可可FAT基因家族进化及表达模式分析
2020-06-19李付鹏邓云梅伍宝朵秦晓威闫林赖剑雄
李付鹏 邓云梅 伍宝朵 秦晓威 闫林 赖剑雄

摘 要:在植物中,酰基-ACP硫酯酶(fatty acyl-ACP thioesterase, FAT)是调控脂肪酸合成的關键酶。为解析可可FAT基因家族成员的特点与功能,本研究从可可基因组中筛选鉴定出FAT基因家族的6个成员,分别命名为TcFATA、TcFATB1、TcFATB2、TcFATB3、TcFATB4、TcFATB5。6个基因外显子数目为6~7个,编码区(CDS)长度介于1128~1263 bp,预测蛋白分子量介于42.72~46.47 kDa,等电点介于6.57~9.10。进化分析结果表明可可FAT基因家族分成FATA与FATB亚群,FATA亚群包含1个可可TcFATA成员,FATB亚群包含5个可可TcFATBs成员。不同可可种子发育时期表达分析结果表明:TcFATA和TcFATB1伴随果实发育成熟,表达量呈下降趋势,TcFATA和TcFATB1在不同种质中表达量与油酸(C18:1)和棕榈酸(C16:0)比例呈正相关,表明其与脂肪酸组分比例调控有紧密关联。
关键词:可可;酰基载体蛋白硫酯酶(FAT);系统进化;表达模式
中图分类号:S571.3 文献标识码:A
Phylogeny and Expression Profile of Fatty Acyl-ACP Thioesterase (FAT) Gene Family in Cacao (Theobroma cacao L.)
LI Fupeng1, DENG Yunmei1, 2, WU Baoduo1, QIN Xiaowei1, YAN Lin1, LAI Jianxiong1
1. Spice and Beverage Research Institute, Chinese Academy of Tropical Agricultural Sciences / Key Laboratory of Genetic Resources Utilization of Spice and Beverage Crops, Ministry of Agriculture and Rural Affairs / Hainan Provincial Key Laboratory of Genetic Improvement and Quality Regulation for Tropical Spice and Beverage Crops, Wanning, Hainan 571533, China; 2. College of Tropical Crop Science, Yunnan Agricultural University, Pu'er, Yunnan 665099, China
Abstract: Fatty acyl-ACP thioesterase (FAT) is the key enzyme regulating the synthesis of plant lipid. In order to better understand the characteristics of FAT gene family in Theobroma cacao, six novel FAT genes were identified from economically important cacao trees, designated as TcFATA, TcFATB1, TcFATB2, TcFATB3, TcFATB4, TcFATB5, respectively. Sequences analyses revealed that CDS of TcFATs was 1128–1263 bp, containing 6–7 exons. The molecular weight of the six predicted protein was 42.72–46.47 kDa, and pI of the proteins was 6.57–9.10. FAT family could be divided into FATA (one TcFATA) and FATB (five TcFATBs) subfamily. The expression patterns of the genes were investigated via real-time PCR in various developmental phases. The transcription level of TcFATA and TcFATB1 was continuously decreased along with pod development. Furthermore, the expression level of TcFATA and TcFATB1 was positively associated with the ratio of oleic acid (C18:1) and palmitic acid (C16:0) respectively. The results indicate that TcFATA and TcFATB1 are significantly related with the regulation of fatty acid component.
