1,25-二羟维生素D3通过调节性T细胞延缓肾间质纤维化的作用研究
2020-07-04高峰郭珲
高峰 郭珲


[摘要] 目的 研究1,25-二羥维生素D3[1,25(OH)2D3]对单侧输尿管梗阻(UUO)大鼠肾间质纤维化的影响。 方法 采用随机数字表法将54只wistar大鼠分为假手术组(A组)、UUO组(B组)和1,25(OH)2D3治疗组(C组),每组18只。C组灌胃1,25(OH)2D3 3 ng/(d·100 g),A组和B组灌胃等量花生油。B组和C组左侧输尿管结扎,A组输尿管只游离不结扎,每组术后第1、3、7天分批处死6只大鼠,并取留取左侧肾脏。常规检测血肌酐水平,苏木精-伊红(HE)染色观察肾脏病理形态,免疫组化法检测大鼠肾脏平滑肌动蛋白(a-SMA)、叉头P3蛋白(FOXP3),Western blot法测磷酸化腺苷酸活化蛋白激酶(p-AMPK)。 结果 术后第1、3、7天B组血肌酐、a-SMA水平均高于A组,C组血清肌酐、a-SMA水平均低于B组(均P < 0.05)。A组大鼠肾脏组织形态正常,B组大鼠肾脏组织随梗阻时间延长,可见肾小管扩张及间质水肿,而C组较B组肾小管变性减轻。术后第1、3、7天B组p-AMPK、FOXP3水平均低于A组,C组p-AMPK、FOXP3水平均高于B组(均P < 0.05)。 结论 1,25(OH)2D3可延缓肾间质纤维化,其机制可能与激活AMPK进而促进调节性T细胞的活化有关。
[关键词] 1,25-二羟维生素D3;肾间质纤维化;腺苷酸活化蛋白激酶;调节性T细胞
[中图分类号] R692 [文献标识码] A [文章编号] 1673-7210(2020)05(a)-0041-04
Effect of 1,25-dihydroxyvitamin D3 delaying renal interstitial fibrosis by T regulatory cell
GAO Feng1 GUO Hui2
1.The Second Clinical Medical College, Shanxi Medical University, Shanxi Province, Taiyuan 030001, China; 2.Department of Nephrology, the Second Hospital of Shanxi Medical University, Shanxi Province, Taiyuan 030001, China
[Abstract] Objective To study the effect of 1, 25-dihydroxyvitamin D3 [1,25(OH)2D3] on renal interstitial fibrosis in rats with unilateral ureteral obstruction (UUO). Methods According to random number table method, 54 wistar rats were divided into sham operation group (group A), UUO group (group B) and 1,25 (OH)2D3 treatment group (group C), with 18 rats in each group. Group C was gavaged 3 ng/(d·100 g) of 1,25(OH)2D3 every day. Group A and group B were gavaged the same amount of peanut oil. The left ureter ligation was performed in group B and group C. Group A was treated with ureteral free, but not ligated. On the 1st, 3rd and 7th day after operation, 6 rats were killed in batches, and the left kidney was retained. Serum creatinine were measured in each group. Renal pathological changes were observed by hematoxylin-eosin (HE) staining. The expression of a-smooth muscle actin (a-SMA) and forkhead P3 protein (FOXP3) in the kidney was detected by immunohistochemistry. Western blot was used to detect the protein expression of phosphorylated adenosine monophosphate activated protein kinase (p-AMPK). Results On the 1st, 3rd and 7th day after operation, the serum creatinine,a-SMA levels of group B was higher than those of group A, and the serum creatinine,a-SMA levels of group C was lower than those of group B (all P < 0.05). The renal tissue morphology of group A was normal, while the renal tissue of group B was prolonged with the obstruction time, showing renal tubules dilatation and interstitial edema, while the tubular degeneration of group C was reduced compared with that of group B. On the 1st, 3rd and 7th day after operation, p-AMPK and FOXP3 levels in group B were lower than those in group A, p-AMPK and FOXP3 levels in group C were higher than those in group B (all P < 0.05). Conclusion 1,25(OH)2D3 can delay renal interstitial fibrosis, and its mechanism may be related to the activation of AMPK to promote the activation of regulatory T cells.
