亚临床认知障碍慢性阻塞性肺疾病患者脑白质改变基于体系分析统计学分析
2020-07-04汪春荣刘勇詹亚峰高德宏邹立秋彭珂文陈济明邱士军
汪春荣 刘勇 詹亚峰 高德宏 邹立秋 彭珂文 陈济明 邱士军
[摘要] 目的 探討稳定期亚临床认知障碍慢性阻塞性肺疾病(COPD)患者脑白质结构的改变。 方法 选择2012年7月~2015年3月华中科技大学协和深圳医院(以下简称“我院”)就诊COPD患者60例作为COPD组,另选择同期于我院进行健康体检人群62名作为对照组。收集两组人群一般资料、生理及神经心理学资料,并利用基于体素水平分析(VBA)分析两组人群全脑脑白质微观结构改变。 结果 COPD组吸烟时间长于对照组,差异有统计学意义(P < 0.05)。简易精神状态量表、蒙特利尔认知评估量表总分及视空间与执行功能、命名及延迟记忆得分显著低于对照组,差异有统计学意义(P < 0.05)。COPD组左侧枕中回、右侧辅助运动区、左侧颞上回及右侧小脑后叶区域白质FA值显著低于对照组,差异有高度统计学意义(P < 0.01)。 结论 稳定期COPD患者脑白质FA值异常,纤维微观结构完整性被破坏,应引起临床重视。
[关键词] 基于体素水平分析;脑白质;慢性阻塞性肺疾病;认知障碍
[中图分类号] R563.05 [文献标识码] A [文章编号] 1673-7210(2020)05(a)-0114-04
Statistical analysis of white matter changes in patients with chronic obstructive pulmonary disease and subclinical cognitive impairment based on voxel analysis
WANG Chunrong1 LIU Yong2 ZHAN Yafeng3 GAO Dehong1 ZOU Liqiu1 PENG Kewen1 CHEN Jiming4 QIU Shijun5
1.Department of Radiology, Huazhong University of Science and Technology Union Shenzhen Hospital, Guangdong Province, Shenzhen 518052, China; 2.Brainnetome Center, Institute of Automation, Chinese Academy of Sciences, Beijing 100190, China; 3.School of Biomedical Engineering, Southern Medical University, Guangdong Province, Guangzhou 510515, China; 4.Department of Respiration, Huazhong University of Science and Technology Union Shenzhen Hospital, Guangdong Province, Shenzhen 518052, China; 5.Department of Radiology, the First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangdong Province, Guangzhou 510405, China
[Abstract] Objective To investigate the changes of white matter structure in patients with stable subclinical cognitive impairment chronic obstructive pulmonary disease (COPD). Methods From July 2012 to March 2015, 60 patients with COPD treated in Huazhong University of Science and Technology Union Shenzhen Hospital (hereinafter referred to as “our hospital”) were selected as the COPD group, while a total of 62 healthy people who underwent a physical examination in our hospital during the same period were selected as the control group. The general data, physiological and neuropsychological data of the two groups were collected, while the changes in the whole brain white matter microstructure of the two groups were analyzed by the voxel level analysis (VBA). Results The smoking time of the COPD group was longer than that of the control group, and the difference was statistically significant (P < 0.05). The total scores of mini mental state examination, Montreal cognitive assess ment and the visual space and executive function, naming and delayed memory scores were significantly lower than those of the control group, the differences were statistically significant (P < 0.05). In the COPD group, the white matter FA values in the left middle occipital gyrus, right auxiliary motor area, left superior temporal gyrus, and right posterior cerebellar area were significantly lower than those in the control group, and the differences were highly statistically significant (P < 0.01). Conclusion FA value of the white matter in patients with COPD in the stable phase is abnormal, and the microstructural integrity of the fibers is destroyed, which should be paid attention to clinically.