Keywords: Theobroma cacao; fatty acyl-ACP thioesterase (FAT); phylogenetic; expression profile
DOI: 10.3969/j.issn.1000-2561.2020.05.014
在高等植物中,脂肪酸合成在细胞的质体脂肪酸合酶复合体(fatty acid synthetases, FAS)中进行,FAS由乙酰CoA-ACP转移酶、丙二酸单酰CoA转移酶、β-酮脂酰ACP合酶、β-酮脂酰ACP还原酶、β-羟脂酰ACP脱水酶、烯脂酰ACP还原酶和1个含巯基的酰基载体蛋白(acyl carrier protein, ACP)组合形成的复杂而松散结构[1]。FAS催化乙酰辅酶A与酰基-ACP缩合成4:0-ACP,进而连续催化酰基碳链以每个循环增加2个碳单位进行延伸[2]。对于多数植物而言,仅有少量的硬脂酸(C18:0)被转运出质体,因此硬脂酸很少在种子中富集[3];FAS虽偏爱合成18:0-ACP,部分16:0-ACP也会提前从FAS中释放出来,从而形成棕榈酸(16:0),一些植物会合成更短的癸酸(C10:0)或月桂酸(C12:0)[4-5]。18:0-ACP在十八烷酰-ACP去饱和酶(stearoyl-ACP desaturase, SAD)的催化下生成18:1-ACP,进而形成油酸(C18:1)。
酰基-ACP硫酯酶(fatty acyl-ACP thioesterase, FAT)水解酰基和ACP 之间的硫酯键,释放出游离脂肪酸和ACP,終止脂肪酸合成[6-7]。脂肪酸之后被运送到细胞质中进一步酯化形成脂酰-CoA,在甘油-3-磷酸酰基转移酶(glycerol-3-phosphate acyltransferase, GPAT)和溶血磷脂酸酰基转移酶(lysophosphatidic acid acyltransferase, LPAT)的催化下,分别转移到甘油-3-磷酸(G3P)sn-1和sn-2位置,依次生成溶血磷脂酸(LPA)和磷脂酸(PA),sn-3位置上的磷酸由磷脂酸磷酸酶(phosphatidic acid phosphatase, PAP)催化脱去,生成二酰甘油(diacylglycerol, DAG)。在二酰甘油酰基转移酶(diacylglycerol acyltransferase, DGAT)催化下,将酰基CoA的脂肪酸转移到DAG上sn-3位置,生成三酰甘油(TAG)[8-9]。
FAT是由核基因编码,在质体中发挥作用的靶向可溶性酶,根据氨基酸序列和底物偏好性分为FATA和FATB 2个亚族[10]。FATA偏好不饱和酰基-ACP,尤其对18:1-ACP活性最高;而FATB通常对饱和酰基-ACP显示出高活性,对18:1-ACP也有一定的活性[11-12]。FAT的功能在很大程度上决定植物体内脂肪酸链的长度和不饱和度,并影响脂肪酸组分及含量[13-14]。拟南芥AtFATB功能缺失后,不同组织中饱和脂肪酸的总量相比于野生型减少了40%~50%,并且棕榈酸比例明显降低[15]。将麻风树JcFATB1在拟南芥中过表达会导致饱和脂肪酸含量增加,尤其是棕榈酸比例大幅增加,而不饱和脂肪酸比例显著降低[16]。
