Rb 掺杂Li4Ti5O12 作为锂离子电池负极材料的合成及电化学性能*
2020-07-06SMOLIANOVAInna张聪聪赵欣悦张灵志
SMOLIANOVA Inna,张聪聪,赵欣悦,张灵志†
(1.中国科学院广州能源研究所,广州 510640;2.中国科学院可再生能源重点实验室,广州 510640;3.中国科学院大学,北京 100049)
0 Introduction
Nowadays,lithium-ion batteries (LIBs) are the main power supply sources for portable electronic devices[1].Challenging by the rising demands,especially for the applications of electric vehicles and hybrid electric vehicles,tremendous efforts have been devoted to improving the cycling life and safety of LIBs.Developing new electrode materials or modifying and improving the existing ones can be an effective approach to attain this goal.Lithium titanate (Li4Ti5O12,LTO) has been attracting considerable attention due to its unique “zero-strain”structure (lattice parameter changes only 0.2% during phase transformation Li4Ti5O12↔ Li7Ti5O12)[2-3].A flat high-voltage charge/discharge plateau (≈ 1.55 V vs.Li+/Li) hampers the formation of solid electrolyte interface (SEI) and the occurrence of lithium dendrites at the electrode surface during cycling providing excellent cycling stability[4–6].But the low electronic conductivity(ca.10−13S/cm) and poor lithium diffusion properties (ca.10−9-10−13cm2/s)along with low power density limit the practical applications of LTO[7].
Several strategies have been developed to overcome the above-stated problems.(1) Reducing particle size can shorten the lithium diffusion distance and increase the electrode/electrolyte contact area[8].(2) Coating conductive material on the surface of LTO can increase electronic conductivity[9-10].(3) Heteroatom doping into Li,Ti,O sites[11-14],self-doping[15-17]and co-doping[18-20]can facilitate electronic conductivity and lithium-ion diffusivity through influencing lattice structure and chemical bonds and generating abundant structural defects[21-23].Various metal ions have been investigated as efficient heterovalent dopants to increase the electronic conductivity through unbalancing the charge in LTO structure[4,11,15-16,20-21,24-36].
However,monovalent alkali metal doping is rarely reported in literature so far.Having the same valence with Li+,alkali metals ions do not introduce any charge interference in LTO structure.ZHAO et al.reported Na-doped LTO (NaxLi4−xTi5O12) for sodium-ion batteries[12].LIU et al.investigated the effects of Na+,K+doping and Na+-K+codoping on Li4Ti5O12for lithium-ion batteries[37].Another alkali metal,Rb,was previously used as a dopant only for cathode materials (LiNi0.8Co0.15Al0.05O2,Li1.27Cr0.2Mn0.53O2,Li1.2Mn0.54Co0.13Ni0.13O2) for lithiumion[38-40]and sodium-ion batteries[41]and proved to be beneficial for the electrochemical performance.
We previously reported the synthesis of nanospherical LTO doped with rare earth calcium as anode materials for lithium-ion batteries.The Ca-doped lithium titanate shows excellent rate performance,delivering a capacity of 155.1 mA∙h/g after 1 000 cycles at 10 C[24].In this work,we report our progress on the synthesis of a new alkali metal Rb-doped lithium titanate (Li4−xRbxTi5O12;x=0.010,0.015,0.020).The structures and the morphologies of these Rb-doped lithium titanates are characterized and their electrochemical performances are investigated in detail.The full cell test,using LiFePO4as cathode and Li4−xRbxTi5O12(x=0.010) with an optimized Rb doping level as anode,is conducted.
1 Experimental
1.1 Preparation of Rb-doped Li4Ti5O12
Rb-doped lithium titanates,Li4−xRbxTi5O12(x=0.010,0.015,0.020),were synthesized through a solid-state reaction using stoichiometric amounts of TiO2(≥99.8%,Aladdin Shanghai China),Rb2CO3(≥99.8%,Aladdin Shanghai China) and Li2CO3(≥ 99%,Aladdin Shanghai China) as Ti,Rb,Li sources.In a typical procedure,starting materials were mixed by ball milling(8 h,350 r/min) in water/ethanol slurry and then dried by vacuum spinning at 60°C.The collected powder was further dried in air at 60°C overnight and calcined in muffle oven at 400°C for 2 h;then the temperature was further increased to 850°C for 4 h.The heating rate was kept at 5°/min.Neat Li4Ti5O12(x=0) was prepared under the same conditions for comparison.
