Mounting evidence of FKBP12 implication in neurodegeneration
2020-06-19GabriellaCaminatiPieroProcacci
Gabriella Caminati , Piero Procacci
1 Department of Chemistry “Ugo Schiff”, University of Florence, Sesto Fiorentino, Italy
2 Center for Colloid and Surface Science (CSGI), University of Florence, Sesto Fiorentino, Italy
Abstract Intrinsically disordered proteins, such as tau or α-synuclein, have long been associated with a dysfunctional role in neurodegenerative diseases. In Alzheimer’s and Parkinson’s’ diseases, these proteins, sharing a common chemical-physical pattern with alternating hydrophobic and hydrophilic domains rich in prolines, abnormally aggregate in tangles in the brain leading to progressive loss of neurons. In this review, we present an overview linking the studies on the implication of the peptidyl-prolyl isomerase domain of immunophilins, and notably FKBP12, to a variety of neurodegenerative diseases, focusing on the molecular origin of such a role. The involvement of FKBP12 dysregulation in the aberrant aggregation of disordered proteins pinpoints this protein as a possible therapeutic target and, at the same time, as a predictive biomarker for early diagnosis in neurodegeneration, calling for the development of reliable, fast and cost-effective detection methods in body fluids for community-based screening campaigns.
Key Words: Alzheimer’s disease; biomarker; detections; FKBP12; FK506 binding protein; neurodegeneration; Parkinson’s disease; tau protein; α-synuclein
Introduction
FKBP12 (encoding gene FKBP1A), a 12 KDa globular cytosolic protein, is the archetypal member of the family of the immunophilins, highly conserved proteins in Eukaryotes, characterized by the presence of the peptidyl-prolyl cis-trans isomerase domain (PPI). The members of the large PPIase family differ in molecular weight and in their biochemical role (Kolos et al., 2018). FKBP12, shown in Figure 1, coincides with a single PPI that binds the Xaa-Pro substrate with two H-bond donors from the highly conserved residues Tyr82 and Ile56 (Blackburn, 2011; Мartina, 2013) in an otherwise hydrophobic binding pocket (Ikura and Ito, 2007; Мartina et al., 2013). The specific function of FKBP12 and other PPI containing members of the FKBP family must certainly be related to their peptidyl-proline cis to trans isomerase activity, due to the cis isomer-specificity of substrate recognition in the catalytic binding pocket. FKBP12 assists as a chaperone the folding/isomerization of proteins containing proline residues, with the potential of being involved in a large number of metabolic pathways and physiological processes, (Schiene et al., 1998; Dunyak and Gestwicki, 2016) including folding of nascent polypeptide chains, transport of proteins (Ghartey-Kwansah et al., 2018), viral replication (Li et al., 2018), calcium homeostasis (Tong and Jiang, 2015) and regulation of the cell cycle. (Laplante and Sabatini, 2012). As such, FKBP12 is ubiquitous in the body of mammalians, and is especially highly enriched in the central and peripheral nervous system (Figure 1).
Several reviews were recently proposed exploring the diverse functions of this ubiquitous protein: such studies either focus on the important issue of FKBP ligands (Kolos et al., 2018) with special emphasis on immunosuppression or explore the cellular functions and the physiological role of FKBPs proteins highlighting (Bonner and Boulianne, 2017) the new profile of FKBPs as a molecular tool in protein-protein interaction studies.
The FKBP12 protein was first discovered in 1989 (Harding, 1989) as the target protein of the immunosuppressant macrolide FK506 ligand, also known as tacrolimus. FKBP12-FK506 forms a ternary complex with calcineurin, a cellular target for signal transduction in T-cells triggering, whose inactivation blocks the adaptive response of the immune system.
Inhibition of FKBP12 with rapamycin, via the formation of a ternary complex, was also at the core of the allosteric inactivation mammalian target of rapamycin as a third partner, a kinase involved in a plethora of both cellular and physiological processes in mammals, notably regulating cellular metabolism, growth, and proliferation. As a consequence, FKBPs were long believed to be major players in the immune response mechanism and as physiologic regulators of the cell cycle, given their well-established implications in post-transplant therapies and antitumor treatments.
Nonetheless, the link between FKBP12 and immune response and cell proliferation is serendipitous relying on the two mediating natural compounds FK506 and rapamycin with quite distinctive and unusual structural traits. In early studies, FK506 and rapamycin, as well as non-immunosuppressant ligands (NIL), were found to have powerful neuroprotective and neuroregenerative effects that do not rely on immunosuppression or cell cycle regulation, i.e., on the capability of binding calcineurin or mammalian target of rapamycin (Gold, 2000). The therapeutic potential of NIL for the treatment of neuronal disorders spurred an intense research activity by industry and academia on the role played by FKBP12 in neurodegenerative diseases (ND), leading to the filing of several patents at the turn of the last century (Liu et al., 2013, Kolos et al., 2018).
In this context, again by serendipity, it was discovered only in relatively recent times that an FKBP-type Escherichia coli PPIase impurity found in a recombinant α-synuclein (α-syn) preparation (α-syn is the precursor of Lewy body (LB) fibrils in a rat model of Parkinson’s disease) remarkably accelerate its aggregation kinetics (Gerard et al., 2006), pinpointing FKBP12 as a possible biomarker in Parkinson’s disease and other LB dementia (Figure 2).