可可(Theobroma cacao L.)与咖啡、茶并称为世界三大饮料作物,有悠久的栽培历史,被誉为“上帝的食物”。可可属于梧桐科(Stercu liaceae)可可属(Theobroma),多年生常绿小乔木,茎干上开花结果,果实为纺锤形,成熟呈橙黄色,内含30~50粒种子,种子富含油脂(可可脂)、蛋白、纤维、多酚等成分。可可脂作为最重要的成分,含量占可可豆的50%左右。可可脂具备独特的物理与化学性质,熔点在35~36.5 ℃之间,入口即化,并具有舒缓、保湿功效,是制作巧克力、化妆品、药品等的重要原料[17]。成熟可可种子中棕榈酸(16:0)、硬脂酸(18:0)、油酸(18:1)总量占到可可脂含量的90%以上[18];脂肪酸聚合形成1,3-二棕榈酸-2-油酸甘油酯(1,3-dipalmitoyl-2-oleoylglycerol, POP)、1,3-二硬脂酸-2-油酸甘油酯(1,3-distearoyl-2-oleo ylglycerol, SOS)、1(3)-棕榈酸-2-油酸-3(1)-硬脂酸甘油酯(1(3)-palmitoyl-3(1)stearoyl-2-oleoylgl ycerol, POS)[19]。三者在可可种子中配比稳定,合成的POP、POS、SOS比例趋于22∶46∶32,归因于三酰甘油结构与配比,可可脂具体独特的理化性质,熔点刚低于人体温度。
目前,FAT基因已在拟南芥[15]、麻风树[16]、蓖麻[20]、椰子[21]、花生[22]等植物中相继被克隆。目前为止,可可FAT基因家族的研究尚未见报道。本研究对可可FAT基因家族进行进化、表达模式系统分析,为研究可可FAT基因特征及可可种子脂肪酸比例调控提供理论依据。
1 材料与方法
1.1 材料
试验所用种质材料,保存于中国热带农业科学院香料饮料研究所试验基地,为前期鉴定筛选出的性状差异可可种质‘Z216和‘Z115;2份种质幼果颜色均为绿色,其中种质‘Z216鲜果重为653.6 g、果长为153.9 cm、种子厚为6.1 mm、种子可可脂含量为49.1%,种质‘Z115鲜果重为504.0 g、果长为165.7 cm、种子厚为8.6 mm、种子可可脂含量为42.5%。可可树开花成果时期,挂牌标记,在果实不同发育时期分别剥取种子,立即置于液氮中速冻后,保存于?80 ℃冰箱中备用。试验用RNA提取试剂盒(R6827-01)购自Omega公司,cDNA反转录试剂盒(K1621)购自Fermentas公司,实时荧光定量PCR试剂SYBR? Green Realtime PCR Master Mix(QPK- 201)购自TaKaRa公司,其他生化试剂均为进口或国产分析纯试剂。
1.2 可可FAT基因序列获取及分析
依托可可全基因组序列,利用BLAST工具搜索分析可可FAT基因家族成员序列。具体方法如下,利用油棕已知FAT基因序列在可可全基因组数据库(http://www.cacaogenomedb.org)进行BlastP分析,筛选鉴定可可FAT基因序列。利用GSDS软件分析可可FAT基因的外显子/内含子基因结构。在ExPASy网站(http://expasy.org/tools/)利用在线工具对可可FAT蛋白保守性、等电点、分子量、跨膜结构、亚细胞定位等进行分析预测。
1.3 可可FAT基因进化分析
基于可可FAT蛋白序列,在NCBI GenBank数据库搜索出木本棉(Gossypium arboretum,8条)、辣椒(Capsicum annuum,3条)、巴西橡胶(Hevea brasiliensis,10条)、海岛棉(Gossypium barba dense,14条)、蓖麻(Ricinus communis,5条)、凤梨(Ananas comosus,1条)、金钱橘(Citrus clementine,2条)、猕猴桃(Actinidia chinensis,5条)、中粒种咖啡(Coffea