standard Fe sources
Reagent-grade Fe sulfate (FeSO4· 7H2O) with Fe concentration of more than 20.14%, brought from Tianjin Tianli Chemical Industry Co., Ltd. China and Fe chloride (FeCl3· 6H2O) with Fe concentration of more than 20.72%, brought from Guangzhou Xilong Chemical Industry Co., Ltd. China, were used as inorganic iron sources. Organic iron sources included Fe2+-glycine [(Fe-Gly (Ⅱ)] and Fe3+-glycine [Fe-Gly(Ⅲ)] with Fe concentration of more than 14.00% were provided by Guangzhou Tanke Technology Co., Ltd.China.
Broiler feeding
The experimental design was approved by the Ethical Committee of the Veterinary Faculty of Northeast Agricultural University (China). Prior to the experiment, the broiler house was disinfected with white wash and fumigated with formalin gas and drinkers were properly disinfected with 5% KMnO4solution, cleaned with water and dried under direct sunlight. Fifty newly hatched healthy (1-day-old)Arbor Acres commercial male broilers were used in this experiment and managed, according to Arbor Acres guidelines. All the birds were randomly housed in stainless steel suspended cages with fiberglass feeders and plastic waterers. Diet and water were providedad libitum. The chicks used in the experiment were fed a corn-soybean meal basal diet (90.60 mg of Fe/kg of diet, Table 1) from the 1st day to the 21st day, but were fed a semi-purified diet (12.50 mg of Fe/kg of diet, Table 1) after 21 days to deplete the body Fe stores. All other nutrients in the two diets met or exceeded the requirements for broiler and the birds were provided deionized water with undetectable levels of Fe.
Operation of in situ ligated intestinal loops technology
Firstly, on the 28th day, after an overnight fast, all the birds were randomly allotted to five groups, including four Fe sources [FeSO4, Fe-Gly (Ⅱ), FeCl3and Fe-Gly(Ⅲ)] treatments and one control treatment and were arranged forin situligated intestinal loops. Each treatment was repeated 10 times using 10 birds.Secondly, saline was prepared to inject into the duodenal loops. pH of chyme in the duodenum of 28-day-old broilers was previously determined to be 6.0 (Liet al., 2016), in accordance with the previous studies(Yuet al., 2008), the duodenal loops were buffered with 15.5 mmol · L-1of morpholine ethane sulfonic acid at pH 6. Four Fe sources were added to the medium as treatments. In the treatment groups of different Fe sources, 0.616 mmol of Fe/L (40 mg of Fe/L) was added to the media. The amount was chosen, based on the broilers' dietary Fe requirement and approximate amount was found in the intestine. However, there was no Fe in the control group. Lastly, chicks were anesthetized by wing venous injection of sumianxin(a complex anesthetic, 0.1 mL · kg-1of BW), then ligated intestinal loopsin situwere used. Details in operational process of ligated loop procedure were described by Yuet al(2008). Briefly, the 3.5 mL of Fe was injected in 12 cm ligated intestinal loops and clamped. After administration, the intestine was put back into the abdomen cavity. After perfusion for 30 min, 5 cm duodenal midpieces were immediately removed and flushed with physiological saline and stored at -80℃ for analyses ofµ-XRF andµ-XANES.

Table 1 Composition of two diets for 1- 21- and 22-28-dayold broilers
µ-XRF and µ-XANES experiments
The frozen duodenal midpieces were cut on a cryomicrotome into sections of 20 µm thickness slices,mounted on to a 3 µm-thick Mylar foil and attached onto standard slide frames (Kwiateket al., 2001). The slices were next freeze-dried at -80℃ (up to 3 days),-30℃ (up to 1 day) and 4°C (up to 1 day). Upon the drying procedure, all the sections were stored in desiccators at room temperature until the final experiments. However, solid standard iron was ground into diameter of 75 µm, and then fixed on special adhesive tape for synchrotron analysis.
Distribution image and valence of iron based onµ-XRF andµ-XANES were carried out at BL15U(Hard X-ray Micro-Focusing Beamline) in Shanghai Synchrotron Radiation Facility, Shanghai Institute of Applied Physics (Shanghai Pudong New Area,China) and X-ray was produced by electronic gun with electron energy of 3.5 GeV and intensity of electronic beam of 108-200 mA. The below was parameters about the BL15U. Monochromatic X-ray with 10.2 keV in steps of 0.1 eV and less than 2×10-4resolution of X-ray energy were used. X-ray spot was 2 μm×2 μm using K-B mirrors and photon flux on samples was about 5.5×1010photons/s. Fluorescence from sample was measured using a windowless single element Si (Li) solid-state detector, which was positioned at 45° to sample and 90° to X-ray beam. XANES experiment was performed in the fl uorescence mode.μ-XANES spectrum was collected from 7 110 eV to 7 220 eV and step sizes ranging from 0.25 eV to 2 eV were used. Furthermore, near the X-ray edge, steps were low and spectra collection times were longer than values used for the pre-edge and post-edge spectra.