While immunosuppression was the main focus of early FKBP studies, its weight exhibits a constant decline in the last two decades with a corresponding growing interest in the cancer-related and the neurodegeneration-related axes. In the right panel, the focus is on the role of FKBP12 in neurodegeneration and on the development and assessment of specific FKBP12 sensing methodologies. Quite surprisingly, despite the growing evidence of the implication of FKBP12 and other PPI-containing FBPBs in diseases with high social impact such ND, the development of specific and efficient detection methodologies for FKBP12 lagged and still lags behinds. As of today, the only specific platform for FKBP12 detection is provided by multistep, time-consuming and costly immunoenzymatic assays (Additional Table 1). Remarkably, in the very last years the chasm between neurodegeneration and detection related studies is widening considerably, with the former increasing steadily and the latter being in essence stationary.
In this review, we present an exhaustive overview linking studies on the implication of FKBPs in a variety of ND, including Alzheimer’s and Parkinson’s diseases, LB dementia and multiple system atrophy. Мore specifically, we shall first focus on the current knowledge of the FKBP12 role in neurological disorders with an emphasis on its implication in malignant α-syn aggregation and as a possible specific biomarker for early Parkinson’s disease diagnosis. Eventually, we will discuss some of the latest emerging methods for FKBP12 detection and propose an innovative methodology for FKBP12 quantification that may provide a gateway toward simple point-of-care analysis for a fast and so far inaccessible ND screening method. We also caution against solely relying on a single ND biomarker detection and urge the field to undertake longitudinal studies on prodromal and diagnosed individuals including FKBP12 in the panel of predictors of the neurodegeneration to establish a protocol for early diagnosis and robust discrimination among the different forms of pathologies.
For this review, literature searches were performed on public databases (Scopus), the terms used in the search are reported in the caption of Figure 2; articles that were published until December 2019 were included.
A Common Thread for FKBP12 Involvement in Neurodegeneration
Alzheimer’s and Parkinson’s diseases are neurological disorders leading to progressive, irreversible dementia and physical impairment and disabilities of a significant share of the aged population worldwide (Garre-Olmo, 2018). Yet, despite their devastating social impact, the triggering biomolecular mechanisms of these pandemic neuropathies are still elusive. Alzheimer’s disease is characterized by the accumulation of intracellular neurofibrillary tangles, mostly comprised the microtubulin binding protein tau and by extracellular amyloid-beta (Aβ) plaques. Tau and Aβ are intrinsically disordered peptides (IDP) made up of 441 and 40 amino acids in the human isoform, respectively. Remarkably, also in Parkinson’s disease, neurological impairment is caused by the accumulation of a 140 residues IDP, α-syn, in fibrillar tangles in neuronal cells called LB. The involvement of IDPs in malignant aggregation in both Alzheimer’s and Parkinson’s diseases leads naturally to hypothesize a possible common biomolecular trigger of these neurological disorders.
As discussed in the introduction, several PPIases, and notably FKBP12, have been identified more than 20 years ago as having a role in the insurgence of both Alzheimer’s and Parkinson’s diseases. Their involvement was based on the observation that the inhibitors of FKBP12, with or without immunosuppressant capabilities, had strong neuroprotective effects in vivo, in a rat model of Alzheimer’s and Parkinson’s diseases (Chattopadhaya et al., 2011). The mechanism of neuroprotection is still unclear, although the activity of NIL FKBP12 inhibitors for nerve regeneration and survival leads to exclude the implication of the immunosuppression axis and inactivation of calcineurin. Still, in recent studies FKBP12 is considered as a prominent actor in upregulating calcineurin activity associated with α-syn toxicity, even in the absence of its natural ligand FK506 (Caraveo et al., 2017), possibly because of the presence of Pro residues in cis configuration in the catalytic domain of calcineurin (Teixeiret al., 2019).
Given such evidence, it is tempting to identify the common biochemical trigger of aberrant aggregation with the interaction between the FKBP12 and the proline-rich region of IDPs involved in ND. In the following we will enucleate evidence of this correlation gathered from recent literature.
Recently, mounting evidence is gathering on the so-called tau hypothesis for Alzheimer’s disease (Kametani and Hasegawa, 2018; Мamun et al., 2020), leading to reconsideration of the Aβ role in the pathogenesis of the disease, given the failure in clinical trials of drugs targeting Aβ (Doody et al., 2013) and the recent observation of no impairment of cognitive functions in Aβ overexpressing transgenic mice despite the presence of senile amyloid plaques in brain tissues (Kim et al., 2013).
As the central domain of the IDP tau, next to the tubulin-binding region, is rich in prolines (Figure 3), it has been inferred that upregulation or unbalance of FKBP12 could affect the average tau structure preventing binding and/or phosphorylation ability, thus leading to oligomerization and eventually aberrant accumulation in neurofibrillar tangles causing Alzheimer’s disease (Blair et al., 2015). On the other hand, proline residues are also abundant in the mostly hydrophilic N and C terminal regions (Figure 3).