canephora,2条)、海枣(Phoenix dactylifera,2条)、苹果(Malus domestica,8条)、油桐树(Vernicia fordii,2条)、油棕(Elaeis guineensis,1条)、花生(Arachis hypogaea,10条)、陆地棉(Gossypium hirsutum,18条)、谷子(Setaria italic,1条)、芝麻(Sesamum indicum,4条)、芒果(Mangifera indica,1条)、番茄(Solanum lycopersicum,1条)、大豆(Glycine max,7条)、山竹(Garcinia mangostana,1条)、油菜(Brassica napus,2条)、长蒴黄麻(Corchorus olitorius,6条)、藜麦(Chenopodium quinoa,2条)、胡桃(Juglans regia,8条)、甜橙(Citrus sinensis,6条)、莴苣(Lactuca sativa,1条)、亚麻荠(Camelina sativa,1条)、欧洲橄榄(Olea europaea,4条)、拟南芥(Arabidopsis thaliana,1条)、烟草(Nicotiana tabacum,3条)、葡萄(Vitis vinifera,9条)、榴莲(Durio zibethinus,13条)FAT同源序列,结合可可(Theobroma cacao,6条)FAT序列,利用MEGA 5.0软件[23]对以上33种植物169条FAT序列进行分子系统学分析;采用Neighbour-joining聚类方法,进行1000次bootstrap统计学检测。
2.4 可可FAT基因表達量与脂肪酸组分关系
GC-MS生成的可可脂肪酸质谱数据,经数据库检索分析和面积归一化计算,结果表明棕榈酸(C16:0)、硬脂酸(C18:0)、油酸(C18:1)和亚油酸(C18:2)是可可脂的主要组分,4种主要脂肪酸占比达可可脂的98%以上。在‘Z115种质中,棕榈酸、硬脂酸、油酸和亚油酸比例分别是29.16%、30.30%、35.78%、3.01%;在‘Z216种质中,棕榈酸、硬脂酸、油酸和亚油酸比例分别是38.76%、29.56%、28.58%、2.14%。‘Z115种质的油酸比例比‘Z216种质高7.20%,而棕榈酸比例低9.6%,与TcFATA和TcFATB1表达趋势一致(图4)。
3 讨论
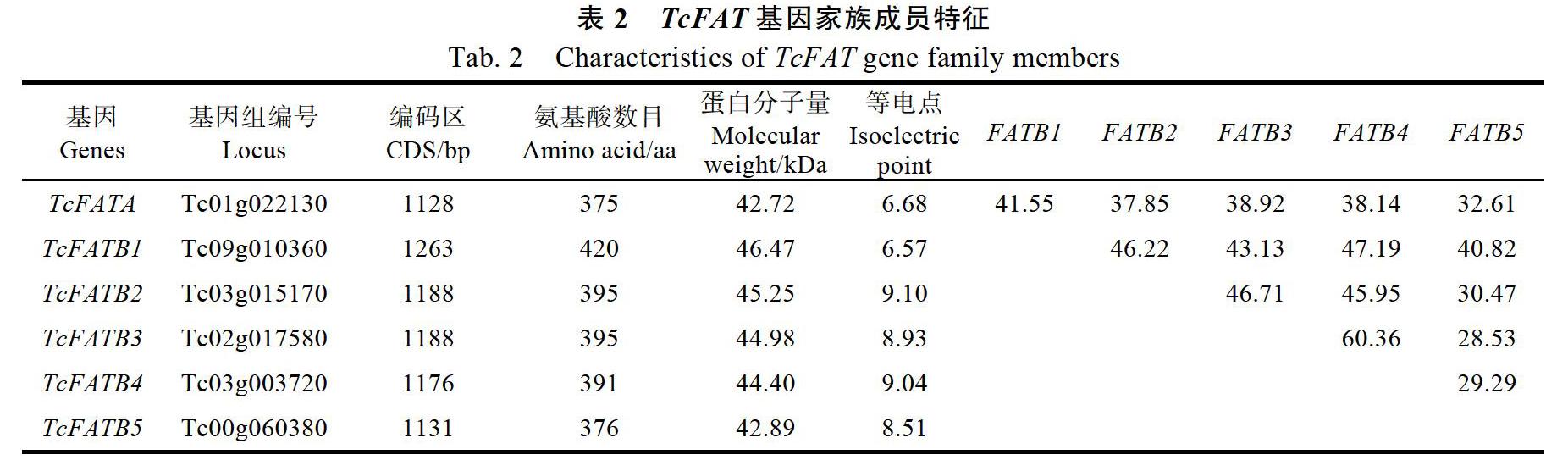
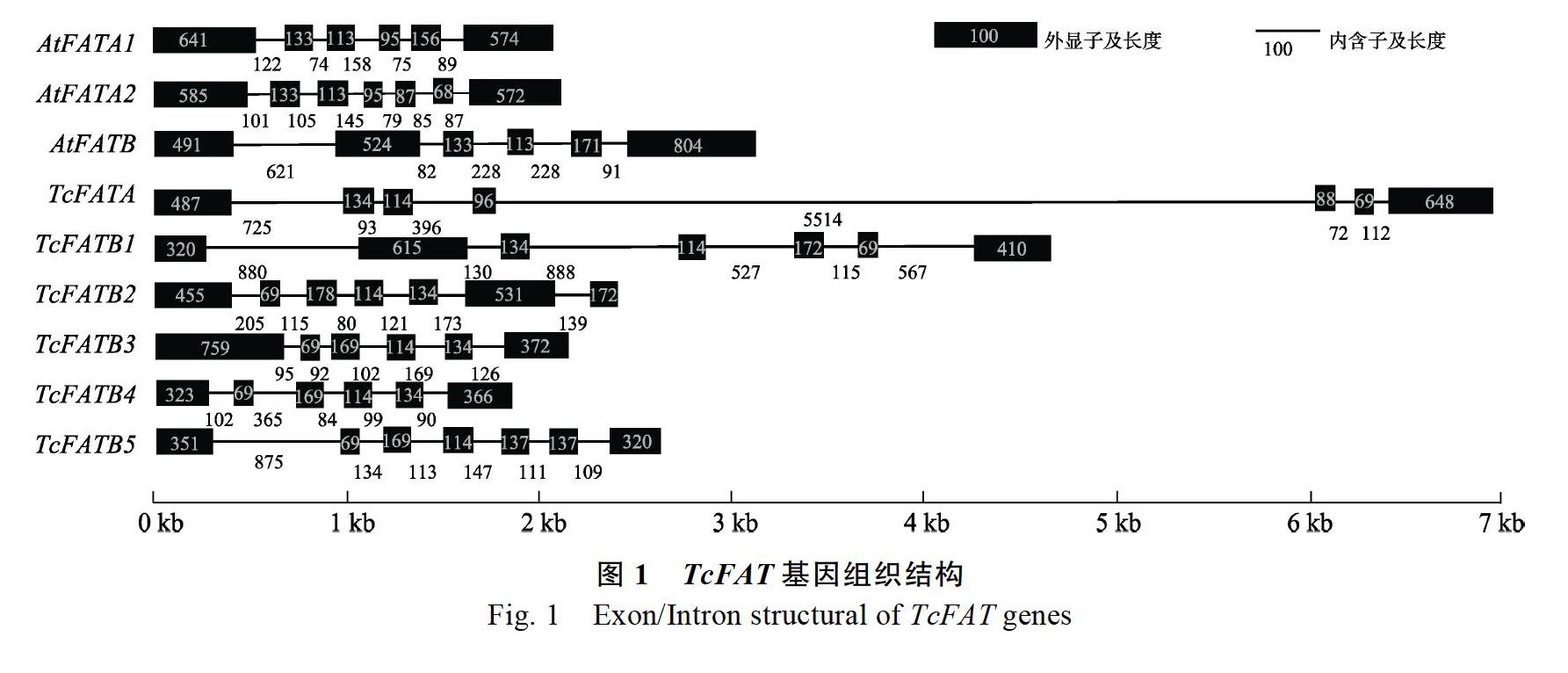
可可是世界重要的饮料作物,也是一种油料作物,可可脂中主要的组分是棕榈酸、硬脂酸和油酸,因其脂肪酸组分的特定比例,表现出特定的理化性质,熔点35~37 ℃,是制作巧克力等主要原料。克里奥洛(Criollo)和弗拉斯特洛(Forastero)可可全基因组已经测序完成,为可可基因家族的克隆提供了便利,本研究从可可基因组中获得6条FAT序列;然而,已测得的可可基因组序列分别占到克里奥洛和弗拉斯特洛可可的76%和92%,在未测得的可可基因组序列中仍可能存在未知的可可FAT基因。获得的6条FAT基因序列,TcFATB3和TcFATB4含有5个内含子,其余4条序列均含有6条内含子,基因的内含子数目保守;6个预测蛋白平均含有392个氨基酸,平均分子量为44.5 kDa,与植物中其他FAT成员类似[21, 25]。