1.2 Materials characterization
Crystal structure and chemical composition of the samples were studied by X-ray diffraction (XRD PANALITICAL,2θ=5-80°,V=40 kV,I=40 mA) and X-ray photoelectron spectroscopy (XPS ESCALAB 250 Thermo Fishing Scientific).Morphology and microstructure were observed using scanning electron microscopy (SEM Hitachi S-4800).The electronic conductivity of pristine powders was measured by a powder conductivity measurement system (FD-300) at 25°C.
1.3 Electrochemical tests
The anodes were made by mixing Li4−xRbxTi5O12(x=0,0.010,0.015,0.020),acetylene black and polyvinylidene fluoride (PVDF5130,Shenzhen Micro Electron Co.,China) in a weight ratio of 8:1:1,using a suitable amount of N-methyl-2-pyrrolidone solvent (99.99%,Aladdin Industrial Corporation,China) to obtain a homogeneous slurry.The slurry was coated on a Cu foil substrate,dried at 60 °C,cut into 14 mm diameter circular disks and further dried at 110°C overnight.The active material loading level was 1.8 -2 mg/cm2.Coin half-cells(CR 2025) were assembled in Ar-filled glove box (H2O,O2≤ 0.1 ppm) using Celgard 2400 as separator,pure lithium foil as a counter electrode,and 1 M LiPF6EC:DMC:EMCv/v/v=1:1:1 (Zhangjiagang Guotai-Huarong New Chemical Materials Co.,China) as electrolyte.Full cells were assembled using commercial LiFePO4as cathode and Li4−xRbxTi5O12(x=0.010) as anode.The capacity of the full cell was calculated based on the LiFePO4mass.The mass of Li3.99Rb0.01Ti5O12was adjusted to be slightly higher than LiFePO4mass.Galvanostatic charge/discharge was conducted at a Shenzhen Neware battery cycler (China) at various current rates in a voltage window between 1.0-2.5 V (vs.Li/Li+) for the half cells and 1.0-3.0 V for the full cells.Electrochemical impedance spectroscopy (EIS) was measured for the half cells using an IM6e electrochemical workstation (Zahner,Germany) in a frequency range between 10 mHz and 100 kHz with an amplitude of 5 mV.
2 Results and discussion
2.1 Phase characterization
Rb-doped LTOs with various doping levels(Li4−xRbxTi5O12;x=0.010,0.015,0.020) were prepared via ball milling and subsequent solid-state reaction at 850°C.For comparison,neat Li4Ti5O12(x=0) was synthesized under the same conditions.XRD patterns of Li4−xRbxTi5O12(x=0,0.010,0.015,0.020) are displayed in Fig.1a.Diffraction peaks at 2θ=18.4°,35.6°,43.2°,47.4°,57.2°,62.8°,66.1°,74.3°,79.3° are attributed to(111),(311),(400),(331),(333),(440),(531),(533),(444) planes of Li4Ti5O12spinel (JCPDS#49-0207)[5,42].The magnification of (111) crystal panel peak at 18.4°shows the shift of the peak to the lower angle with the increase of doping level,indicating that Rb successfully incorporated into the LTO lattice,occupying Li 8a site,and increased its lattice parameter (Fig.1b).XPS survey spectra of Li4−xRbxTi5O12(x=0,0.010,0.015,0.020)identify O 1s,Ti 2p,Li 1s peaks of main elements (Fig.1c).In addition to these elements,Rb 3d peak is detected for all Rb-doped samples Li4−xRbxTi5O12(x=0.010,0.015,0.020),indicating the successful doping of Rb+in the structure[23].Fig.1d shows the two peaks at 109.9 eV and 111.4 eV corresponding to Rb 3d5/2and Rb 3d3/2.SEM shows that Li4−xRbxTi5O12(x=0,0.010,0.015,0.020) particles all have similar morphologies with almost cubic shape and the particle size is in the range of 0.2-1.5 μm (Fig.2).The electronic conductivity was measured using a powder conductivity apparatus at 25°C.The obtained values are 3×10−8S/cm,11.2×10−8S/cm,12.5×10−8S/cm,17.5×10−8S/cm for Li4Ti5O12,Li3.99Rb0.01Ti5O12,Li3.985Rb0.015Ti5O12,and Li3.98Rb0.02Ti5O12,respectively.Obviously,Rb doping enhances the electronic conductivity of LTO,which can be attributed to the increase of lattice parameter due to the replacement of Li+atom by Rb+with larger ionic radius[12,37].