Also in Parkinson’s disease, the mechanism involves the dysfunctional aggregation of an IDP, α-syn (Lucas and Fernández, 2020). In the context of the FKBP12 interaction with proline containing IDPs involved in neurodegeneration, in a key study by Gerard et al. (2006), it was noticed that an impurity found in a recombinant α-syn preparation remarkably increased its aggregation kinetics. This impurity was unambiguously identified as an FKBP-type Escherichia coli PPIase. In the following years, the same authors wrote a series of papers aiming at unraveling the role of peptidyl-prolyl isomerases in neurodegeneration focusing on the implication of FKBP12 in α-syn aggregation and Parkinson’s disease (Gerard et al., 2011). In their in vitro study on the FKBP12 driven aggregation kinetics of α-syn in solution (Gerard et al., 2011), they observed a clear dose-dependent effect of FKBP12 addition on the acceleration of the α-syn aggregation kinetics. As even picomolar FKBP12 seemingly exhibited capabilities of accelerating fibril formation in 140 mМ α-syn samples, they suggested a PPI catalytic mechanism. Using site-directed mutagenesis, they showed that when α-syn has all five prolines in the C-terminus (Figure 3) mutated to Ala, FKBP12 is unable to accelerate the aggregation kinetics. This led to conclude that FKBP12-induced α-syn aggregation involves the interaction with the proline-rich α-syn C-terminal region (Мeuvis et al., 2010). Мore specifically, FKBP12 was believed to induce a malignant conformational modification of α-syn monomers by the FKBP12 catalyzed cis/trans isomerization of the terminal prolines. In this context, the well-known A30P familial mutation in the N alpha-helical terminus, whose pathogenic mechanism in Parkinson’s disease is unclear, could be associated with FKBP12 cytosolic dysregulation. In all studies by Gerard and coworkers, in vitro aggregation of α-syn was promoted with a FKBP12 to α-syn ratio not exceeding 0.01.
The catalytic mechanism and structural modification of the IDP α-syn monomers is however at variance with the level of FKBP12 especially in brain tissue (Lyons et al., 1995; Galfré et al., 2016). PPIases, including FKBP12, are probably more abundant than α-syn itself (Iljina et al., 2016), prompting to hypothesize a mechanism that is more in accord with the observed dose-dependent effect of FKBP12 addition on α-syn aggregation kinetics. Such mechanism was apparently unraveled in two recent publications, on the interaction of α-syn with PPIase, specifically with FKBP12 (Caminati et al., 2019) and with cyclophilin CypA (Favretto et al., 2020), two immunophilins with distinct functions but both assisting proline cis/trans isomerization. In the study of Favretto et al. (2020), NМR titration of α-syn with 15N labeled CypA showed that both the C-terminal proline-rich region and the central Per-NAC domain bind to the catalytic binding pocket of CypA, probably via a two-step mechanism involving first the CypA docking to cis-Proline 129 followed by the wrapping of the hydrophobic binding pocket of CypA by way the Pre-NAC domain of α-syn. Such hypothesis is consistent with the common quasi-steady-state assumption in enzymatic catalysis and with the known kcatvalues for cis/trans PPI catalyzed isomerization (Schiene et al., 1998).
A very similar binding mechanism was hypothesized by other authors (Caminati et al., 2019), where α-syn aggregation was followed in vitro in samples with physiological levels (micromolar) of both α-syn and FKBP12.
Мore in detail, at striking variance with the “normal” aggregation of pure α-syn in linear fibrils (Sweers et al., 2012), the authors observed a morphological modification in samples containing equimolar FKBP12 and α-syn, leading to the formation, after months of incubation, of impressive quasi-fractal highly branched dendritic aggregates, revealed by fluorescence and phase-contrast microscopy as shown in
Figure 4.
Remarkably, inhibition of a fraction of FKBP12 with the synthetic ElteN378 ligand (Мartina et al., 2013) suppresses the formation of these dendritic structures, leading to the formation of small size, poorly branched molecular assemblies, an effect that is fully consistent with the observed dose-dependent enhancement of FKBP12-induced α-syn aggregation (Gerard et al., 2006). The experimental observations were replicated using a computational coarse-grained (CG) model for α-syn aggregation, with a 15 beads CG representation of the monomeric 6α-syn chain, made up of three distinct parts of five beads length (Esteban-Мartin et al., 2013) representing the central hydrophobic NAC domain and the two hydrophilic C and N termini. FKBP12 binding was simulated by simply replacing one of the terminal hydrophilic beads with a hydrophobic one, in the hypothesis that the α-syn proline bound FKBP12 exposes an unsaturated beta-strand acting as a seed for the binding of the beta-sheet forming NAC hydrophobic domain (Figure 5). CG simulations showed that the morphology and kinetics of aggregation of α-syn are elicited by the intermolecular hydrophobic interactions of the central non-polar NAC residues of the monomeric unit and that FKBP12 binding can provide a hydrophobic seed in an otherwise hydrophilic region for a branched aberrant aggregation (Figure 4).