系统进化树分析结果表明FAT拥有2个类群,FATA类群成员明显少于FATB类群成员,在33个物种中FATA成员比例仅占整个FAT家族的19.53%;FATB类群包含3个亚类群,其分支构成较为复杂,可能是由于FATB有多种进化途径从而导致其类群成员较多[26]。可可FATA和FATBs成员分别归属FATA和FATB两大类群;拟南芥中含有3个FAT基因,其中2个AtFATAs基因、1个AtFATB基因,而6个可可TcFAT基因中有5个是FATB类基因[27-28],表明可可TcFATB基因发生复制多元化,与脂肪酸组分及比例特异性可能存在一定关系。
在本研究中,TcFATA和TcFATB1伴随种子发育成熟,表达量呈下降趋势,表明在种子发育前中期基因大量表达,决定着可可脂肪酸组分。然而,不同可可种质的脂肪酸组分比例存在一定差异,‘Z216可可种质棕榈酸(C16:0)比例较高,‘Z115可可种质油酸(C18:1)比例较高,这与TcFATA和TcFATB1表达模式相同。FAT是调控脂肪组分的关键酶,FATA对油酯-ACP(C18:1-ACP)有较高活性,决定C18:1转运到质体外的水平,对C18:0-ACP和C16:0-ACP活性较低;而FATB对饱和油酯-ACP活性较高,偏向于水解C18:0的脂酰-ACP[29-30]。在种子发育的不同时期,TcFATA在‘Z115种质中的表达量均高于‘Z216,而TcFATB1在‘Z216种质中的表达量均高于‘Z115,从一定程度印证了FATA和FATB对底物的偏向性。
本研究对可可FAT基因家族成员的基因组织结构、进化关系、基因表达模式及与脂肪酸组分间关系进行了系统的分析,结果可为分析FAT基因家族成员理化功能提供研究基础,为解析可可脂特定理化性质提供参考。
参考文献
Chen G, He Q, Xuan N, et al. Functional expression analysis of an acyl-ACP thioesterase FatB1 from Arachis hypogaea L. seeds in Escherichia coli[J]. Journal of Food Agriculture and Environment, 2012, 10: 332-336.
Chen G, Peng Z, Shan L, et al. Cloning of acyl-ACP thioesterase FatA from Arachis hypogaea L. and its expression in Escherichia coli[J]. Journal of Biomedicine and Biotechnology, 2012: 652579.
Pidkowitch M S, Nguyen H T, Heilmann I, et al. Modulating seed β-ketoacyl-acyl carrier protein synthase II level converts the composition of a temperate seed oil to that of a palm-like tropical oil[J]. Proceedings of the National Academy of Sciences, 2007, 104(11): 4742-4747.
Hellyer A, Leadlay P F, Slabas A R. Induction, purification and characterization of acyl-ACP thioesterase from developing seeds of oil seed rape (Brassica napus)[J]. Plant Molecular Biology, 1992, 20(5): 763-780.