[Key words] Voxel-based analysis; White matter; Chronic obstructive pulmonary disease; Cognitive impairment
慢性阻塞性肺疾病(COPD)以持续不完全可逆气流受限为主要特征,呈持续进行性发展,主要累及肺,也可损伤脑认知[1]。以往关于稳定期亚临床认知障碍COPD患者脑结构的研究较少,常规磁共振(MRI)检查多正常,但脑灰白质结构、灌注、代谢等已经改变,如果能及早发现并干预,可以延缓或逆转脑认知障碍进程。本研究利用基于体素的统计学分析(VBA)探讨稳定期亚临床认知障碍COPD患者脑白质纤维微观结构完整性是否受到破坏。
1 资料与方法
1.1 一般资料
选取2012年7月~2015年3月华中科技大学协和深圳医院(以下简称“我院”)COPD患者60例作为COPD组以及同期于我院体检的62名健康人群作为对照组。其中COPD组男43例,女17例,平均年龄(53.68±12.30)岁;对照组男41名,女21名,平均年龄(50.95±12.18)岁。纳入标准:①COPD患者符合全球协议诊断标准;②存在慢性咳嗽/咳痰、呼吸困难,和/或长期接触危险因素;③吸入支气管舒张剂后,第1秒用力呼气容积(FEV1)<80%预计值,且FEV1/用力肺活量(FVC)<70%可确定为不完全可逆性气流受限;④简易精神状态量表(MMSE)评分均≥26分;⑤既往住院而近6个月无急性加重。排除标准:①合并其他呼吸系统疾病;②合并脑外伤及器质病变、癫痫、肿瘤等;③药物滥用。收集受试者病史、生理及神经心理学资料。对COPD患者进行圣乔治呼吸问卷调查(SGRQ)评估呼吸系统症状,用蒙特利尔认知评估量表(MoCA)量表评估并记录得分。
1.2 MRI扫描及数据预处理