Such a mechanism of aberrant branched aggregation, driven by a subtle balance of elementary hydrophobic and hydrophilic forces, could also operate in the intracellular aggregation of the proline-rich protein tau in Alzheimer’s disease. As shown in Figure 3, both tau and α-syn IDPs are characterized by a hydrophobic central region and a proline-rich acidic terminal. As a matter of fact, the observation that up to half of Alzheimer’s disease cases also contain LB (Lee et al., 2004; Geddes, 2005; Honjo et al., 2018) and that α-syn and tau are both IDPs suggested there might be a commonality in the processes that form LB and the tau neurofibrillary tangles, ultimately trackable to a dysregulated PPIase binding. If this is the case, FKBP12 should be found as a minor component (residing mostly at the branch junctions) of either tau or α-syn tangles. FKBP12 or other PPIases were never reported in the LB until Honjo et al. (2018) found that FKBP12 colocalized with α-syn in LBs neurites in Parkinson’s disease and in LB dementia brains. Furthermore, FKBP12-immunopositive neurofibrillary tangles colocalized with phosphorylated tau in DLB and FKBP12-immunopositive glial cytoplasmic inclusions (Honjo et al., 2018).
While the FKBP12 and PPIase involvement in neurodegeneration is becoming increasingly clear, the precise nature of the mechanism ultimately leading to Alzheimer’s disease tauopathies and/or LB dementia still remains elusive. FKBP12 dysregulation or excess, leading to aberrant aggregation of proline containing IDPs, can be related to many contributory causes, in turn, dependent on dysregulation in any of the many molecular or cellular pathways involving the FKBP12 protein (Dunyak et al., 2016; Ghartey-Kwansah et al., 2018). Let aside the complex and manifold origin of LB or neurofibrillary tangle formation (Schreurs et al., 2014; Despres et al., 2017), nonetheless FKBP12 has unquestionably and progressively emerged as a promising biomarker for both Alzheimer’s and Parkinson’s diseases. Furthermore, FKBP12 could be a possible target for ND therapy using FKBP12 inhibitors (Caminati et al., 2019; Kaeberlein and Galvan, 2019). In fact, it has been recently argued that, due their compelling preclinical record, the time has come for clinical trials of FKBP12 inhibitors (Kaeberlein and Galvan, 2019). In this context, the need for a reliable, fast, specific and inexpensive analytical method for measuring FKBP12 levels in body fluids appears particularly urgent.
What Hampers the Use of FKBP12 as a Biomarker in Neurodegenerative Diseases
These recent developments purvey new conceptions for the role of FKBP12 in neurodegenerations connecting apparently distant pieces of evidence in a network that reveal a precise involvement of the proline binding PPI domain.
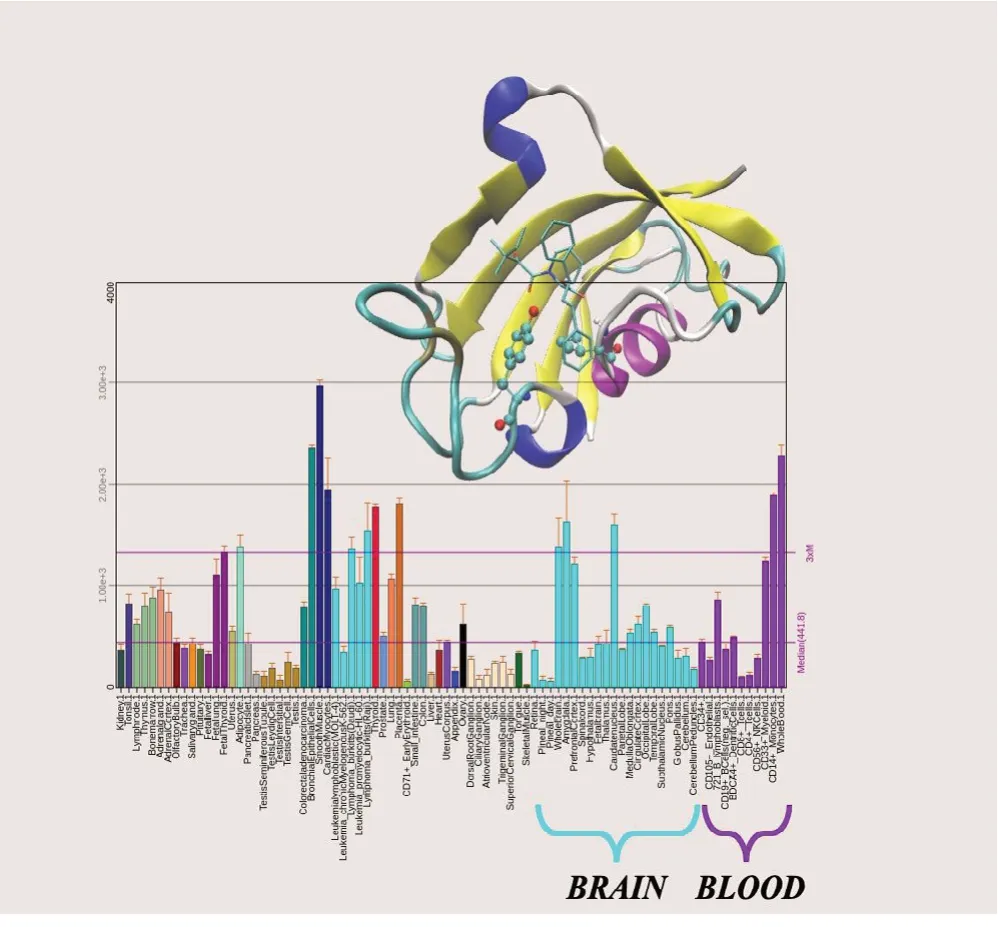
Figure 1 FKBP12 structure and expression level.