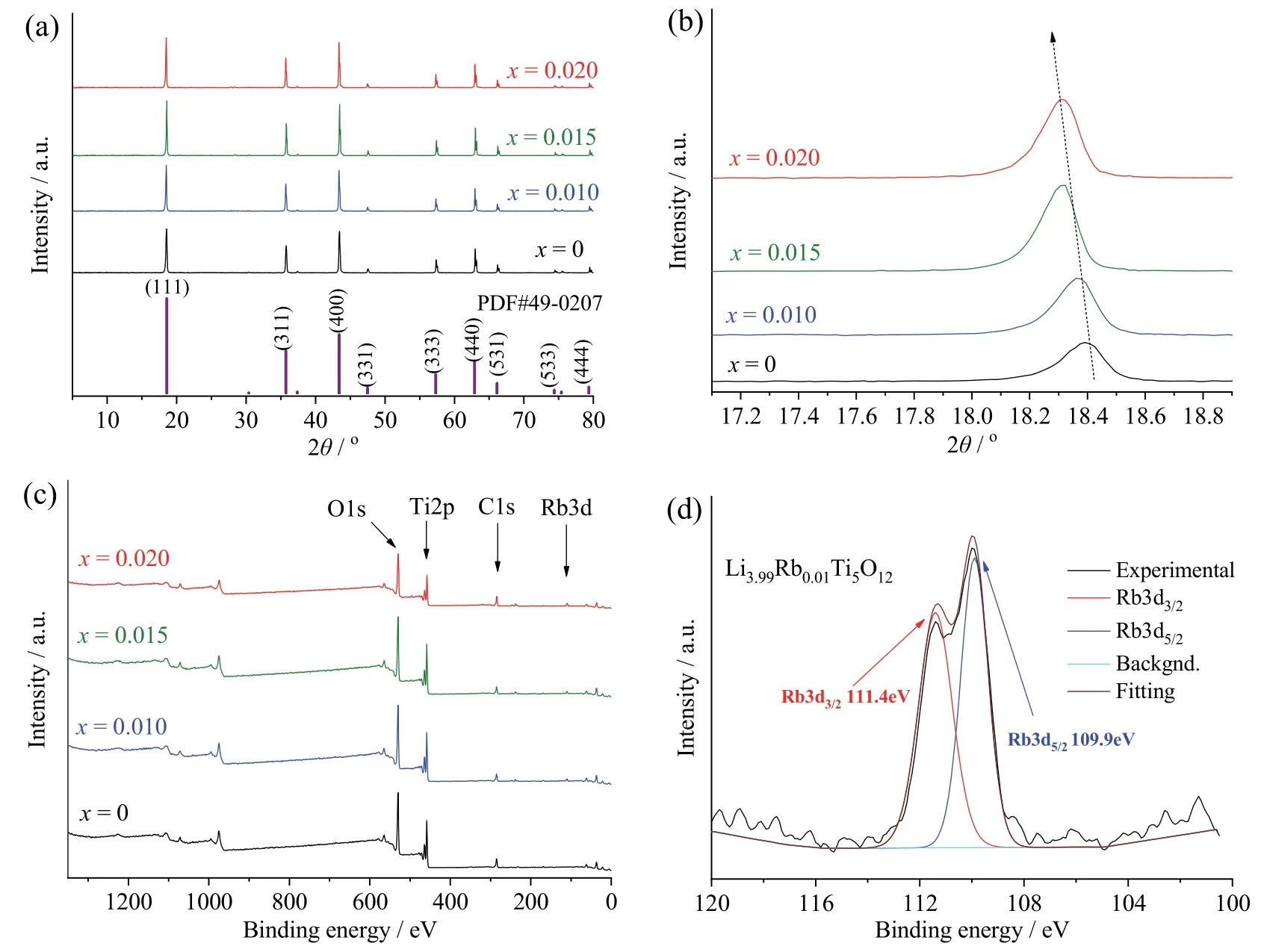
Fig.1 (a) XRD patterns for Li4−xRbxTi5O12 (x=0,0.010,0.015,0.020);(b) magnifigcation of (111) peak at 18.4°;(c) XPS survey spectra for Li4−xRbxTi5O12 (x=0,0.010,0.015,0.020);(d) binding energy of Rb 3d peak in Li3.99Rb0.01Ti5O12
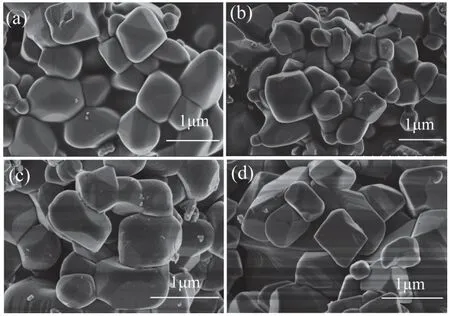
Fig.2 SEM images of Li4−xRbxTi5O12:(a) x=0;(b) x=0.010;(c) x=0.015;(d) x=0.020
2.2 Electrochemical tests
The initial charge-discharge curves of Li4−xRbxTi5O12(x=0,0.010,0.015,0.020) were measured at a rate of 1 C in a voltage range of 1-2.5 V (Fig.3a).All samples show a flat charge plateau at~1.6 V.However,the polarization of Li3.99Rb0.01Ti5O12,characterized by the voltage difference ΔVbetween charge (Vch) and discharge(Vdsch) plateaus,is smaller than Li4Ti5O12,Li3.985Rb0.015Ti5O12,and Li3.98Rb0.02Ti5O12(Table 1).Between all samples,Li3.99Rb0.01Ti5O12(x=0.010) delivers the initial charge/discharge capacity of 161.2/175.9 mA∙h/g,which exceeds the capacity of neat LTO and