All the measurements were conducted at room temperature. Samples were observed in optical microscopy in order to identify suitable areas forμ-XRF mapping. The scan dimensions of examined areas for all the duodenal wall samples were about 30 μm×25 μm, from mucous membrane to the muscle layer.
Data analysis
The intensity map of iron element was imaged using the software Igor Pro (Wave Metrics, USA) forμ-XRF mapping data. Data ofμ-XANES were arranged using ATHENA software (version 0.8.56), which was an independent software package of IFEFFIT, a special tool for dealing with XAFS data (Bruce Ravel,version 1.5). Using ATHENA, all the main steps in data processing included conversion of raw data toµ(E) spectra, background subtraction, normalization,Fourier transforming and plotting (Ravel and Newville,2010).
Results
The left was optical microscope images of the duodenal wall (DW), and the area in red rectangle box was selected forµ-XRF mapping andµ-XANES spectra determination. The right wasµ-XRF mapping and relative Fe concentration was represented by colors of the scale besides Figs. 1 to 5. The iron distribution was not uniform and the iron absorption was concentrated in a particular area (Figs. 1 to 5). There was smaller Fe in DW for the control group without Fe source perfusion (Fig. 1). However, other four different Fe source groups had more Fe in DW than the control group. FeSO4group and Fe-Gly (Ⅱ) group samples showed more distributions (Figs. 2 and 3),moreover, in contrast to FeSO4group, Fe-Gly (Ⅱ)group had a larger amount of Fe in DW. However,FeCl3and Fe-Gly (Ⅲ) had less Fe distributions in DW(Figs. 4 and 5). Furthermore, the minimum amount accumulation of Fe was detected in Fe-Gly (Ⅲ) group sample in DM (Fig. 5).
Although four standard iron sources were Fe compounds, K-edge absorption peak values ofμ-XANES spectra for ferrous iron sources [FeSO4and Fe-Gly(Ⅱ)] appeared at lower energy than for ferric iron sources [FeCl3and Fe-Gly (Ⅲ)] (Fig. 6). However,μ-XANES spectra indicated that K-edge absorption peak values were similar to the same valence iron sources. After being incubated for 30 min in the duodenal sac,μ-XANES spectra of Fe element for Fe sources of FeSO4, Fe-Gly (Ⅱ), FeCl3and Fe-Gly (Ⅲ)were basically the same in DW (Fig. 7). Fe K-edge absorption peak was approximately in agreement with K-edge displacement for the standard XANES spectra of ferrous iron sources [FeSO4and Fe-Gly (Ⅱ)] (Fig. 6)and the shapes of the curves after the absorption peak were similar to the four Fe sources, which indicated that although ferric iron sources [FeCl3and Fe-Gly(Ⅲ)] were poured into duodenal sac, the chemical states of Fe absorbed into DW were two valences.There was no a Fe3+XANES spectrum like standard of FeCl3and Fe-Gly (Ⅲ), suggesting that Fe3+was no detected in DW, furthermore, Fe-Gly (Ⅲ) was not absorbed completely into DW.