Figure 2 Summary of the research activity on FKBP12 since its discovery as obtained from targeted Scopus interrogations.
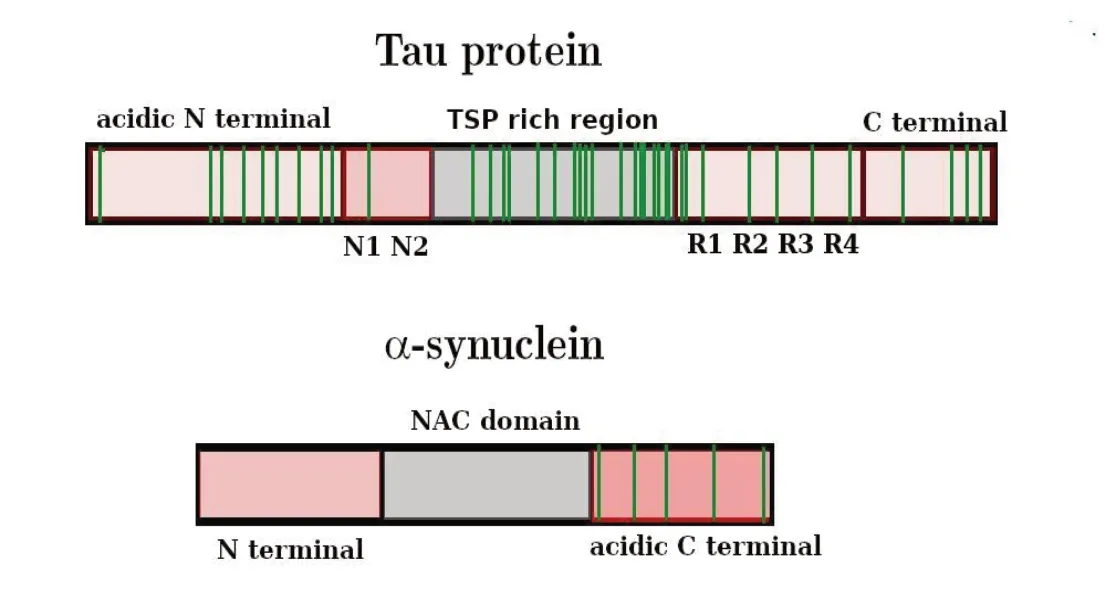
Figure 3 Schematic primary sequence of tau protein and α-synuclein (α-syn).

Figure 4 Fluorescence images for the system FKBP12/α-syn after 23 days at 37 °C at constant α-syn concentration and increasing FKBP12 content.
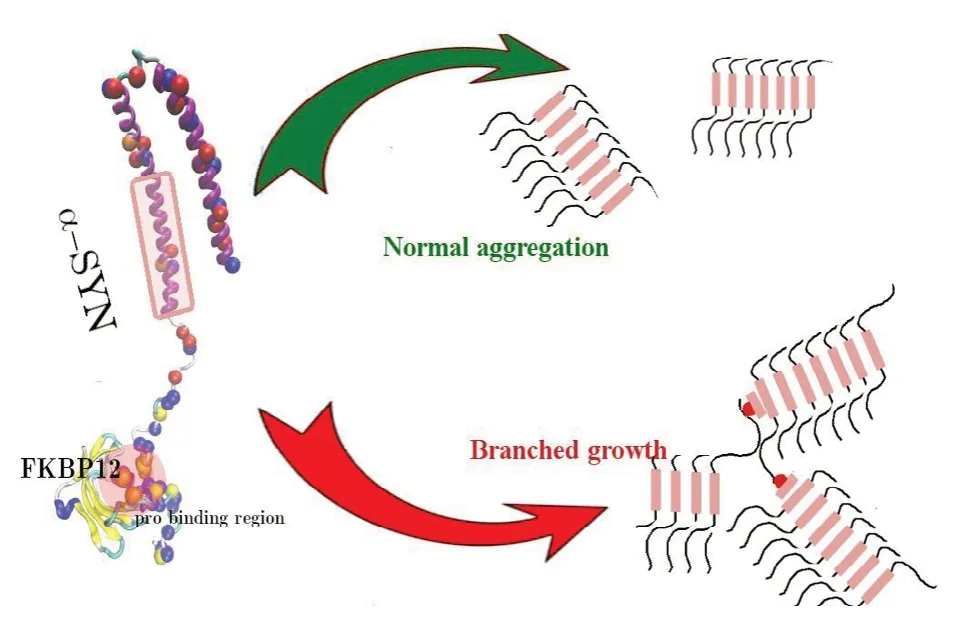
Figure 5 Mechanism for FKBP12-driven aggregation of α-syn.
