湿润烧伤膏对寻常型银屑病小鼠IL23/Th17轴及其相关因子表达水平的影响
2020-06-28任晓燕王李雯张丽丽孙丽萍
任晓燕 王李雯 张丽丽 孙丽萍
作者单位:712000 陕西 咸阳,陕西中医药大学附属医院皮肤科
寻常型银屑病是一种常见的慢性、复发性、炎症性皮肤病[1]。研究发现,白细胞介素-23(IL-23) /辅助性T细胞17(Th17) 轴失调是银屑病的主要发病机制之一[2], IL23/Th17轴相关因子参与炎症反应与银屑病的复发及持续密切相关[3]。临床上针对银屑病IL-23/Th17通路的靶向药物已被证实有效[4],但却存在症状反复、药物不良反应较多等弊端[5]。近年来,部分研究学者将湿润烧伤膏应用于银屑病的治疗,取得了一定的临床疗效,且未见明显不良反应[6],但具体作用机制尚不明确。本研究通过观察湿润烧伤膏对寻常型银屑病小鼠皮损组织中白细胞介素-17(IL-17)、白细胞介素-23(IL-23)、 肿瘤坏死因子-α (TNF-α) 及干扰素-γ(IFN-γ)水平的影响,探讨了其对寻常型银屑病的作用机制,以期为湿润烧伤膏的临床应用提供理论依据。
Psoriasis vulgaris is a common chronic,recurrent and inflammatory skin disease[1].Studies have shown that the imbalance of interleukin-23 (IL-23) /helper T cell 17 (Th17) axis is one of the main pathogenesis of psoriasis[2].The involvement of IL23 /Th17 axisrelated factors in the inflammatory reactions is closely related to the recurrence and persistence of psoriasis[3].Targeted drugs for IL-23 /Th17 pathway in psoriasis have been proven effective in clinical practice[4], but such drugs still have some drawbacks, because they may lead to the recurrence of the disease and some adverse drug reactions[5].In recent years, some research scholars have adopted MEBO to treat psoriasis.Certain clinical effect has been obtained and no obvious adverse reactions have been observed[6].However, the specific mechanism of action of MEBO remains unclear.In this study,the effect of MEBO on the expression levels of interleukin-17(IL-17), interleukin-23 (IL-23), tumor necrosis factor-α (TNF-α)and interferon-γ (IFN-γ) in skin tissues on the backs of mice with psoriasis vulgaris was observed and the mechanism of its action on psoriasis vulgaris was explored with a view to provide experimental evidence for its clinical application.
1 材料与方法
1.1 实验动物
SPF级健康雌性近交系 BALB/c小鼠40只,6~7周龄,体重 (18±2)g,成都达硕实验动物有限公司提供,动物合格证号SCXK(川)2015-030,饲养于SPF级动物室,自由进食水。本研究经陕西中医药大学附属医院动物伦理委员会批准,符合动物实验的伦理学要求。
1.2 主要药品及试剂
湿润烧伤膏:汕头市美宝制药有限公司生产,国药准字Z20000004;卡泊三醇软膏:香港澳美制药有限公司生产,批准文号HC20150020;5%咪喹莫特乳膏:四川明欣药业有限责任公司生产,国药准字H20030128;兔多抗 IL-17 (ab79056)、 兔多抗 IL-23(ab45420)、 兔多抗 TNF-α (ab9739)、 兔多抗IFN-γ(ab9657):英国Abcam公司生产;小鼠单抗β-actin:武汉博士德生物工程有限公司生产,货号BM0627。
1.3 模型建立
用硫化钠脱去小鼠背部面积约3 cm×2 cm的毛发,裸露背部皮肤并涂抹5%咪喹莫特乳膏,每次62.5 mg,每天1次,连续涂抹7 d[7]。随机选取5只小鼠在无菌操作下取其背部皮损组织,10%福尔马林溶液固定,制备石蜡切片,并于HE染色后,光镜下观察皮肤角化程度,颗粒层、棘层、表皮突、真皮乳头层厚度及排列情况,淋巴细胞、中性粒细胞分布情况等病理变化。
模型评价:小鼠背部可见暗红色或红褐色斑块,表面覆有较厚银白色鳞屑,有不同程度浸润增厚,符合寻常型银屑病的皮损表现 (图1);病理切片显示皮肤角化不全,颗粒层变薄,棘层明显增厚,表皮突延长,嗜中性粒细胞浸润 (图2),类似人寻常型银屑病的病理组织学表现。
1.4 分组与给药
模型建立成功后,采用随机数表法将35只小鼠随机分为对照组、模型组、MEBO低剂量组、MEBO中剂量组及MEBO高剂量组,每组7只。
对照组:小鼠背部裸露皮肤均匀涂抹卡泊三醇乳膏 (1 mg/cm2);模型组:小鼠背部裸露皮肤均匀涂抹适量麻油;MEBO低剂量组:小鼠背部裸露皮肤均匀涂抹湿润烧伤膏与麻油混合物 (混合比例为1∶3,厚约1 mm);MEBO中剂量组:小鼠背部裸露皮肤均匀涂抹湿润烧伤膏与麻油混合物 (混合比例为1∶1,厚约1 mm);MEBO高剂量组:小鼠背部裸露皮肤均匀涂抹湿润烧伤膏 (厚约1 mm)。5组小鼠均每天换药2次,连续用药28 d。
1.Materials and methods
1.1.Experiment animals
The experiment animals were forty 6-to 7-week-old specificpathogen free (SPF) healthy inbred BALB/c female mice, each weighing(18±2) g,provided by Chengdu Dossy Experimental Animals Co., LTD.Animal certificate No.: SCXK (Sichuan) 2015-030.They were kept in SPF animal room with sufficient food and water.This study has been approved by The Animal Ethics Committee of Affiliated Hospital of Shaanxi University of Chinese Medicine and complies with the ethical requirements of animal experiments.