Rb-doped samples with higher doping levels (x=0.015,0.020).To demonstrate the advantage of Rb doping on enhancing rate capability,samples were tested at different current rates from 1 C to 10 C (Fig.3b).At the current rate of 1 C,the charge capacity is 150.0 mA∙h/g for Li4Ti5O12and 159.1 mA∙h/g,133.4 mA∙h/g,121.8 mA∙h/g for Li3.99Rb0.01Ti5O12,Li3.985Rb0.015Ti5O12,and Li3.98Rb0.02Ti5O12,respectively.With the increase of current density,the capacity decreases significantly to only 57.2 mA∙h/g,62.3 mA∙h/g,49.6 mA∙h/g,45.2 mA∙h/g at 10 C,which corresponds to the capacity retention of 38.1%,39.2%,37.2% and 37.1%for Li4Ti5O12,Li3.99Rb0.01Ti5O12,Li3.985Rb0.015Ti5O12,and Li3.98Rb0.02Ti5O12,respectively.The long-term cycling of Li4−xRbxTi5O12(x=0,0.10,0.015,0.020) was performed at 1 C in a voltage range of 1-2.5 V (Fig.3c).After 1 000 cycles,Li4−xRbxTi5O12still maintained 97.5% (x=0),90.9% (x=0.010),83.9% (x=0.015) of its initial capacity,indicating an excellent reversibility (vs.2ndcycle).