[Key words] 1,25-dihydroxyvitamin D3; Renal interstitial fibrosis; Adenosine monophosphate activated protein kinase; T regulatory cell
肾间质纤维化是终末期肾脏病的共同肾脏病理改变,近期发现腺苷酸活化蛋白激酶(AMPK)在肾间质纤维化中扮演着重要角色,在高糖和高脂作用下,AMPK的激活可延缓肾脏纤维化[1-2]。AMPK可促进调节性T细胞(Treg)分化,而Treg在改善慢性肾脏病中起重要作用[3-5]。1,25-二羟维生素D3[1,25(OH)2D3]不仅可调节钙磷代谢,还可阻断肾小管上皮细胞间充质转分化(EMT)[6]。因此,本实验研究1,25(OH)2D3对肾间质纤维化大鼠AMPK及Treg的影响,既而探讨1,25(OH)2D3延缓肾间质纤维化的可能机制。
1 材料与方法
1.1 实验动物
健康清洁雄性Wistar大鼠54只,3~4周龄,质量约150 g,购于山西医科大学实验动物中心,许可证编号SCXK(晋)2015-0001,合格批号:0107010。饲养条件:相对湿度37%~39%,室温25℃左右。自由饮水摄食,实验前饲养7 d,适应环境。
1.2 主要试剂
抗兔β-action(美国Cell Signaling公司,货号:3700S);羊抗兔IgG抗体(碧云天公司,货号:A0208);叉头蛋白P3(FOXP3)抗体(invitrogen公司,生产批号:2044725);BCA蛋白浓度测定试剂盒(博士德公司,生产批号:13G18B46);SDS-PAGE凝胶盒(博士德公司,生产批号:14E17B38);抗平滑肌动蛋白(a-SMA)抗体(美国Cell Signaling公司,生产批号:04120 18);抗p-AMPK抗体(美国Cell Signaling公司,生产批号:0312018);1,25(OH)2D3(Sigma公司,货号:D1530)。
1.3 实验分组与处理
采用随机数字表法分成假手术组(A组)、单侧输尿管梗阻(UUO)组(B组)和1,25(OH)2D3治疗组(C组),每组18只。C组灌胃1,25(OH)2D3 3 ng/(d·100 g),A组和B组灌胃等量花生油。B组和C组左侧输尿管结扎,A组输尿管只游离不结扎,每组术后第1、3、7天分批处死6只大鼠,并取左侧肾脏。
1.4 观察指标
1.4.1 血肌酐检测 终点酶法检测大鼠血肌酐。