1.2.Main drugs and reagents
MEBO:produced by Shantou MEBO Pharmaceutical co.,LTD,SFDA approval number: Z20000004; Capotriol cream: produced by BrightFuture PharmaceuticalLaboratoriesLtd., approvalNo.HC20150020;5%imiquimod cream: produced by MED.SHINE,China, SFDA approval No.: H20030128; Rabbit polyclonal antibody IL-17 (ab79056),rabbit polyclonal antibody IL-23(ab45420),rabbit polyclonal antibody TNF-α (ab9739) and rabbit polyclonal antibody IFN-γ (ab9657): produced by British Abcam company; mouse monoclonal antibody: produced by Boster Biological Technology Co.,LTD in Wuhan,article No.BM0627.
1.3.Model establishment
3 cm×2 cm area of hairs on the backs of the mice was removed with sodium sulfide to expose their back skin,on which 5% imiquimod cream was applied, 62.5 mg each time, once a day for 7 days[7].Five mice were randomly selected.Tissues of skin lesions on their backs were taken under aseptic operation and then fixed with 10%formalin solution to prepare paraffin sections.The degree of skin keratinization, the thickness and order of the granular layer, spinous layer, trochanterellus and dermal papilla layer, and the distribution of lymphocytes and neutrophils were observed under the light microscope after HE staining.
Model evaluation:dark red or reddish-brown plaques could be seen on the backs of the mice,the surface of their back skin was covered with thick silvery white scales,varying degrees of infiltration and thickening could be observed,and all of these symptoms were consistent with skin lesions in psoriasis vulgaris(Fig.1); pathological sections showed that the skin keratinization was incomplete,the granular layer got thinned, the spinous layer became significantly thickener,the trochanterellus prolonged and the neutrophil infiltration was found(Fig.2), which were similar to the pathohistological manifestations of human psoriasis vulgaris.
1.4.Grouping and drug administration
After the models were established, 35 mice were divided, according to random number table, into a control group, a model group,a MEBO low-dose group, a MEBO medium-dose group, and a MEBO high-dose group.
The control group: calpatriol cream (1 mg/cm2) was evenly applied on the exposed back skin of the mice; the model group: appropriate volume of sesame oil was evenly applied on the exposed back skin of the mice; the MEBO low-dose group: the mixture of MEBO and sesame oil(mixing ratio=1∶3, thickness=1 mm) was evenly applied on the exposed back skin of the mice; the MEBO medium-dose group: the mixture of MEBO and sesame oil(mixing ratio=1∶1, thickness=1 mm) was evenly applied on the exposed back skin of the mice;the MEBO high-dose group:MEBO was evenly applied on the exposed back skin of the mice(thickness=1 mm).Dressing change was performed twice a day for 28 days in the five groups of mice.