使用德国西门子3.0T(skyra)MRI机及头颅32通道线圈对所有受试者行全脑磁化强度预备梯度回波序列高分辨率T1WI成像。DTI扫描用单次激发自旋回波序列:TR/TE=11 400/84 ms;matrix=128×128;FOV=230×230 mm3;NEX=1;层厚=2 mm,层间隔=0,共67层,64个方向,b=1000 s/mm2。本研究已通过我院医学伦理委员会审查。采用牛津大学脑功能MRI中心开发的Functional Magnetic Resonance Imaging of the Brain Soft Library(FSL,http://www.fimib.ox.ac.uk/fsl)软件对DTI数据处理:①格式转换;②灰白质分割;③头动矫正;④梯度矫正;⑤得到各项异性分数(FA);⑥线性配准;⑦配准到MNI152标准空间;⑧图像平滑。
1.3 统计学方法
采用SPSS 22.0、SPM8软件对所得数据进行分析。计量资料以均数±标准差(x±s)表示,采用t检验。计数资料以例数或百分比表示,采用χ2检验。以P < 0.05为差异有统计学意义。
2 结果
2.1 两组人群一般资料比较
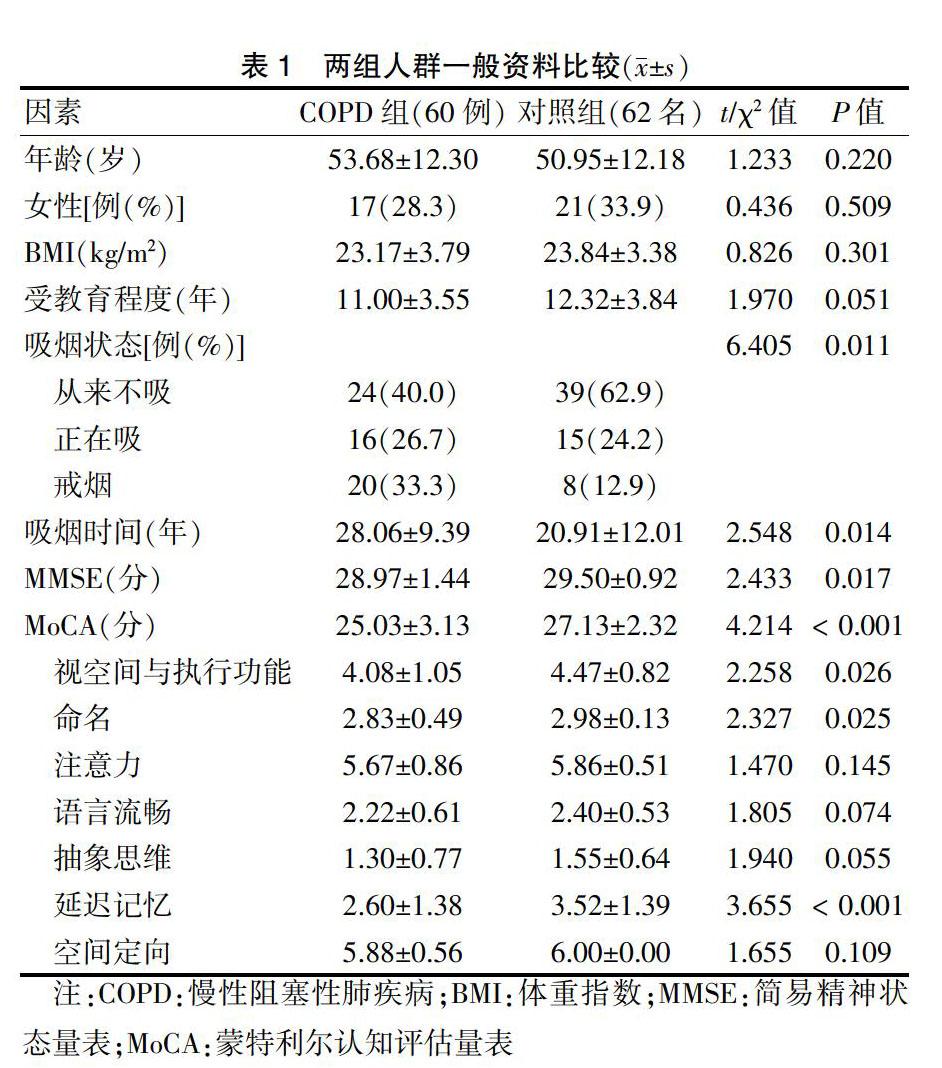
两组人群年龄、性别、BMI、受教育程度比较,差异无统计学意义(P > 0.05),具有可比性。两组人群吸烟状态比较,差异有统计学意义(P < 0.05)。COPD组吸烟时间长于对照组,差异有统计学意义(P < 0.05)。MMSE、MoCA总分及是空间执行能力、命名及延迟记忆得分显著低于对照组,差异有统计学意义(P < 0.05)。见表1。
2.2 两组人群脑白质FA值比较
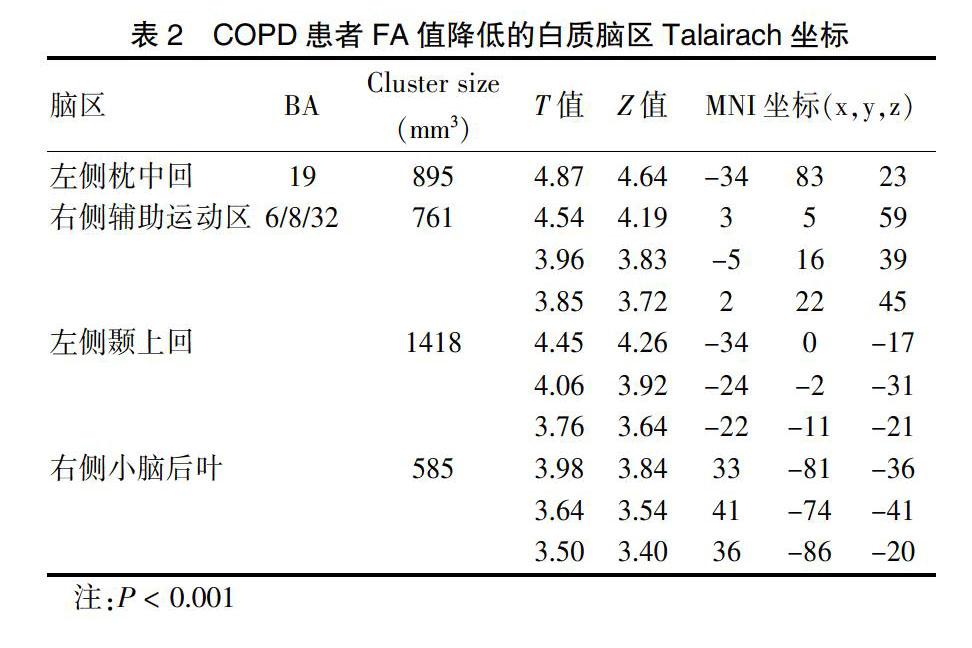
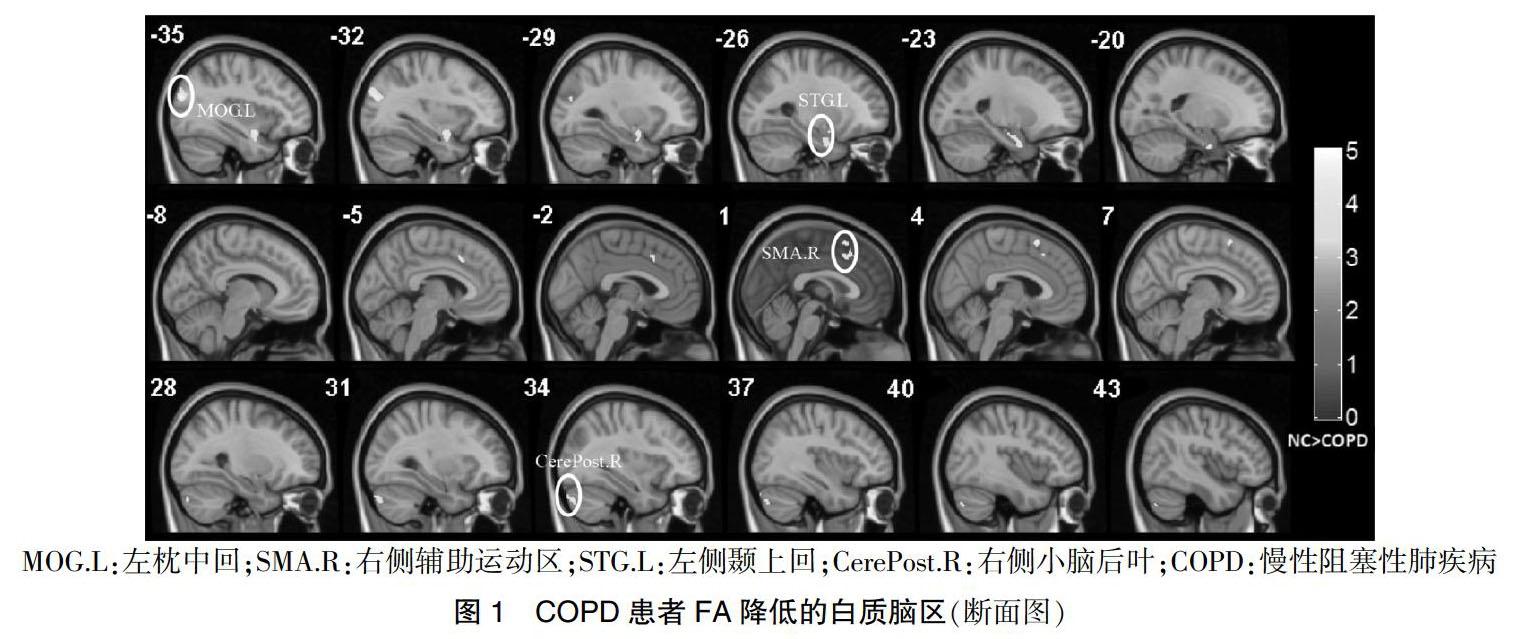
COPD组患者左侧枕中回、右侧辅助运动区、左侧颞上回及右侧小脑后叶区域白质FA显著低于对照组,差异有高度统计学意义(P < 0.01)。见表2、图1。
3讨论