1.4.2 肾组织病理形态观察 常规石蜡包埋切片,苏木精-伊红(HE)染色,200倍显微镜下观察肾组织切片。
1.4.3 免疫组化检测a-SMA、FOXP3水平 组织蜡块切片脱蜡进行抗原修复,加一抗,37℃水浴2 h,PBS洗3次,再加二抗,37℃水浴40 min,PBS洗3次,再加辣根过氧化物酶,37℃,20 min,PBS洗3次,后加入显色剂,蒸馏水冲洗,复染、脱水、透明后封片。随意取10个视野,Image Pro Plus 6.0计算阳性染色区域的积分光密度值和测量区域的总面积,并对免疫组织化学染色进行分析。
1.4.4 Western blot法检测P-AMPK水平 肾组织加入细胞裂解液,冰上裂解30 min,4℃、12 000 r/min离心5 min,离心半径15 cm,取上清液,BCA法测定蛋白浓度,SDS-PAGE凝胶盒分离,转膜后用2%牛血清蛋白(BSA)37℃封闭2 h,一抗4℃孵育过夜,漂洗后加入二抗孵育,后加入增强化学发光法曝光显影,胶片扫描后用Image Lab软件对各条带灰度值进行测定。
1.5 统计学方法
采用SPSS 22.0统计学软件进行数据分析,符合正态分布计量资料的数据用均数±标准差(x±s)表示,多组间比较采用单因素方差分析,两组间比較采用t检验;不符合正态分布的组间比较采用非参数检验(秩和检验)。以P < 0.05为差异有统计学意义。
2 结果
2.1 三组血清肌酐水平比较
术后第1、3、7天B组血肌酐水平均高于A组,C组血清肌酐水平均低于B组(均P < 0.05)。见表1。
2.2 三组肾组织a-SMA水平比较
术后第1、3、7天B组a-SMA水平均高于A组,C组a-SMA水平均低于B组(均P < 0.05)。见表2。
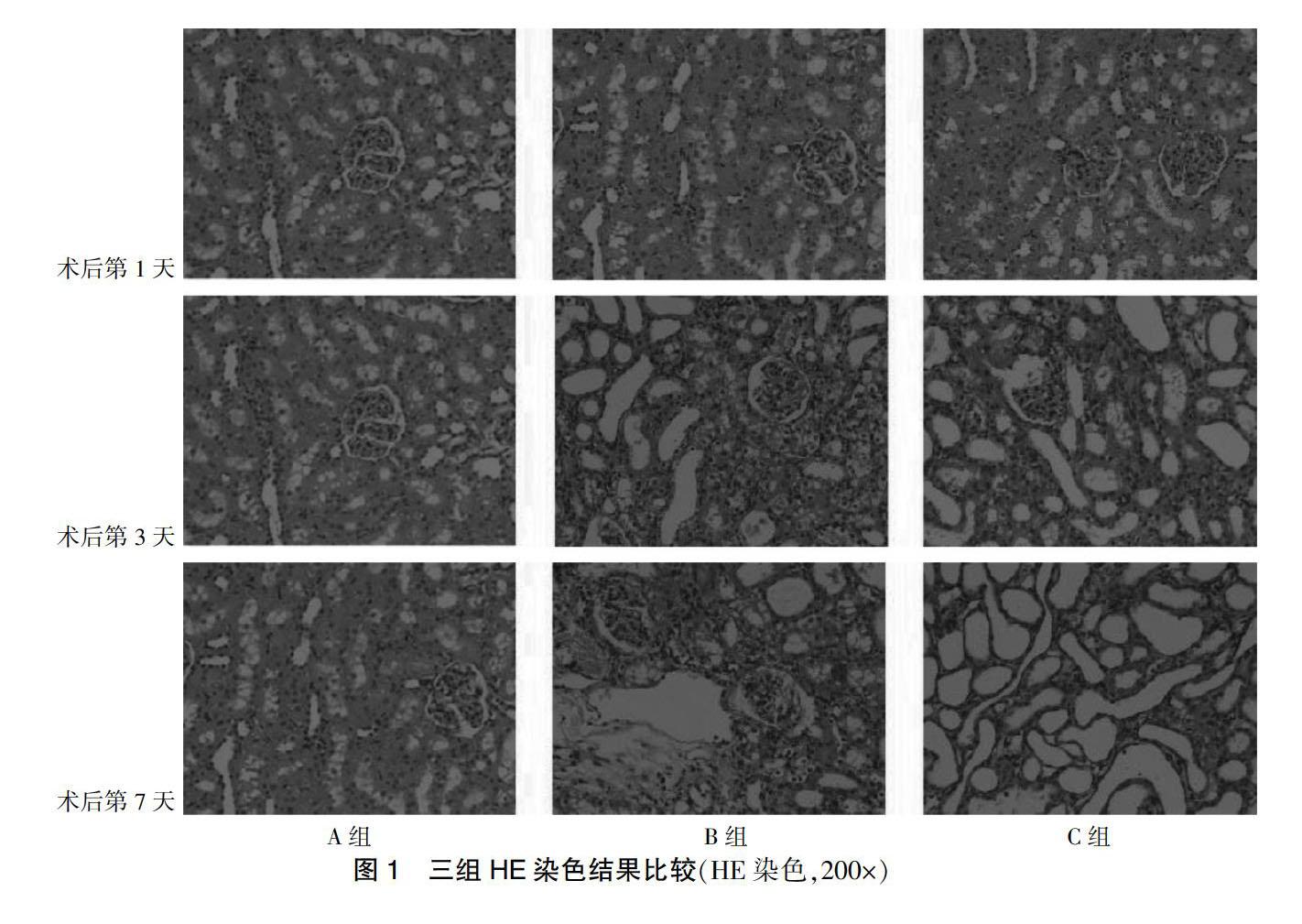
2.3 三组HE染色结果比较
A组大鼠肾脏组织形态正常,B组大鼠肾脏组织随梗阻时间延长,可见肾小管扩张及间质水肿,而C组较B组肾小管变性减轻。见图1。
2.4 三组肾组织p-AMPK水平比较
术后第1、3、7天B组p-AMPK水平均低于A组,C组p-AMPK水平均高于B组(均P < 0.05)。见图2、表3。
AMPK:腺苷酸活化蛋白激酶
2.5 三组肾组织FOXP3水平比较
术后第1、3、7天B组FOXP3水平均低于A组,C组FOXP3水平均高于B组(均P < 0.05)。见表4。
3 讨论