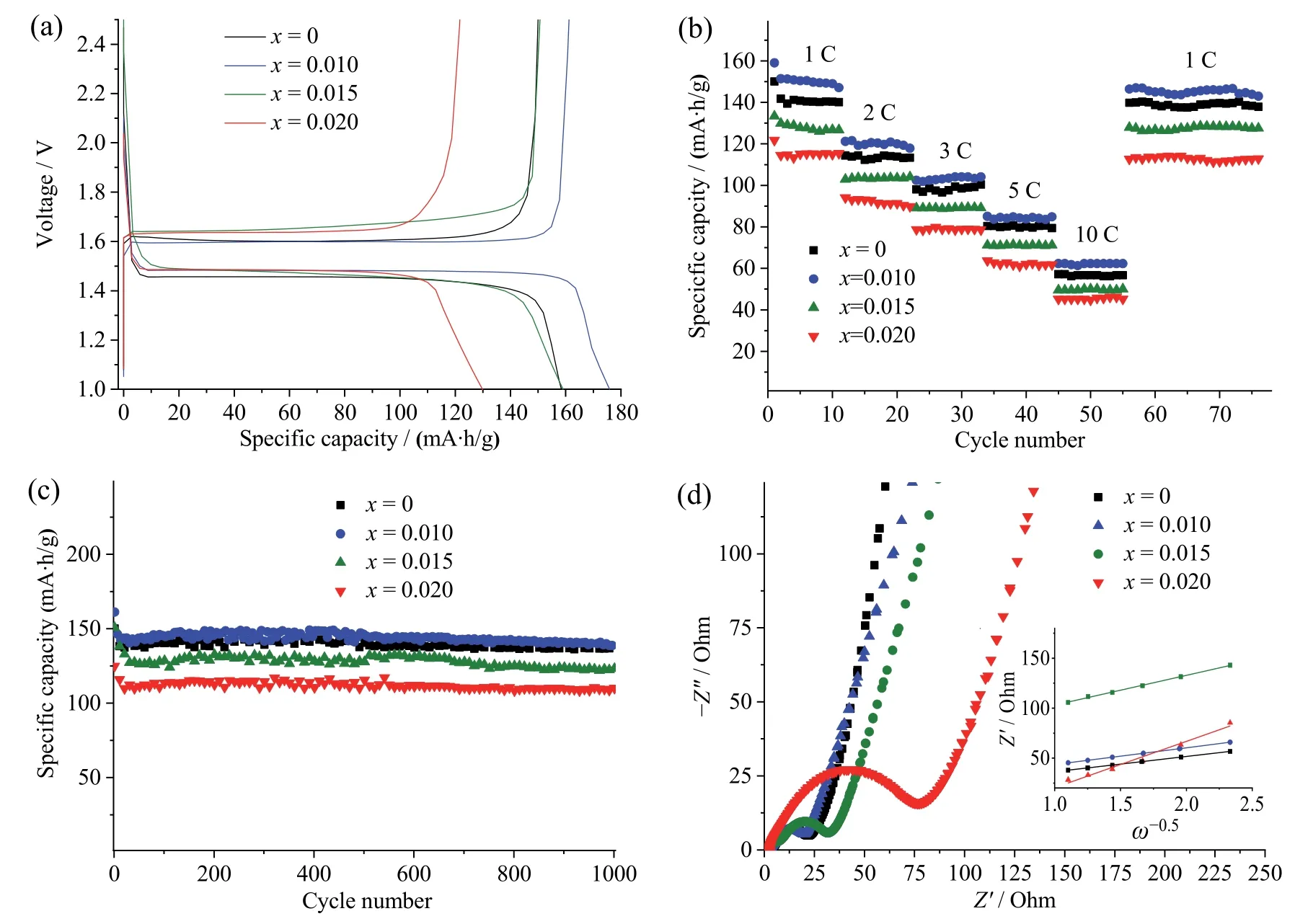
Fig.3 (a) The initial charge-discharge curves for Li4−xRbxTi5O12 (x=0,0.01,0.015,0.020);(b) rate capability of Li4−xRbxTi5O12 (x=0,0.010,0.015,0.020);(c) cycling performance of Li4−xRbxTi5O12 (x=0,010,0.015,0.020) at 1 C;(d) Nyquist plots and (insets) real impedance Z′ plotted against frequency ω−0.5 in the low-frequency region
EIS can further clarify the effect of the doping level on the charge-transfer resistance (RCT) and lithium diffusion coefficient ().Measurements were carried out after 10 cycles (Fig.3d).Nyquist plots consist of a semicircle in a high-frequency region and inclined line in the low-frequency region,corresponding to charge transfer resistance (and surface film formation)RCTand lithium-ion transfer resistance,respectively.