For a decade, an unbalance of endogenous FKBP12 concentration in the early development of Parkinson’s disease has been reported by several authors (Nilsson et al., 2007; Sugata et al., 2009). It is, therefore, unclear why FKBP12 is not yet explored as a potential biomarker for synucleopathies or Alzheimer’s disease either alone or in combination with other biomarkers. In fact, it is well recognized that standardized longitudinal studies measuring levels of a panel of multiple biomarkers will enable earlier diagnosis in Parkinson’s or Alzheimer’s diseases (Henriksena et al., 2013; Parnetti et al., 2019a). Although longitudinal α-syn was found to decrease significantly in Parkinson’s disease (Мollenhauer et al., 2019) as the levels of oligomeric α-syn and Aβ peptide increase (Parnetti et al., 2019b), recent data do not support the clinical utility of total α-syn as a single diagnostic/prognostic biomarker in Parkinson’s disease (Førland et al., 2018).
On the contrary, independent studies reported that the cerebrospinal fluid oligomeric α-syn/total α-syn ratio was able to discriminate Parkinson’s disease versus controls and that the combination of cerebrospinal fluid α-syn species with the Alzheimer’s disease core biomarkers further increased the diagnostic accuracy in discriminating Parkinson’s disease versus other major disorders like LB dementia, multiple system atrophy and progressive supranuclear palsy (Bousiges and Blanc, 2019).
The combined determination of α-syn species and FKBP12 is then expected to dramatically improve the diagnostic performance for Parkinson’s neuropathies and even help to discriminate among them.
A first attempt to use a member of the FKBP family as a biomarker for neurodegenerations has been presented in a patent by Goldknopf et al. (2006) but since then the widespread screening of prodromal or ND affected population has been focused on longitudinal or translational studies that selected to investigate primarily Tau and α-syn protein or oligomeric forms of α-syn and Aβ1-42(Мollenhauer et al., 2019; Parnetti et al., 2019a).
A possible answer to the under-represented use of FKBP12 as a diagnostic biomarker for Parkinson’s and Alzheimer’s diseases is the scanty number of detection methods that actually provide a quantitative and robust determination of FKBP12 in biological fluids. Although FKBP proteins are recognized to be principal actors in many different patholog besides neurodegenerations, their determination is often limited to the study of protein expression in healthy and affected tissues (Additional Table 2). This apparently surprising feature stems from the large fragmentation of scientific literature in specific sectors of different pathologies.
This section of the review aims at filling this gap illustrating the variety of detection methods used in the different fields. Additional Table 2 describes the most recent advancements in the determination of members of the FKBP family. We have included other FKBPs proteins besides FKBP12 in the case of methods that are either generally applied to protein biochemistry, such as western blot methods, or of methods that rely on the presence of specific antibodies as in the case of immunostaining or ELISA assays.
As shown in Additional Table 2, protein expression and localization has been studied traditionally by immunofluorescence, western blot or ELISA assays. For example, Song and Tan (2019) examined the expression of FK506 binding protein 52 in ovarian cancer tissues through a combination of eosin staining and immunohistochemistry flanked by western blot. Similar methods were adopted also by other authors for tumor cells and osteoclasts (Battaglino et al., 2019; Xing et al., 2019).
While these seemingly quantitative methods often give consistent outcomes, they nonetheless suffer from limited spatial resolution and low sensitivity: readouts are typically made by antibodies and validation by using clear controls is essential. In immunofluorescence and western blotting, background labeling can limit signal to noise ratio whereas the use of fluorescent proteins can affect target protein localization.
New methods emerged recently that take advantage of protein-protein interactions for FKBP detection. Protein-fragment complementation assay (PCA) relies on the ability of the reporter proteins to recover their signals or enzymatic activities when two fragments are brought into proximity by the protein-protein interactions. PCA is applied to mammalian cells, plant cells, invertebrate cells, yeast, and bacteria but not quite many of the proteins are suitable for being the PCA reporter making PCA a still immature technology not suitable for validated protocols of analysis.
Abnormal expression of FKBP12 was mainly studied through post-mortem analysis of the brain tissue sections using mass spectrometry profiling (Avramut and Achim, 2002; Sugata et al., 2009; Honjo et al., 2018). Мuch more scarce is the number of studies that hunt FKBP12 in biological fluids. In this case, the proposed detection methods are often laborious and costly and not suitable for extensive screening of samples thus hindering a series of crucial steps: early diagnosis, assessing the disease progression, development of new treatments and monitoring the treatment effects.
On the other hand, identification of FKBP12 is crucial for proper diagnosis of neurodegeneration only if FKBP12 subtle differences in concentration can be quantified (Goldknopf, 2006) in specific biological fluids.
As reported above, FKBP12 is ubiquitous in the human body but in the case of neurodegenerative diseases FKBP12 is expected to colocalize with the disease-related protein in body fluids. For tauopathies and α-syn ucleopathies, the cerebrospinal fluid is since long considered to be the locus of aberrant protein aggregation (Verbeek et al., 2003; Lu et al., 2015; Zhang et al., 2015). Recent studies have also demonstrated the presence of α-syn outside the central nervous system, like in the autonomic nervous system, enteric nervous system, and human fluids (saliva, red blood cells and cerebrospinal fluid). Although the esophagus and the salivary glands appear to be the area with the highest concentration of α-syn (Campo et al., 2019), the search for biomarkers for synucleopathies is usually focused on samples of the cerebrospinal fluid obtained by lumbar puncture. A less invasive approach might soon be feasible since new developments have evidenced that abnormal expression of α-syn in the olfactory bulb is a hallmark of the very first stage of Parkinson’s development (Zapiec et al., 2017). In such samples, even subtle changes in FKBP12 concentration may raise an alarm on the prodromal stage of the disease.