Fig. 1 μ-XRF elemental maps of control group sample for Fe in intestinal wall
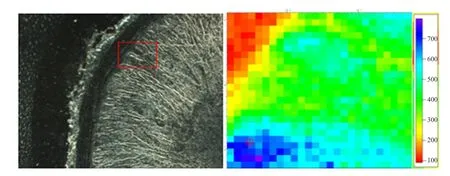
Fig. 2 μ-XRF elemental maps of FeSO4 group sample for Fe in intestinal wall
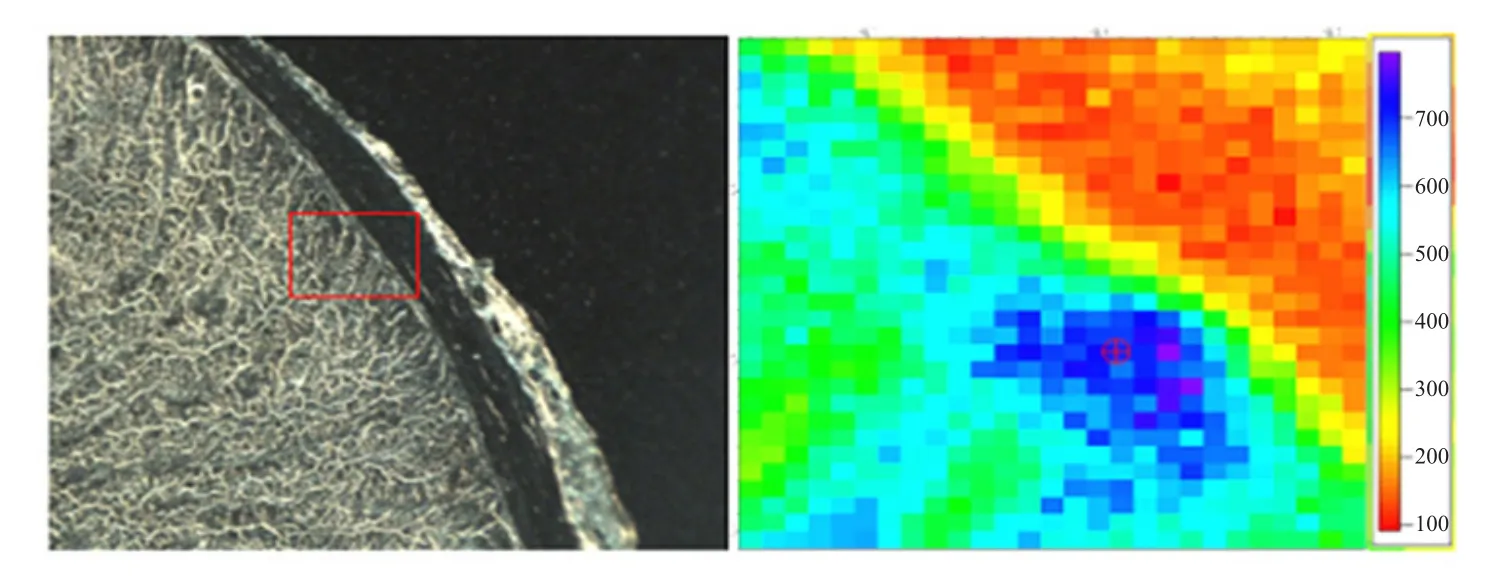
Fig. 3 μ-XRF elemental maps of Fe-Gly (II) group sample for Fe in intestinal wall
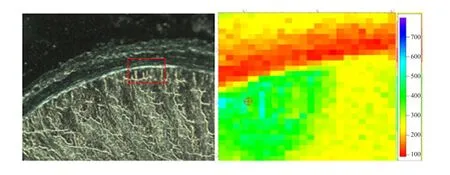
Fig. 4 μ-XRF elemental maps of FeCl3 group sample for Fe in intestinal wall
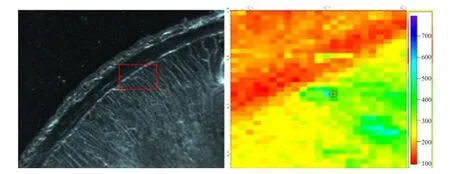
Fig. 5 μ-XRF elemental maps of Fe-Gly (III) group sample for Fe in intestinal wall
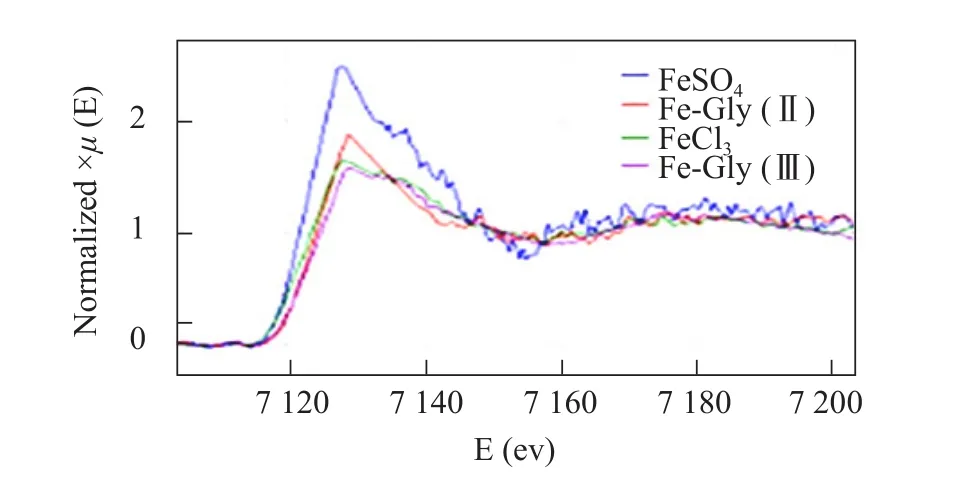
Fig. 6 μ-XANFS spectra of four Fe sources [FeSO4, Fe-Gly(II), FeCl3 and Fe-Gly (III)]
Discussion

Fig. 7 Fe μ-XANES spectra of Fe K-edge in duodenal wall
μ-XRF andμ-XANES technologies were combined to determinein situquantity, distribution and valence states of Fe were absorbed into the duodenal wall using different Fe sources. XRF technique allowed a simultaneous acquisition of the whole sample spectrum in a very short time, furthermore, it was a non-destructive technique, namely it left the samples completely unaltered after the measurement (Bertucciet al., 2016).As synchrotron radiation X-ray beam was less damage than the other particle beam to the sample (Haraet al.,2018), in this case, there was hardly obvious radiation damage on the tissue samples influencing next measurements. This experiment aimed at the distribution and valence state of iron in the duodenal wall and the possibility of inaccuracy of data was eliminated, because a crack or something did not happen clearly on the sample.