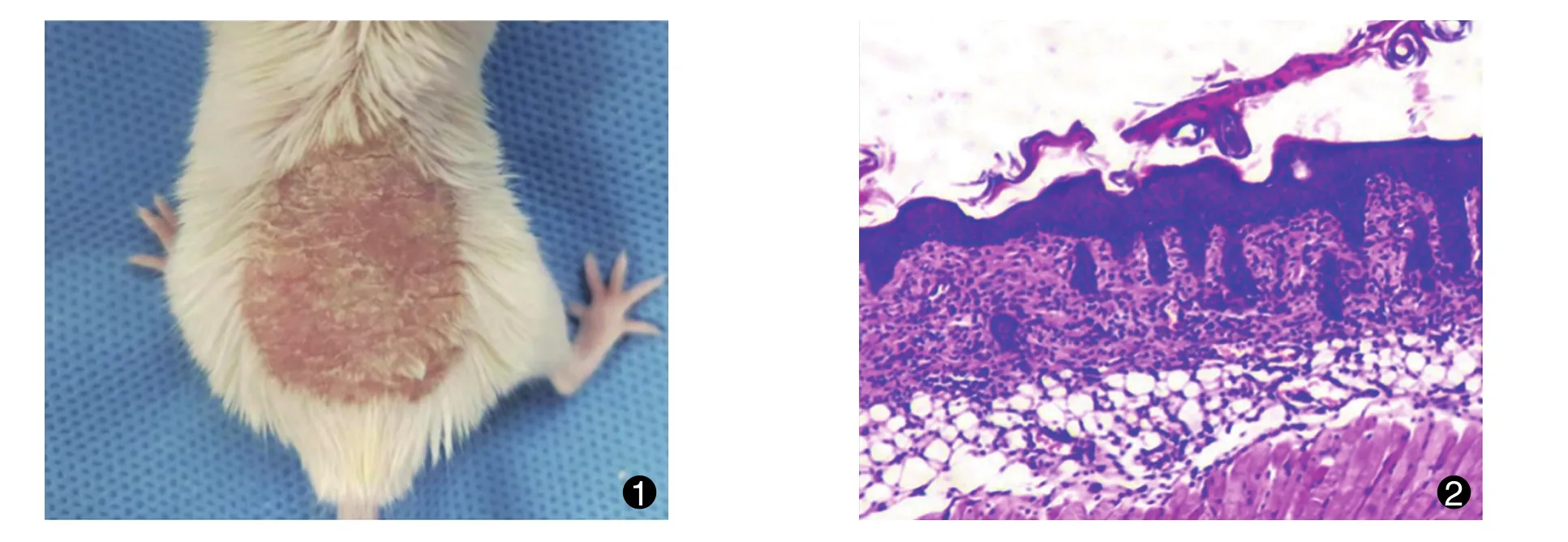
图1 典型寻常型银屑病小鼠模型皮损表现;图2 典型寻常型银屑病小鼠模型皮损组织病理表现(HE, ×100)Fig.1 Skin lesions of typical mouse model of psoriasis vulgaris; Fig.2 Pathological manifestations of skin lesions of typical mouse model of psoriasis vulgaris(HE, ×100)
1.5 标本采集与检测
1.5.1 皮损严重程度评分 分别于治疗第7、14天,参照银屑病皮损面积与严重程度指数 (psoriasis area and severity index, PASI)评分标准[8]评估皮损严重程度,PASI评分标准包含红斑、鳞屑及浸润增厚程度3项,每项0~4分,3项之和为皮损严重程度总评分,分值越高表示皮损越严重。
1.5.2 Western blotting法检测IL-17、IL-23、TNF-α及IFN-γ蛋白表达水平 分别于治疗第14、28天,每组分别选取4只、3只小鼠以脱颈法处死后,在无菌操作下取其背部皮损组织置于-80℃冰箱中保存备用。待所有标本收集完毕,取出冷冻组织置于冰上称重后,置于研钵内研磨至粉末状;将研磨后的组织粉末置于EP管中行RIPA裂解,提取总蛋白,并采用紫外分光光度计测定总蛋白浓度,绘制蛋白浓度标准曲线。取蛋白上样经SDS-聚丙烯酰氨凝胶 (SDS-PAGE)电泳分离及转模后,TBST洗膜3次;洗膜后,5%脱脂奶粉封闭2 h,4℃孵育相应目的抗体(IL-23、 IL-17、 TNF-α 混合比例均为1∶1000;IFN-γ 混合比例为1∶2000; β-actin混合比例为1∶200)过夜;孵育过夜后,TBST洗膜3次,室温孵育 HRP-羊抗鼠二抗 (混合比例为1∶5000)2 h;二抗孵育后,TBST洗膜3次,ECL显影,并用BandScan 5.0软件分析目的条带灰度值。每组小鼠标本重复检测3次,取均值进行对比分析。
1.5.Specimen collection and detection
1.5.1.Skin lesion severity score On day 7 and day 14 of treatment,skin lesion severity was assessed based on the psoriasis area and severity index (PASI) scoring system[8].Under the PASI score criteria, erythema, scales, and infiltration and thickening are scored between 0 to 4 points.The sum of the 3 items is total score of skin lesion severity.Higher score means more severe lesion.