越来越多研究报道COPD可导致脑认知障碍[2],对有临床认知障碍的COPD患者弥散张量成像(DTI)研究较少且不一致。Ryu等[3]报道重度COPD患者大脑皮层和额顶叶脑室周围脑白质FA值减低;张曦等[4]报道COPD组胼胝体、双侧丘脑前辐射、皮质脊髓束、左侧额枕下束及上纵束FA值明显下降;而关于亚临床认知障碍COPD患者的DTI报道更是极少。
本研究COPD患者较对照组左侧枕中回FA值明显降低与以往报道基本一致[5-6],提示COPD组枕叶脑白质完整性明显降低,FA值下降与髓鞘结构受损。枕中回是视觉皮层、视觉背侧通路的一部分,负责视觉及识别记忆[7]。本研究COPD患者枕叶白质存在结构异常可能和视空间障碍及认知功能障碍有关。COPD患者较对照组右侧辅助运动区FA值降低,辅助运动区前部涉及认知及执行控制,后部參与动作计划和选择[8],对视空间处理起重要作用,还参与语言产生、执行语义任务和文字产生[9]。本研究结果显示,COPD患者视空间与执行功能、命名能力评分下降可能与辅助运动区白质结构受损有关,空间执行功能下降与以往报道基本一致[10-12],命名能力下降与本研究不完全一致[12]。COPD患者较对照组左侧颞上回FA值明显降低,与以往报道基本一致[5-6]。颞上回是运动相关脑区,参与运动准备、规划及信息处理和思维,COPD患者视空间与执行功能和记忆能力下降可能与左侧颞上回脑白质完整性受损有关。此外,COPD患者较对照组右侧小脑后叶FA值明显降低,小脑参与执行功能、学习和记忆、视空间能力、语言处理等[13]。值得注意的是,视空间与执行功能、命名与延迟记忆能力评分下降可能与右侧小脑后叶脑白质完整性受损有关。此外,小脑还能通过发送网状纤维结构影响心血管系统进而改变血压和心率[14]。本研究中,COPD组较对照组心率稍高,可能与小脑调节作用相关。