肾间质纤维化与持续存在的肾脏炎症有关,肾脏炎症激活免疫细胞,进而释放转化生长因子(TGF-β),TGF-β诱导EMT,而a-SMA可反映EMT程度[7-11]。本研究结果显示,术后第1、3、7天C组a-SMA水平均低于B组(均P < 0.05),提示1,25(OH)2D3可延缓大鼠肾间质纤维化。
1,25(OH)2D3可调节钙磷代谢,近期其肾脏保护作用受到人们的重视。研究发现[4,12],AMPK有抑制多条纤维化通路及决定T细胞激活和分化的作用。AMPK作为能量传感器,当细胞能量缺乏时,三磷酸腺苷(ATP)供能后被分解成一磷酸腺苷(AMP),AMP与AMPK的调节亚基结合,进而抑制催化基团上苏氨酸的脱磷酸作用激活AMPK[13]。Lu等[14]研究发现二甲双胍可激活AMPK,减少TGF-β诱导的I型胶原产生。Bakhshalizadeh等[15]发现1,25(OH)2D3可激活AMPK,调节多囊卵巢综合征小鼠模型颗粒细胞中类固醇的生成。本研究结果显示,术后第1、3、7天C组p-AMPK水平均高于B组(均P < 0.05),提示1,25(OH)2D3可作为AMPK的激活剂。
辅助性T细胞(Th17)与Treg的动态平衡在维持炎症中至关重要,本课题组前期发现UUO模型中Th17被激活[16]。Treg中表现出更高水平的AMPK,而AMPK活化后可抑制糖酵解和增强脂质氧化来控制T细胞分化[17-19]。Tian等[20]研究发现吡格列酮可调节AMPK依赖机制中的Th17/Treg平衡来稳定动脉粥样硬化斑块。Treg可特异性表达FOXP3,所以FOXP3水平可反映Treg的分化程度。本研究结果显示,C组FOXP3水平均高于B组(均P < 0.05),提示1,25(OH)2D3可能通过诱导AMPK激活来促进Treg的分化延缓肾间质纤维化。
[参考文献]
[1] Zhang B,Shi YQ,Zou JJ,et al. High glucose stimulates cell proliferation and Collagen Ⅳ production in rat mesangial cells through inhibiting AMPK-KATP signaling [J]. Int Urol Nephrol,2017,49(11):2079-2086.
[2] Lee MJ,Feliers D,Mariappan MM,et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy [J]. Am J Physiol Renal Physiol,2007,292(2):F617-F627.