1.5.2.Detection of the protein expression levels of IL-17, IL-23, TNF-α and IFN-γ with Western blotting On day 14 and day 28 of treatment, 4 and 3 mice were selected from each group and killed by cervical dislocation.After that,the skin tissues on their backs were taken under aseptic operation and stored in a refrigerator at the temperature of-80℃.After all the specimens were collected,the frozen tissues were taken out and put on ice to be weighted and then grounded into powder in a mortar.The grounded tissue powder was placed in the EP tube to be cracked with RIPA lysis buffer for total protein extraction.Ultraviolet spectrophotometer was used to measure the concentration of total protein,based on which the standard curve for protein concentration was drawn.The protein was loaded and then separated by using SDS-PAGE gel electrophoresis and then transferred to membrane,which was washed 3 times with TBST; after washing, it was sealed for 2 hours with 5%skim milk powder,and the corresponding target antibody was incubated overnight at the temperature of 4 ℃ (the mixing ratio of IL-23,IL-17, and TNF-α was 1∶1000; the mixing ratio of IFN-γ was 1∶2000; the mixing ratio of β-actin was 1∶200) ; after overnight incubation, the membrane was washed with TBST for 3 times,and HRP-goat anti-mouse secondary antibody(mixing ratio=1∶5000) was incubated at room temperature for 2 hours; after secondary antibody incubation, the membrane was washed with TBST for 3 times, ECL was used for its development, and Band-Scan 5.0 software was adopted to measure the gray value of target band.The specimens of each group of mice were detected three times and the mean value of the test was taken for comparative analysis.
1.6 统计学处理
采用SPSS 19.0统计软件对所得数据进行统计学分析,计量资料符合正态分布用均数±标准差 ()表示,多样本间比较采用单因素方差分析;均以P<0.05为差异具有统计学意义。
2 结果
2.1 5组小鼠背部皮损PASI评分对比
治疗第7天,与模型组相比,对照组、MEBO中剂量组及MEBO高剂量组小鼠背部皮损红斑颜色较浅,浸润增厚不明显,鳞屑较少,PASI评分均较低 (P均<0.05),且对照组、MEBO中剂量组及MEBO高剂量组组间两两对比,PASI评分无明显差异 (P均>0.05)。治疗第14天,5组小鼠背部皮损症状均明显改善,斑块颜色呈淡红色,无明显浸润增厚,可见极少量鳞屑,PASI评分无明显差异 (P>0.05),详见表1。
2.2 5组小鼠背部皮损组织中IL-17、IL-23、
TNF-α及IFN-γ蛋白表达水平对比
治疗第14天,小鼠背部皮损组织中IL-17、 IL-23、 TNF-α及 IFN-γ蛋白表达水平对比,对照组显著低于其他各组 (P均 <0.05),且MEBO高剂量组显著低于模型组(P均<0.05),详见表2、图3。治疗第28天,小鼠背部皮损组织中 IL-17、IL-23、TNF-α及IFN-γ蛋白表达水平对比,对照组显著低于模型组、MEBO低剂量组及MEBO中剂量组 (P均 <0.05),与 MEBO高剂量组无明显差异 (P均>0.05);MEBO高剂量组和MEBO中剂量组显著低于模型组和MEBO低剂量组 (P均<0.05),且MEBO高剂量组与MEBO中剂量组无明显差异 (P均>0.05), 详见表3、 图3。
1.6.Statistical processing
SPSS 19.0 statistical software was used to analyze the obtained data.The measurement data conforming to the normal distribution were expressed as mean±standard deviation(), and multi-sample comparison was performed using one-way ANOVA;P<0.05 was considered as statistically significant.
2.Results
2.1.Comparison of PASI scores of skin lesions on the backs of the five groups of mice
On day 7 of treatment, the back skin lesions in the control group,the MEBO medium-dose group and the MEBO high-dose group was lighter in color, had less obvious infiltration and thickening and fewer scales,and lower PASI score(all P<0.05) compared with that in the model group, and pairwise comparison among the control group, the MEBO medium-dose group and the MEBO high-dose group showed no significant difference in PASI scores(all P >0.05).On day 14 of treatment, the skin lesions on the back of the five groups of mice improved significantly:the plaques were reddish in color, no obvious infiltration and thickening,only a very small amount of scales were observed,and no significant difference was observed in PASI score(P>0.05),as shown in table 1.