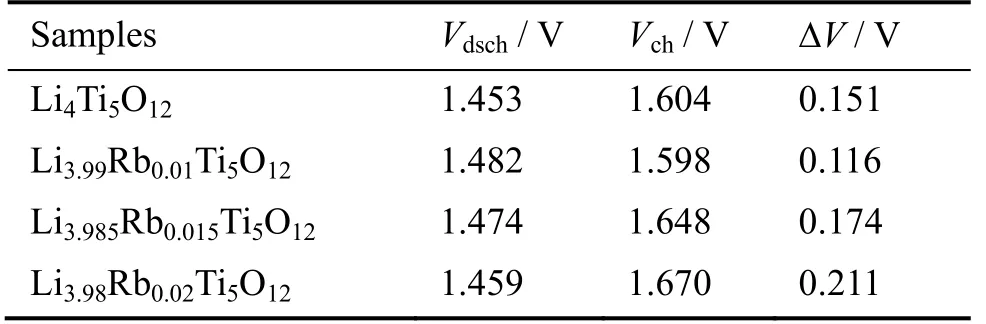
Table 1 Voltage differences between charge and discharge plateaus for Li4−xRbxTi5O12 (x=0,0.010,0.015,0.020)a
Li3.99Rb0.01Ti5O12exhibits the smallest semicircle and thus the lowestRCTbetween all samples,inferring the smallest electrochemical polarization of Li3.99Rb0.01Ti5O12,which leads to the best cycling and rate performance.
The lithium diffusion coefficientcan be calculated from on EIS results in low-frequency region based on equations (1) and (2):
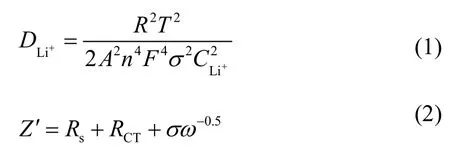
whereR–gas constant,T–absolute temperature,A–contact area of the electrode,n–the amount of electrons per molecule,F–Faraday constant,–concentration of Li-ions,σ–Warburg coefficient associated with real impedance (Z′) in low frequency (ω) region by equation(2),whereRs– electrolyte resistance.is calculated to be 2.17×10−9cm2/s,1.81×10−9cm2/s,2.36×10−10cm2/s,5.17×10−10cm2/s for Li4Ti5O12,Li3.99Rb0.01Ti5O12,Li3.985Rb0.015Ti5O12,Li3.98Rb0.02Ti5O12,respectively.Li3.99Rb0.01Ti5O12has the highest lithium diffusion coefficient,which is favorable for electrode kinetics,while the values for Li3.985Rb0.015Ti5O12and Li3.98Rb0.02Ti5O12are even lower than for neat Li4Ti5O12.
Li3.99Rb0.01Ti5O12shows the highest specific capacity,smallest polarization,and best rate performance among all LTO samples.This indicates that the optimal level of Rb-doping isx=0.010,while the effect of higher doping amounts (x=0.015,0.020) is adverse.Since the capacity of the cell is coming from the number of lithium ions that are involved in charge/discharge process,it can be seen that at higher Rb-doping levels,a significant amount of lithium ions are eliminated from cycling,which affects the specific capacity values.On the top over that,the excessive doping hampers lithium ions transport[18].
The fundamental difference between monovalent and heterovalent atom doping is enclosed in the charge distortion,which it introduces into lithium titanate structure.Atoms with the valence if II,III,V,VI cause a partial transition of insulating Ti4+to conductive Ti3+,enhancing the electronic conductivity.At the same time,the majority of investigated dopants have larger ionic radii,than Li+or Ti4+,those sides they occupy.It results in the expansion of the lattice parameter,and thus,facilitates lithium ion transport.Dopants with the valence of I can not introduce the charge distortion.Thus,the enhancement in electrochemical performance for Li3.99Rb0.01Ti5O12is mainly attributed to the improvement of lithium diffusivity due to the increased lattice parameter.Regarding to higher doping levels (x=0.015,0.020),lithium transport can be hampered due to the excessive amount of Rb forming impurities in the structure of LTO.