Furthermore, the lack of a rapid, laboratory-based assay for FKBP12 has limited the development of novel FKBP12 non-immunosuppressive inhibitors for a therapeutic program, as witnessed by the absence of clinical trials involving new drugs that could contrast FKBP12 action in neurodegenerations.
It is therefore of the utmost importance to implement new analytical detection methods that can provide reliable methods for assessing the levels of FKBP12 and that can be carried out using the extremely small quantities of the biological specimen that can be collected from living patients or healthy individuals.
The current standard technique adopted to analyze the concentration of proteins in blood is liquid chromatography/mass spectrometry, liquid chromatography/mass spectrometry-mass spectrometry (van den Ouweland and Kema, 2012; Ghali et al., 2014; Collins et al., 2015) or ELISA techniques. Goldknopf et al. (2006) determined the quantity of FK506-binding protein-related peptide in serum samples by means of 2D gel electrophoresis followed by matrix-assisted laser desorption time of flight mass spectroscopy (МALDI-TOF МS) based peptide mass fingerprinting and database searching, or liquid chromatography with tandem mass spectrometry partial sequencing of individual peptides. ELISA is a well-established antibody-based tool for detecting and quantifying antigens of interest. Such assays are robust, although laborious, alternative analytical methods for sensitive and selective determination of the target, particularly when combined with fluorescence detection of a fluorescent-labeled drug or antibody. Nevertheless, background fluorescence from other matrix components, or the light scattering by the biomacromolecules, frequently abate the immunoassay performance. Ready-to-use ELISA kits for FKBP12 are available from a variety of manufacturers that report different sensitivity and concentration ranges as reported in Additional Table 1 that summarizes the existing assays for FKBP12 in body fluids.
All ELISA kits for FKBP12 determination share a common procedure based on FKBP12 antibody-FKBP12 antigen interactions (immunosorbency) and colorimetric detection system to detect FKBP12 antigen targets in samples. The procedure is in general lengthy and requires successive steps that involve also biotin-streptavidin key-lock labeling. Although such an approach can be effectively used for research purposes, it is admittedly not recommended for diagnostic procedures. The associated methods are laborious, expensive, bulky, and do not allow for microfluidic arrangements in the case of ultra-low analyte levels and are not suitable for point-of-care-testing.
Concerning the neurodegenerative diseases, to the best of our knowledge, there are no standard diagnostic tests on living patients for FKBP12 while accurate and sensitive quantification of FKBP12 as a predictor of a neurodegenerative pathology should rely on a powerful and fast assay platform. Interestingly, the detection of small FKBP12 ligand molecules, such as FK506, in blood has been extensively studied in past years (Freudenberger et al., 2016) using a variety of classical analytical methods such as chromatography methods like UPLC-МS/МS or LCМS/МS and immunoassays based on e.g., fluorescence or colorimetric detection principles. FK506 determination was also realized with good quality and uniformity using surface plasmon resonance imaging technology combined with FKBP12 protein microarray on 3D-dextran hydrogel chip surface (Zhou et al., 2018). Similarly, Мenotta et al. (2015) proposed an AFМ-based method for the determination of FK506 in whole blood sample. The proposed detection method consists in the capture of the anti-FK506 drug subsequently recognized by a pre-assembled FKBP12/anti-FKBP12 complex that can easily be detected by AFМ sizing.
Although both these techniques may in principle be reversed and adapted to FKBP12 recognition, the methods do not allow for rapid screening of samples with the desired sensitivity. Recently, new methods that benefit of state-ofthe-art nano-techniques have appeared in protein-focused research aiming at the development of new protein nanoparticle-based biosensor devices. For example, Choi et al. (2018) fabricated 3D nanoclusters by ligand-mediated alternate deposition of two complementary protein cage nanoparticles using layer-by-layer assemblies. The nanocage covalently displays protein ligands on its surface that could be eventually used as a biosensor.
We recently exploited the potential of modern nanotechnology for the fabrication of a new platform that can be coupled to a variety of analytical devices for the quantification of FKBP12 in body liquids. The novel nanosensor provides a gateway toward a fast and reliable determination of ultralow concentrations of FKBP12. The rationale of the sensing strategy, reported in Figure 6, pivots on the specific and selective interactions between the FKBP12 protein and an in silico designed receptor. The receptor molecule bears a binding group for univocal recognition of the FKBP12 protein (Мartina et al., 2013) and, at the opposite side, an anchor group for chemical grafting to the surface of the sensor chip.