2.2.Comparison of the protein expression levels of IL-17, IL-23, TNF-α and IFN-γ in the skin lesions on the backs of the five groups of mice
On day 14 of treatment, the protein expression levels of IL-17,IL-23, TNF-α and IFN-γ in the skin tissues on the backs of the five groups of mice were compared:their levels in the control group was significantly lower than other groups(all P<0.05) and their levels in the MEBO group were significantly lower than the model group(all P<0.05).See Table 2 and Fig.3 for details.On day 28 of treatment, the protein expression levels of IL-17, IL-23, TNF-α and IFN-γ in the skin tissues on the backs of the five groups of mice were compared:their levels in the control group was significantly lower than the model group,the MEBO low-dose group and the MEBO medium-dose group(all P<0.05)and no significant difference was observed between the control group and MEBO high-dose group (all P >0.05);their levels in the MEBO high-dose group and MEBO medium-dose group were significantly lower than the model group and MEBO low-dose group (all P <0.05),but there was no significant difference between MEBO high-dose group and MEBO medium-dose group (all P >0.05).See Table 2 and Fig.3 for details.
表1 5组小鼠背部皮损PASI评分对比 ()Table 1 Comparison of PASI scores of skin lesions on the backs of the five groups of mice()

表1 5组小鼠背部皮损PASI评分对比 ()Table 1 Comparison of PASI scores of skin lesions on the backs of the five groups of mice()
注:5组小鼠背部皮损PASI评分组间两两对比,其中与对照组对比,aP<0.05,差异具有统计学意义;与模型组对比,bP<0.05,差异具有统计学意义;与MEBO低剂量组对比,cP<0.05,差异具有统计学意义Note:Pairwise comparison was made among the five groups in the PASI scores.Among them,comparison with that of the control group(aP<0.05)showed statistically significant difference;comparison with that of the model group(bP <0.05) showed statistically significant difference;comparison with that of the MEBO low-dose group(cP<0.05) showed statistically significant difference
组别Group鼠数 (只)Number of mice (n)第7天 (分)Day 7 (point)第14天 (分)Day 14 (point)对照组Control group 7 5.29±0.75 1.71±0.49模型组Model group 7 7.00±0.81a 2.00±0.82 MEBO低剂量组MEBO low-dose group 7 6.86±0.69a 1.86±0.69 MEBO中剂量组MEBO medium-dose group 7 5.86±0.69bc 1.71±0.76 MEBO高剂量组MEBO high-dose group 7 5.71±0.95bc 1.57±0.54 F值F value 6.346 0.415 P值P value 0.001 0.797
表2 治疗第14天5组小鼠背部皮损组织中IL-17、IL-23、TNF-α及IFN-γ蛋白表达水平对比 ()Table 2 Comparison of the protein expression levels of IL-17, IL-23, TNF-α and IFN-γ in the skin lesions on the backs of the five groups of mice on day 14 of treatment()
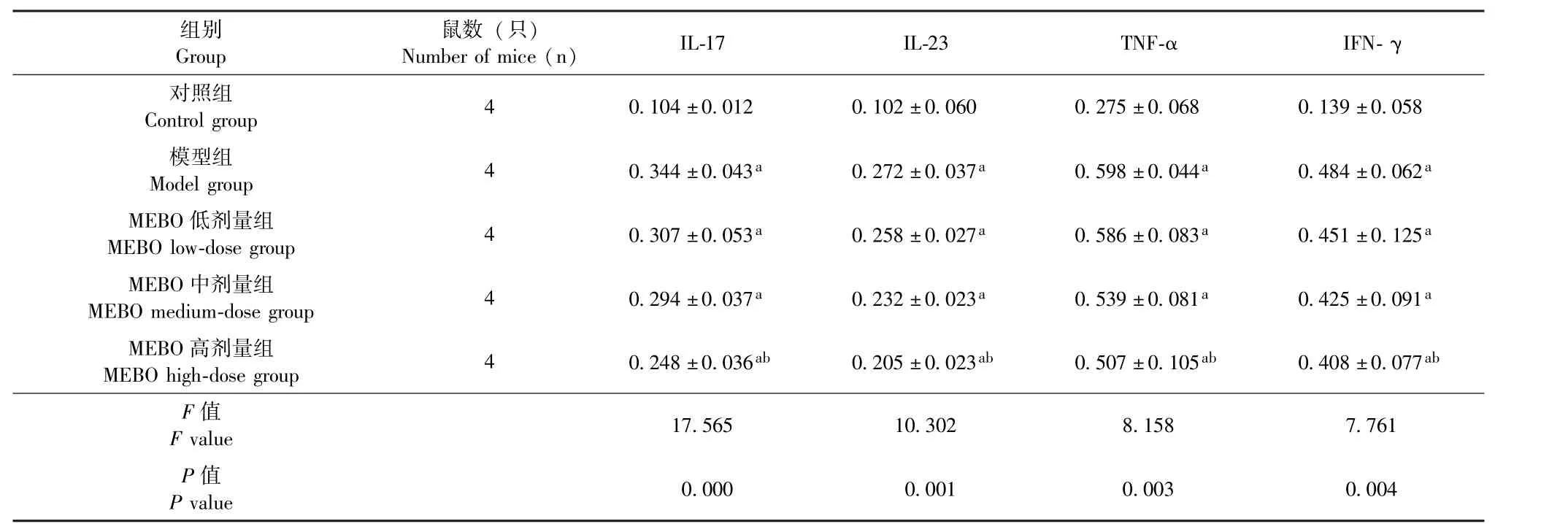
表2 治疗第14天5组小鼠背部皮损组织中IL-17、IL-23、TNF-α及IFN-γ蛋白表达水平对比 ()Table 2 Comparison of the protein expression levels of IL-17, IL-23, TNF-α and IFN-γ in the skin lesions on the backs of the five groups of mice on day 14 of treatment()