To evaluate the potential application of Li3.99Rb0.01Ti5O12electrode,a full cell with a Li3.99Rb0.01Ti5O12as anode and commercial LiFePO4as cathode was tested.Cells were cycled between 1 and 3 V at the current density of 0.1 C(1st,2ndcycle),0.2 C (3rd,4thcycle),and 0.5 C (from 5thcycle onwards).Galvanostatic charge-discharge curves of Li3.99Rb0.01Ti5O12//LiFePO4have a very flat discharge plateaus at 1.8 V at various current rates (1st– 0.1 C,3rd–0.2 C,5th– 0.5 C),implying an ideal battery system[15,43](Fig.4a).Li3.99Rb0.01Ti5O12//LiFePO4cell delivers an initial charge/discharge capacity of 167.0/145.7 mA∙h/g with a coulombic efficiency of 87.2%.The plateau specific discharge capacity in the 1stcycle (at 0.1 C) is 139.7 mA∙h/g,which is 95.9% of its total initial discharge capacity (145.7 mA∙h/g).In the 3rdcycle (at 0.2 C)plateau discharge capacity retains at 139.7 mA∙h/g,maintaining 96.5% of total discharge capacity(144.7 mA∙h/g).When the current density reaches 0.5 C,the plateau discharge capacity decreases to 126.4 mA∙h/g,still delivering 87.8% of 5thdischarge capacity(144.0 mA∙h/g).Fig.4b demonstrates a long term cycling performance of Li3.99Rb0.01Ti5O12//LiFePO4at 0.5 C.After 150 cycles Li3.99Rb0.01Ti5O12//LiFePO4still delivers 113.8/113.5 mA∙h/g,corresponding to the capacity retention of 78.8%.
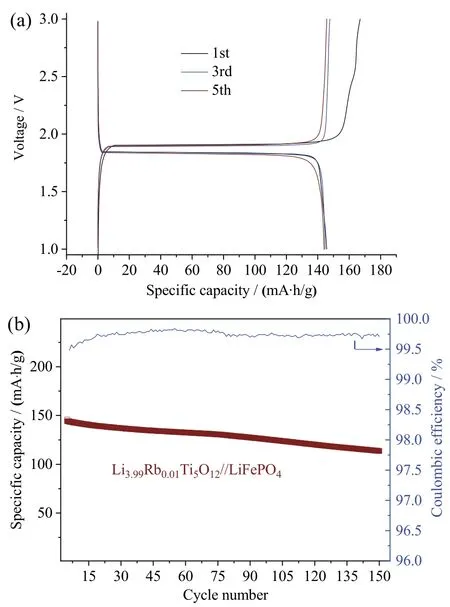
Fig.4 (a) Charge-discharge curves of Li3.99Rb0.01Ti5O12//LiFePO4 at 1st,3rd and 5th cycles at 0.1 C,0.2 C,0.5 C,respectively;(b) the cycling performance and coulombic efficiency of Li3.99Rb0.01Ti5O12//LiFePO4 at 0.5 C
3 Conclusion
Rb-doped lithium titanates with different doping levels,Li4−xRbxTi5O12(x=0.010,0.015,0.020),have been synthesized via a facile solid-state reaction.The electronic conductivity of all Rb-doped samples increases with the increase of the doping level.Li3.99Rb0.01Ti5O12(x=0.010) with the optimized doping level exhibited the best electrochemical performance,delivering an initial charge/discharge capacity of 161.2/175.9 mA∙h/g and 90.9% capacity retention after 1 000 cycles at 1 C.The Li3.99Rb0.01Ti5O12//LiFePO4full cell delivers a capacity of 144 mA∙h/g with a capacity retention of 78.8% after 150 cycles at 0.5 C.