COPD患者脑白质异常发病机制尚不清楚,有学者认为可能是血氧饱和度低、慢性缺氧及高碳酸血症[5,15-16]。Alexandre等[17]认为脑缺氧时会增加脑血流量,但无脑血流量增加会导致神经元损伤。COPD患者夜间氧饱和度下降频繁,所以无缺氧COPD患者也存在间断性夜间缺氧而引起脑认知及脑形态的改变。另有研究显示[18],FEV1与白质病变严重程度相关。此外,吸烟是COPD导致脑白质完整性损伤的独立因素[19],且吸烟持续时间也影响脑白质FA值,即使戒烟也较未吸烟者脑白质有明显损伤。吸烟还可能是COPD相关血管源性水肿的危险因素[20]。
总之,本研究通过VBA方法证实COPD患者存在广泛脑白质纤维异常,可对脑白质超微结构异常量化,为探讨COPD患者脑认知障碍的病因及病理机制提供了新的研究方法,可早期诊断并提前预防保护亚临床脑认知障碍COPD患者认知功能。
[参考文献]
[1] Hu X,Wang H,Tu Y,et al. Alteration of the default mode network and cognitive impairments in patients with chronic obstructive pulmonary disease [J]. Int J Chron Obstruct Pulmon Dis,2018,13:519-528.
[2] Wang C,Ding Y,Shen B,et al. Altered Gray Matter Volume in Stable Chronic Obstructive Pulmonary Disease with Subclinical Cognitive Impairment:an Exploratory Study [J]. Neurotox Res,2017,31(4):453-463.
[3] Ryu CW,Jahng GH,Choi CW,et al. Microstructural change of the brain in chronic obstructive pulmonary disease:a voxel-based investigation by MRI [J]. COPD,2013,10(3):357-366.