注:治疗第14天,5组小鼠背部皮损组织中IL-17、IL-23、TNF-α及IFN-γ蛋白表达水平组间两两对比,其中与对照组对比,aP<0.05,差异具有统计学意义;与模型组对比,bP<0.05,差异具有统计学意义Note: Pairwise comparison in the expression levels of IL-17, IL-23, TNF-α and IFN-γ in the skin tissues on the backs of the five groups of mice was made,comparison with that of the control group(aP<0.05) showed statistically significant difference,comparison with that of the model group(bP<0.05)showed statistically significant difference
组别Group鼠数 (只)Number of mice (n) IL-17 IL-23 TNF-α IFN-γ对照组Control group 4 0.104±0.012 0.102±0.060 0.275±0.068 0.139±0.058模型组Model group 4 0.344±0.043a 0.272±0.037a 0.598±0.044a 0.484±0.062a MEBO低剂量组MEBO low-dose group 4 0.307±0.053a 0.258±0.027a 0.586±0.083a 0.451±0.125a MEBO中剂量组MEBO medium-dose group 4 0.294±0.037a 0.232±0.023a 0.539±0.081a 0.425±0.091a MEBO高剂量组MEBO high-dose group 4 0.248±0.036ab 0.205±0.023ab 0.507±0.105ab 0.408±0.077ab F值F value 17.565 10.302 8.158 7.761 P值P value 0.000 0.001 0.003 0.004
3 讨论
寻常型银屑病临床以红斑、鳞屑为主要表现,是由多种免疫细胞和细胞因子介导的自身免疫性疾病[9],且与皮肤屏障功能不全密切相关[10]。相关研究证实, IL-23/Th17轴失调是银屑病的主要发病机制之一[11],IL-23作为重要的致炎因子,可通过促进Th17细胞的分化及活化而促使IL-17、IL-22、IL-21等细胞因子的大量释放,进而导致角质形成细胞过度增生[12],并向真皮层生长,逐渐衍变为皮肤炎性损害,形成银屑病[13];IL-17可通过促进炎症反应的发生,刺激角质形成细胞分泌IL-6、TNF-α及IFN-γ[14]而加快中性粒细胞的增殖、成熟和趋化,进而导致表皮细胞过度增殖。湿润烧伤膏作为一种油膏制剂,因其内含有的有效成分可抑制TNF-α与IL-6等细胞因子的产生与释放,减轻局部与全身炎症反应[15-17]而被应用于寻常型银屑病的治疗,疗效较为满意,但具体作用机制并未被证实。
3.Discussion
Psoriasis vulgaris is an autoimmune disease mediated by a variety of immune cells and cytokines[9].Its main clinical manifestations are erythema and scales and its occurrence is closely related to skin barrier dysfunction[10].Studies have confirmed that the imbalance of IL-23 /Th17 axis is one of the main pathogenesis of psoriasis[11].As an important inflammatory factor,IL-23 can promote the differentiation and activation of Th17 cells,leading to the release of large amount of cell factors like IL-17, IL-22 and IL-21.As a result, keratinocytes proliferate excessively[12]and start growing towards dermis, causing inflammatory skin lesions and finally psoriasis[13]; IL-17 can accelerate the proliferation,maturation and chemotaxis of neutrophils by promoting inflammatory reactions and stimulating keratinocytes to secrete IL-6,TNF-α and IFN-γ[14], resulting in excessive proliferation of epidermal cells.MEBO is a kind of ointment and has been applied in the treatment of psoriasis vulgoriasis thanks to its active ingredients,which can inhibit the production and release of cytokines such as TNF-α and IL-6 and reduce local and systemic inflammatory reactions[15-17].Despite of its satisfactory effect,its specific mechanism of action has not been proven.