[3] Peter T,Hawley SA,Clarke RG,et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes [J]. J Exp Med,2006, 203(7):1665-1670.
[4] Duan W,Ding Y,Yu X,et al. Metformin mitigates autoimmune insulitis by inhibiting Th1 and Th17 responses while promoting Treg production [J]. Am J Transl Res,2019, 11(4):2393-2402.
[5] MahajanD,Wang Y,Qin X,et al. CD4+CD25+ Regulatory T Cells Protect against Injury in an Innate Murine Model of Chronic Kidney Disease [J]. J Am Soc of Nephrol,2006, 17(10):2731-2741.
[6] Chung S,Kim S,Kim M,et al. Treatment combining aliskiren with paricalcitol is effective against progressive renal tubulointerstitial fibrosis via dual blockade of intrarenal renin [J]. PLoS One,2017,12(7):e0181757.
[7] Djudjaj S,Martin IV,Buhl EM,et al. Macrophage Migration Inhibitory Factor Limits Renal Inflammation and Fibrosis by Counteracting Tubular Cell Cycle Arrest [J]. J Am Soc Nephrol,2017,28(12):3590-3604.
[8] Liu BC,Tang TT,Lv LL,et al. Renal tubule injury:a driving force toward chronic kidney disease [J]. Kidney Int,2018,93(3):568-579.
[9] Epstein FH,Blobe GC,Schiemann WP,et al. Role of Transforming Growth Factor (beta) in Human Disease [J]. N Enql J Med,2000,342(18):1350-1358.
[10] 牛潼,劉琦然,李玲,等.Wnt/β-catenin信号通路对肾小管上皮间充质转分化的调控[J].中华实用儿科临床杂志,2016,31(12):958-960.
[11] 陈红淑,杨元宵,周小杰,等.肾气丸通过TGF-β1/Smads/ILK信号途径干预肾小管上皮细胞间充质转分化的分子机制[J].中华中医药杂志,2016,31(6):2102-2105.
[12] 刘慧铭,王圆圆,郭兵.腺苷酸活化蛋白激酶在肾脏纤维化中的研究进展[J].中国当代医药,2018,25(26):33-36.
[13] Hardie DG. AMP-activated/SNF1 protein kinases:conserved guardians of cellular energy [J]. Nat Rev Mol Cell Biol,2007,8(10):774-785.
[14] Lu J,Shi J,Li M,et al. Activation of AMPK by metformin inhibits TGF-β-induced collagen production in mouse renal fibroblasts [J]. Life Sci,2015,127:59-65.
[15] Bakhshalizadeh S,Amidi F,Shirazi R,et al. Vitamin D3 regulates steroidogenesis in granulosa cells through AMP-activated protein kinase(AMPK)activation in a mouse model of polycystic ovary syndrome [J]. Cell Biochem Funct,2018,36(4):183-193.
[16] 王茜,罗静,郭珲.1,25-二羟维生素D3对肾间质纤维化大鼠IL-17的调节作用[J].临床医药实践,2017,26(4):289-292.
[17] Michalek RD,Gerriets VA,Jacobs SR,et al. Cutting Edge:Distinct Glycolytic and Lipid Oxidative Metabolic Programs Are Essential for Effector and Regulatory CD4+ T Cell Subsets [J]. J Immunol,2011,186(6):3299-3303.
[18] Gwinn DM,Shackelford DB,Egan DF,et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint [J]. Mol Cell,2008,30(2):214-226.
[19] Ma EH,Poffenberger MC,Wong AH,et al. The role of AMPK in T cell metabolism and function [J]. Curr Opin Immunol,2017,46:45-52.
[20] Tian Y,Chen T,Wu Y,et al. Pioglitazone stabilizes atherosclerotic plaque by regulating the Th17/Treg balance in AMPK-dependent mechanisma [J]. Cardiovasc Diabetol,2017,16(1):140.
(收稿日期:2019-10-21 本文編辑:刘明玉)