[4] 张曦,张静娜,秦显莉,等.慢性阻塞性肺疾病患者脑白质微结构变化的初步研究[J].重庆医科大学学报,2017, 42(12):1639-1643.
[5] Zhang H,Wang X,Lin J,et al. Grey and white matter abnormalities in chronic obstructive pulmonary disease:a case-control study [J]. BMJ Open,2012,2(2):1-10.
[6] Dodd JW,Chung AW,Van Den Broek MD,et al. Brain structure and function in chronic obstructive pulmonary disease:a multimodal cranial magnetic resonance imaging study [J]. Am J Respir Crit Care Med,2012,186(3):240-245.
[7] Lajiness-O′neill R,Akamine Y,Bowyer SM. Treatment effects of fast forword demonstrated by magnetoencephalography (MEG) in a child with developmental dyslexia [J]. Neurocase,2007,13(5):390-401.
[8] Kim JH,Lee JM,Jo HJ,et al. Defining functional SMA and pre-SMA subregions in human MFC using resting state fMRI:functional connectivity-based parcellation method [J]. Neuroimage,2010,49(3):2375-2386.
[9] Morgan VL,Mishra A,Newton AT,et al. Integrating functional and diffusion magnetic resonance imaging for analysis of structure-function relationship in the human language network [J]. PLoS One,2009,4(8):1-8.
[10] Lu CQ,Xu W,Zeng CH,et al. Altered amplitude of low-frequency fluctuation in basal ganglia correlates to pulmonary ventilation function in COPD patients:A resting-state fMRI study [J]. Brain Behav,2019,9(7):e01336.
[11] Savage CC,Dixey PHA,Pennington C,et al. Visual rating assessment of cerebral atrophy and its relationship with cognitive function in chronic obstructive pulmonary disease [J]. BMJ Open Respir Res,2018,5(1):e000310.
[12] 閆俊,朱葛敏,屈晓一,等.慢性阻塞性肺疾病患者脑白质疏松及认知功能改变的研究[J].实用医学杂志,2018,34(21):3513-3516.
[13] Schraa-Tam CK,Rietdijk WJ,Verbeke WJ,et al. fMRI activities in the emotional cerebellum:a preference for negative stimuli and goal-directed behavior [J]. Cerebellum,2012,11(1):233-245.
[14] Dai XJ,Gong HH,Wang YX,et al. Gender differences in brain regional homogeneity of healthy subjects after normal sleep and after sleep deprivation:a resting-state fMRI study [J]. Sleep Med,2012,13(6):720-727.
[15] Van Dijk EJ,Vermeer SE,De Groot JC,et al. Arterial oxygen saturation,COPD,and cerebral small vessel disease [J]. J Neurol Neurosurg Psychiatry,2004,75(5):733-736.
[16] Kamba M,Inoue Y,Higami S,et al. Cerebral metabolic impairment in patients with obstructive sleep apnoea:an independent association of obstructive sleep apnoea with white matter change [J]. J Neurol Neurosurg Psychiatry,2001,71(3):334-339.
[17] Alexandre FHN,Varray A. Is nocturnal desaturation a trigger for neuronal damage in chronic obstructive pulmonary disease? [J]. Med Hypotheses,2015,84(1):25-30.
[18] Longstreth WT,Arnold AM,Manolio TA,et al. Clinical correlates of ventricular and sulcal size on cranial magnetic resonance imaging of 3,301 elderly people. The Cardiovascular Health Study. Collaborative Research Group [J]. Neuroepidemiology,2000,19(1):30-42.
[19] Cleutjens F,Ponds R,Spruit MA,et al. The relationship between cerebral small vessel disease,hippocampal volume and cognitive functioning in patients with COPD:An MRI Study [J]. Front Aging Neurosci,2017,9:88.
[20] Wang X,Huang X,Gao Z,et al. Vasogenic cerebral edema associated with the disability in activities of daily living in patients with chronic obstructive pulmonary disease [J]. Brain Behav,2018,8(8):e01065.
(收稿日期:2019-11-04 本文編辑:王晓晔)