表3 治疗第28天5组小鼠背部皮损组织中IL-17、IL-23、TNF-α及IFN-γ蛋白表达水平对比 ()Table 3 Comparison of the protein expression levels of IL-17, IL-23, TNF-α and IFN-γ in the skin lesions on the backs of the five groups of mice on day 28 of treatment()
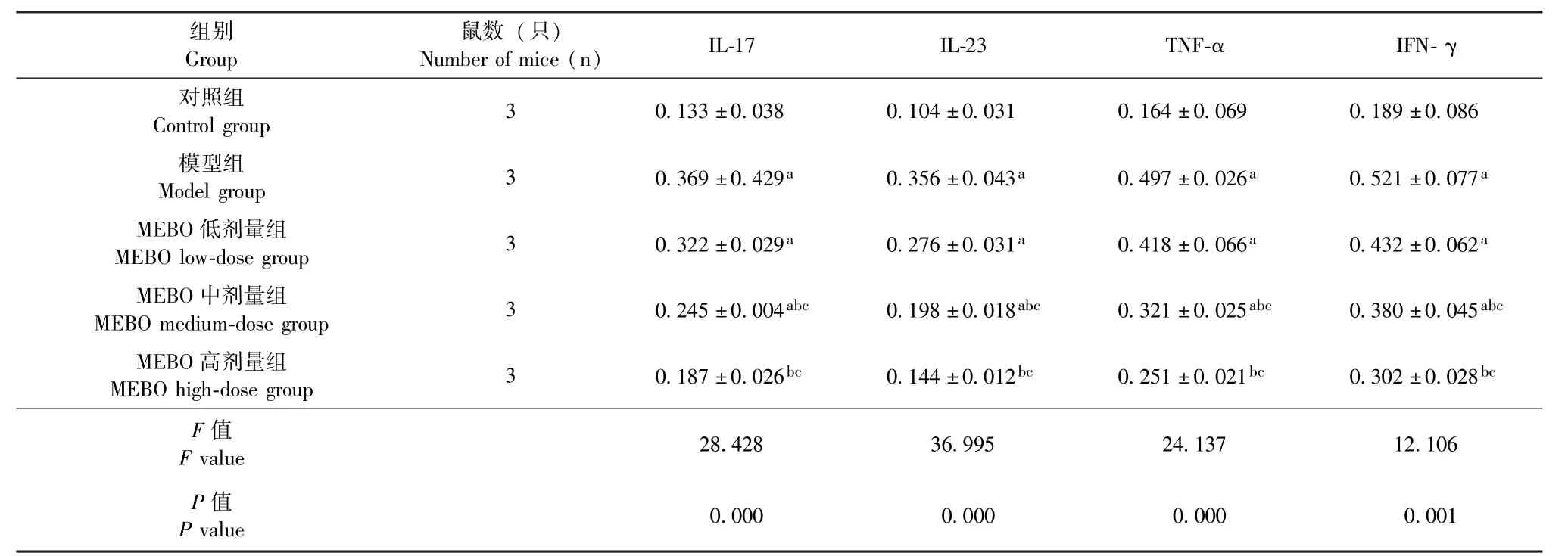
表3 治疗第28天5组小鼠背部皮损组织中IL-17、IL-23、TNF-α及IFN-γ蛋白表达水平对比 ()Table 3 Comparison of the protein expression levels of IL-17, IL-23, TNF-α and IFN-γ in the skin lesions on the backs of the five groups of mice on day 28 of treatment()
注:治疗第28天,5组小鼠背部皮损组织中IL-17、IL-23、TNF-α及IFN-γ蛋白表达水平组间两两对比,其中与对照组对比,aP<0.05,差异具有统计学意义;与模型组对比,bP<0.05,差异具有统计学意义;与MEBO低剂量组对比,cP<0.05,差异具有统计学意义Note: The protein expression levels of IL-17, IL-23, TNF-α and IFN-γ in the skin lesions on the backs of the five groups of mice were compared between each two groups.Among them,comparison with that of the control group(aP<0.05) showed statistically significant difference;comparison with that of the model group(bP<0.05)showed statistically significant difference;compared with the MEBO low-dose group(cP<0.05)showed statistically significant difference
组别Group鼠数 (只)Number of mice (n) IL-17 IL-23 TNF-α IFN-γ对照组Control group 3 0.133±0.038 0.104±0.031 0.164±0.069 0.189±0.086模型组Model group 3 0.369±0.429a 0.356±0.043a 0.497±0.026a 0.521±0.077a MEBO低剂量组MEBO low-dose group 3 0.322±0.029a 0.276±0.031a 0.418±0.066a 0.432±0.062a MEBO中剂量组MEBO medium-dose group 3 0.245±0.004abc 0.198±0.018abc 0.321±0.025abc 0.380±0.045abc MEBO高剂量组MEBO high-dose group 3 0.187±0.026bc 0.144±0.012bc 0.251±0.021bc 0.302±0.028bc F值F value 28.428 36.995 24.137 12.106 P值P value 0.000 0.000 0.000 0.001

图3 5组小鼠背部皮损组织中IL-17、IL-23、TNF-α及IFN-γ蛋白表达条带图Fig.3 Histogram of protein expression levels of IL-17, IL-23, TNF-α and IFN-γ in the skin lesions on the backs of the five groups of mice
本研究通过观察湿润烧伤膏对寻常型银屑病小鼠背部皮损组织中 IL-17、IL-23、TNF-α及IFN-γ水平的影响探讨其对寻常型银屑病的作用机制。结果显示,治疗第7天,对照组、MEBO中剂量组及MEBO高剂量组小鼠背部皮损PASI评分均显著低于模型组,且对照组、MEBO中剂量组及MEBO高剂量组组间两两对比,PASI评分均无明显差异。可见,湿润烧伤膏能够有效改善寻常型银屑病小鼠红斑、鳞屑、浸润增厚等皮损症状,且能够达到临床已证实有效的卡泊三醇软膏同等的疗效。Western blotting检测结果显示,治疗第14天,小鼠背部皮损组织中IL-17、IL-23、TNF-α及 IFN-γ蛋白表达水平对比,对照组显著低于其他各组,且MEBO高剂量组显著低于模型组;治疗第28天,小鼠背部皮损组织中IL-17、IL-23、TNF-α及IFN-γ蛋白表达水平对比,对照组显著低于模型组、MEBO低剂量组及MEBO中剂量组,与MEBO高剂量组无明显差异,MEBO高剂量组和MEBO中剂量组显著低于模型组和MEBO低剂量组,且MEBO高剂量组与MEBO中剂量组无明显差异。可见,湿润烧伤膏可有效降低IL-23/Th17轴相关因子的表达水平,抑制炎症反应及角质形成细胞过度增生,且随着治疗时间的延长,其对炎症反应的抑制作用加强,并达到卡泊三醇软膏同等的作用水平;另外,本结果可见湿润烧伤膏的作用效果具有剂量及时间依赖性,即在一定条件下,药物浓度越高、治疗时间越长,其治疗作用越强。
综上所述,湿润烧伤膏治疗银屑病的疗效与卡泊三醇软膏相当,且调节IL-23/Th17轴及其相关因子的表达水平,抑制炎症反应可能是其作用机制。