Knutzon D S, Bleibaum J L, Nelsen J, et al. Isolation and characterization if two safflower oleoyl-acyl-carrier protein thioesterase cDNA clones[J]. Plant Physiology, 1992, 100(4): 1751-1758.
Qigen W, Yong L, Jiaquan H, et al. Molecular cloning and characterization of an acyl-ACP thioesterase gene (AhFatB1) from allotetraploid peanut (Arachis hypogaea L.)[J]. African Journal of Biotechnology, 2012, 11(77): 14123-14131.
Yuan Y, Chen Y, Yan S, et al. Molecular cloning and characterization of an acyl carrier protein thioesterase gene (CocoFatB1) expressed in the endosperm of coconut (Cocos nucifera) and its heterologous expression in Nicotiana tabacum to engineer the accumulation of different fatty acids[J]. Functional Plant Biology, 2014, 41(1): 80-86.
Graham I A. Seed storage oil mobilization[J]. Annual Review of Plant Biology, 2008, 59: 115-142.
周 丹, 趙江哲, 柏 杨, 等. 植物油脂合成代谢及调控的研究进展[J]. 南京农业大学学报, 2012, 35(5): 77-86.
元冬娟, 吴 湃, 江黎明. 高等植物的酰基-ACP硫酯酶研究进展[J]. 中国油料作物学报, 2009, 31(1): 103-109.
Dong S, Huang J, Li Y, et al. Cloning, characterization, and expression analysis of acyl-acyl carrier protein (ACP)-thioe sterase B from seeds of Chinese Spicehush (Lindera communis)[J]. Gene, 2014, 542(1): 16-22.
Zhou Z, Zhang D, Lu M. Cloning and expression analysis of PtFATB gene encoding the acyl-acyl carrier protein thioesterase in Populus tomentosa Carr[J]. Journal of Genetics and Genomics, 2007, 34(3): 267-274.