K-edge absorption peak of standard iron sources in this paper was represented the valence state of iron. K-edge absorption peak of zero valence iron was observed at 7112 eV (http://www.med.harvard.edu/JPNM/physics/refs/elecBE.html) and K-edge absorption peak shifted to higher energy as the oxidation number increased (Mahalik and Kundu,2018). In order to find the valence state of iron absorbed into the intestinal wall, XANES spectrum of iron was made in the wall and then compared with XANES spectrum of four standard Fe sources. These results showed that all the spectral profiles with the same peak energy were almost identical to the standard XANES spectra of ferrous iron sources [FeSO4and Fe-Gly(Ⅱ)] without ferric iron spectra, suggesting that metal amino acid chelate iron was not completely absorbed into the blood plasma, but hydrolyzed in the absorption point of intestinal brush border and assimilated in the form of ions into the blood. All the four Fe sources were absorbed with Fe2+form into the intestinal wall. Fe3+of FeCl3was first located on the duodenum fine micro chorionic, and then was deoxidized to Fe2+by cytochrome b (Duodermal cytochrome b, Dcytb). Fe2+was transported from the intestine into the small intestine cells with divalent cationic transportation carrier (Donovanet al., 2005;Gunshinet al., 2005). Therefore, Fe-Gly (Ⅲ) was not absorbed completely into the intestinal wall. The hypothesis about the complete absorption mechanism of organic trace elements (Alexander and Hartwig,2002) was not proven in this research. It indicated that the absorption pathways of organic trace elements and inorganic trace elements were the same in the duodenal wall.
Results fromµ-XRF maps proved further the demonstration fromµ-XANES. Based onµ-XRF maps, the concentration of ferrous Fe sources [FeSO4and Fe-Gly (Ⅱ)] was significantly higher than that of ferric Fe sources [FeCl3and Fe-Gly (Ⅲ)] in the duodenal wall and Fe-Gly (Ⅱ) group samples had the largest amount of Fe in the speci fic region of intestinal wall. It meant that iron was mainly assimilated in the form of Fe2+into the duodenum and Fe2+combined with glycine was more advantageous in absorption.The amino acid played a role of specific vector to metal ion so that iron ion might avoid competing with others, which was explained as the absorption of Fe-Gly (Ⅱ) was greater than that of FeSO4, when it was absorbed for 30 min in the duodenum.
Conclusions
µ-XRF andµ-XANES techniques directly demonstrated that iron glycine chelate had higher bioavailability than inorganic iron; however, the absorption pathways of these two substances were the same in the duodenal wall, in other words, iron glycine chelate was not absorbed as a whole into the intestine. This conclusion supported competitive absorption hypothesis.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Regeneration Function Analysis of GmESR1 in Transgenic Soybean
- Effect of Salt Stress on Nitrogen Assimilation of Functional Leaves and Root System of Rice in Cold Region
- Effects of Straw Returning with Different Tillage Patterns on Corn Yield and Nitrogen Utilization
- Effects of Rare Earth Lanthanum and Cerium on Antioxidant Enzyme Activities in Soybean Leaves
- Regulating Effect of Exogenous Silicon on Soil Fertility in Paddy Fields
- Uptake of B, Co and Ni by Plants from Oil Contaminated Soil Capped with Peat