In this study, the effect of MEBO on the levels of IL-17, IL-23,TNF-α and IFN-γ in the skin tissues on the backs of mice was investigated so as to explore its mechanism of action on psoriasis vulgoriasis.The results showed that, on day 7 of treatment, the PASI scores of the skin lesions on the backs of mice in the control group,the MEBO mediumdose group,and the MEBO high-dose group were significantly lower than the model group.Pairwise comparison in the PASI scores among the control group,the MEBO medium-dose group and the MEBO high-dose group was made and no significant difference was observed.It can be concluded that MEBO can alleviate the skin lesions of psoriasis vulgaris on mice such as erythema, scales, and infiltration and thickening and is clinically as effective as calcipotriol cream.The result of western blotting test showed that on day 14 of treatment,the protein expression levels of IL-17, IL-23, TNF-α and IFN-γ in the skin lesions on the backs of the mice were compared:their levels in the control group was significantly lower than other groups and their levels in the MEBO high-dose group was significantly lower than the model group; on day 28 of treatment, the protein expression levels of IL-17, IL-23, TNF-α and IFN-γ in the skin tissues on the backs of the mice were compared:their levels in the control group was significantly lower than the model group,the MEBO lowdose group and the MEBO medium-dose group,no significant difference was observed between the control group and the MEBO high-dose group;their levels in the MEBO high-dose group and the MEBO medium-dose group were significantly lower than the model group and the MEBO lowdose group and no significant difference was observed between the MEBO high-dose group and the MEBO medium-dose group.It can be concluded that MEBO can reduce the expression levels of IL-23/Th17 axis-related factors,inhibit inflammatory response and excessive proliferation of keratinocytes.As treatment time increases,inhibitory effect of MEBO on inflammatory reactions can be strengthened to the same level as calcipotriol cream;in addition,the results showed that the effect of MEBO is closely related to its dosage amount and action time, that is, under certain conditions,the higher the drug concentration and the longer the treatment time are the stronger its therapeutic effect will be.
In summary,the efficacy of MEBO in treating psoriasis is equivalent to calcipotriol cream,and its mechanism of action may lie in its ability to regulate the expression levels of the IL-23/Th17 axis and its related factors and inhibit inflammation.
(收稿日期:2019-12-09)