Tan K W M, Lee Y K. Expression of the heterologous Dunaliella tertiolecta fatty acyl-ACP thioesterase leads to increased lipid production in Chlamydomonas reinhardtii[J]. Journal of Biotechnology, 2017, 247: 60-67.
Dong S, Liu Y, Xiong B, et al. Transcriptomic analysis of a potential bioenergy tree, Pistacia chinensis Bunge, and identification of candidate genes involved in the biosynthesis of oil[J]. Bioenergy Research, 2016, 9(3): 740-749.
Bonaventure G, Bao X, Ohlrogge J, et al. Metabolic responses to the reduction in palmitate caused by disruption of the FATB gene in Arabidopsis[J]. Plant Physiology, 2004, 135(3): 1269-1279.
Wu P Z, Li J, Wei Q, et al. Cloning and functional characterization of an acyl-acyl carrier protein thioesterase (JcFATB1) from Jatropha curcas[J]. Tree Physiology, 2009, 29(10): 1299-1305.
Lehrian D W, Keeney P G. Changes in lipid components of seed during growth and ripening of cacao fruit[J]. Journal of the American Oil Chemists Society, 1980, 57(2): 61-65.
Padilla F C, Liendo R, Quintana A. Characterization of cocoa butter extracted from hybrid cultivars of Theobroma cacao L.[J]. Archivos Latinoamericanos de Nutricion, 2000, 50(2): 200-205.
Shekarchizadeh H, Tikani R, Kadivar M. Optimization of cocoa butter analog synthesis variables using neural networks and genetic algorithm[J]. Journal of Food Science and Technology, 2014, 51(9): 2099-2105.
Sánchez-García A, Moreno-Pérez A J, Muro-Pastor A M, et al. Acyl-ACP thioesterases from castor (Ricinus communis L.): an enzymatic system appropriate for high rates of oil synthesis and accumulation[J]. Phytochemistry, 2010, 71(8-9): 860-869.
肖 勇, 楊耀东, 夏 薇, 等. 椰子Acyl-ACP硫酯酶相关基因的克隆及其表达分析[J]. 广东农业科学, 2013, 40(12): 149-152.
张 会, 单 雷, 李新国, 等. 花生FAT基因家族的全基因组分析[J]. 山东农业科学, 2018, 50(6): 19-26.
Kumar S, Nei M, Dudley J, et al. MEGA: a biologist-centric software for evolutionary analysis of DNA and proteinsequences[J]. Briefings in Bioinformatics, 2008, 9(4): 299-306.
Pinheiro T T, Litholdo Jr C G, Sereno M L, et al. Establishing references for gene expression analyses by RT-qPCR in Theobroma cacao tissues[J]. Genetics and Molecular Research, 2011, 10(4): 3291-3305.
李昊远, 郝翠翠, 潘丽娟, 等. 花生酰基载体蛋白硫酯酶(FATB2)基因的克隆与表达分析[J]. 花生学报2017, 46(4): 7-14.
Huynh T T, Pirtle R M, Chapman K D. Expression of a Gossypium hirsutum cDNA encoding a FatB palmitoyl-acyl carrier protein thioesterase in Escherichia coli[J]. Plant Physiology and Biochemistry, 2002, 40(1): 1-9.
程 涛, 杨建明, 刘 辉, 等. 拟南芥硫酯酶基因(AtFATA)在大肠杆菌中的表达及其对游离脂肪酸合成的影响[J]. 应用与环境生物学报, 2011, 17(4): 568-571.
郝昭程, 王腾飞, 李忠奎, 等. 拟南芥硫酯酶基因在毕赤酵母中的表达[J]. 生物工程学报, 2015, 31(1): 115-122.
Rodríguez-Rodríguez M F, Salas J J, Garcés R, et al. Acyl-ACP thioesterases from Camelina sativa: cloning, enzymatic characterization and implication in seed oil fatty acid composition[J]. Phytochemistry, 2014, 107: 7-15.
Jing F. Characterization of acyl-ACP thioesterases for the purpose of diversifying fatty acid synthesis pathway[D]. Ames: Iowa State University, 2013.