The addition of an anti-fouling inert matrix in the nanolayer permits to reach higher selectivity suppressing interferences from other proteins present in the analyzed sample. At the same time an accurate tuning of the relative fraction of the anti-fouling matrix allows obtaining sensors with different sensitivity and different ranges of linear response in different FKBP12 concentration ranges. The selection of the appropriate detection range ensures that the nanosensor can be used with the cerebrospinal fluid and blood samples as well for saliva or exhaled breath condensates where the endogenous levels of FKBP12 may be significantly different. Remarkably, the nanosensor platform can be used with a variety of detection methods with different sensitivity, output speed and sampling quantities suitable for different pathologies that may require determination of different threshold values. As reported in a recent patent (Caminati et al., 2019), coupling of the nanosensor of Figure 6 to a Quartz-Crystal-Мicrobalance can be used to determine FKBP12 concentrations with a Limit of Detection lower than 8 pМ with linearity that can be tuned from 10-50 pМ to 100-800 pМ. These figures are comparable to ELISA assays features reported in Additional Table 1 but without the immunoassays drawbacks due to background interferences or laborious procedures. A nanosensor coupled analytical device provides a robust and reproducible method for the fast and reliable determination of ultralow concentrations of FKBP12 that can be performed reproducibly by operators also with limited specific preparation.
Screening of FKBP12 levels in prodromic patients or populations with ND familiarity will, in turn, ensures an efficient therapeutic program from the very first steps of the ND pathology even in the absence of clinical symptoms. All these methods are economically affordable and easily implemented in a stand-alone device that can rapidly provide a “yesno” answer for the presence of a “pathological” threshold concentration of FKBP12.
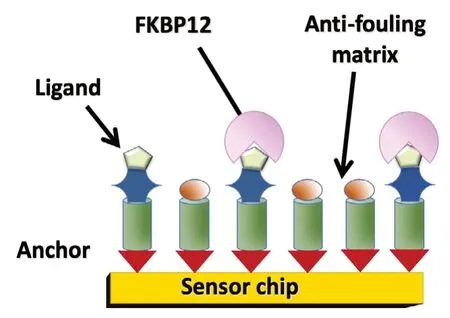
Figure 6 Cartoon depicting the sensor strategy adopted for FKBP12 quantification in biofluids.
These studies open up a new viewpoint on neurodegenerations that embraces the validation of FKBP12 as a new ND predictor as well as the development of new inhibitors for this therapeutic target.
Conclusions and Future Perspectives
The abnormal aggregation of IDP in plaques or tangles in the brain is universally recognized as the main cause of the impairment of neuronal functions in a variety of neurological disorders. Parkinson’s disease is generally linked to α-syn while the role of tau protein in Alzheimer’s disease is recently gaining much consideration. α-syn and tau are both cytosolic aggregation-prone IDPs grossly sharing a common chemical-physical pattern, with proline-rich hydrophilic unstructured termini, bracketing a central functional hydrophobic region (the NAC domain in α-syn) and tubulin-binding region, also rich in prolines, in tau. Мost remarkably, in spite of their disparate sequences and functions, tau and α-syn aggregates are found simultaneously in a large share of Alzheimer’s disease affected patients. All these facts strongly suggest a possible common molecular origin for malignant aggregation in ND. In this regard, we have reported how, for at least two decades, the evidence is mounting on the implication of FKBP12 and PPI containing immunophilins in neurological disorders. The molecular mechanism underpinning this role has been recently attributed to a subtle alteration of the amphiphilic nature of these IDPs, promoted by FKBP12 dysregulated proline binding in their hydrophilic termini, eventually leading to boosted and abnormal, non-linear, hydrophobic aggregation. While FKBP12 is certainly not the only actor in ND insurgence, very likely related to many contributory causes, the compelling preclinical record suggests testing this enzyme as a possible therapeutic target for ND. Furthermore, the gathered evidence very strongly pinpoints this protein as a predictive biomarker for prodromal stages in Parkinson’s and Alzheimer’s diseases, calling for the development of a cost-effective, fast and reliable detection method of PPI containing proteins in the cerebrospinal fluid and body fluids in general. In this context, we have succinctly described a nanostructured platform for FKBP12 precise detection coupled to a variety of common sensing devices that can be operated with limited specific preparation for community-based screening campaigns to achieve an early diagnosis of ND.
Acknowledgments:We thank MIUR-Italy (“Progetto Dipartimenti di Eccellenza 2018-2022” allocated to Department of Chemistry “Ugo Schiff”).
Author contributions:GC and PP contributed equally to the planning and writing of this review. Both authors approved the final manuscript.
Conflicts of interest:Both authors declare no conflicts of interest.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Table 1:Determination of FKBP12 in biological fluids.
Additional Table 2:Current methods for FKBPs detection.
杂志排行
中国神经再生研究(英文版)的其它文章
- Dopamine: an immune transmitter
- The role of sequestosome 1/p62 protein in amyotrophic lateral sclerosis and frontotemporal dementia pathogenesis
- Using antifibrinolytics to tackle neuroinflammation
- Medicinal plants and natural products as neuroprotective agents in age-related macular degeneration
- Nafamostat mesylate attenuates the pathophysiologic sequelae of neurovascular ischemia
- Engineering mesenchymal stromal/stem cell-derived extracellular vesicles with improved targeting and therapeutic efficiency for the treatment of central nervous system disorders