Effect of phacoemulsification on intraocular pressure and bleb function in eyes with previous trabeculectomy
2020-06-08JiaCherngChongSweeSewTeh2HaireenKamaruddin2PohYanOng2TajunisahIqbal
Jia Cherng Chong,2, Swee Sew Teh2, Haireen Kamaruddin2, Poh Yan Ong2, Tajunisah Iqbal
Abstract
INTRODUCTION
Trabeculectomy is the most popular filtering surgery for better intraocular pressure(IOP) control in glaucoma patients. It is often indicated in groups of patients who failed to respond adequately to medical anti-glaucoma treatment, having compliance or tolerance issue with the medication, and/or on patients who are keen to be medication-free. Trabeculectomy creates an alternative pathway for aqueous to flow, where it drains through the surgically created scleral flap into the subconjunctival space.
The principle is straightforward and the surgery itself does not require sophisticated technique, instruments or equipment, though surgical experience may be important. The bleb and the subconjunctival space patency determine the efficacy of the trabeculectomy and it is of utmost importance to maintain it. A patent bleb and subconjunctival space is essential to maintain the drainage of aqueous from the anterior chamber. Inflammation and healing process, which lead to scarring, cause a reduced subconjunctival space and increased resistance for the aqueous outflow, and impair the intraocular pressure control. Mitomycin-C or 5-fluorouracil augmentation during trabeculectomy is an effort to reduce subconjunctival scarring formation and maintain good aqueous outflow[1].
After cataract surgery, variable level of inflammation occurs in the anterior segment. In a trabeculectomized eye, inflammatory cells and cytokines flow within the aqueous, and through the scleral flap into the subconjunctival space. Theoretically, these deposits of inflammatory cells and cytokines will cause inflammation and scarring along its drainage site, from the scleral flap to the subconjunctival space. With scar formation, the aqueous outflow is impaired and bleb survival is at risk. Several studies have been done and it suggest that cataract surgery do have various degree of effect on bleb function over time, evidenced by the increased intraocular pressure and medication usage[2-13]. There is no similar report or publication done from a local institution. In this retrospective study, we analyze the influence of clear corneal phacoemulsification on IOP control, vision outcome and the usage of anti-glaucoma drops in eyes with previous anti-metabolite augmented trabeculectomy.
SUBJECTSANDMETHODS
The retrospective search of patients who had undergone trabeculectomy between 1stJan 2013 to 31stDecember 2015 with subsequent cataract surgery was done with the help from the hospital IT Department. This study was registered with the National Medical Research Registry with the research ID of NMRR-17-1469-36847. It was also approved by the Malaysian Medical Research Ethical Committee. Informed consent was omitted, as it was a retrospective study. The search is from the patient database kept in server (powered by Cerner Power Chart). The list of patients was then filtered to match our inclusion criteria. The main parameters that are of our interest for the study include IOP measurement, number of anti-glaucoma and visual acuity.
The inclusion criteria include uncomplicated clear cornea phacoemulsification; the previous trabeculectomy must be at least a qualified success (details will follow); and the phacoemulsification must be at least 6mo from the trabeculectomy. As we are only looking for the effect of phacoemulsification on the bleb function and IOP control, we only included eyes with uncomplicated phacoemulsification for analysis to minimize the presence of surgical complication related confounding factors that may also affect bleb function, which will make it even more difficult to identify the cause bleb function alteration, if any. Both primary and secondary glaucoma were included into analysis. Exclusion criteria include paediatric patients less than the age of 12, repeated trabeculectomy, and those who undergone combined surgery. A random pick of fifteen eyes (to achieve 1∶1 ratio) with previous uncomplicated trabeculectomy done within the similar period, with no subsequent eye surgery in the next 2y, were used as control group. The random control list consists of 13 primary glaucoma and 2 secondary glaucoma. The indications of trabeculectomy in these cases, which include age, visual requirement, rate of progressionetc. was not relevant to our study.
A complete success trabeculectomy is defined as IOP reduction ofat least >20% (i.e. new upper limit: baseline IOP-20%) in those with pre-trabeculectomy IOP of less than 21 mmHg, or a reduction to less than 21 mmHg (i.e. new upper limit: 21 mmHg) for those who was higher than 21 mmHg before trabeculectomy. A qualified success is defined as achieving >20% IOP reduction (i.e. new upper limit: baseline IOP-20%) in those with pre-operative pressure of less than 21 mmHg, or a reduction to less than 21 mmHg (i.e. new upper limit: 21 mmHg) for those who had pre-operative pressure higher than 21 mmHg with the help of anti-glaucoma medication. Hypotony of less than 6 mmHg (lower limit) is considered complicated trabeculectomy. Failed trabeculectomy refers to case where a trabeculectomy is unable to bring the IOP into the range of upper and lower limit despite being concurrently on anti-glaucoma after 2 consecutive visits to the clinic. Note that the filtering ability of a trabeculectomy might still fail later as a natural progression. This implies that each individual eye has its own new target IOP based on pre-trabeculectomy baseline IOP. This definition of complete success/qualified success is according to that summarized in Guidelines on Design and Reporting of Glaucoma Surgical Trial published by World Glaucoma Association[14].
All the previous trabeculectomy surgeries were done by glaucoma fellow in training or a glaucoma consultant. All trabeculectomy were done with superior limbal-based approach. Corneal traction suture was used to place the eye into downgaze position. Conjunctiva peritomy over the proposed scleral flap was done and the underlying Tenon’s capsule were dissected to exposed bare sclera. A partial thickness rectangular scleral flap measuring 4 mm×3 mm was then made. Mitomycin-C (MMC) soaked sponge (in the concentration of 0.3 mg/mL) was then applied along the subconjunctival space and underneath the scleral flap for about 2-3min. Residual MMC was then flushed with balanced salt solution. Sclerostomy was then performed with a puncher to access the anterior chamber, and peripheral iridectomy was performed to avoid occlusion. The scleral flap was then closed with releasable nylon sutures. The conjunctiva was then closed with absorbable vicryl suture and fluorescein sodium was used to check for leakage. Postoperatively, patient was started on topical ciprofloxacin 0.3% and topical dexamethasone 0.1% 2 hourly. Dexamethasone eye drop was then tapered slowly over a minimum of 6mo duration. Throughout the post op follow up, needling and 5-fluorouracil injection of 5 mg/0.1 mL was done on PRN basis, as decided by the treating consultant.
Phacoemulsification on these patients was again performed by a glaucoma fellow in training or a glaucoma consultant. 2.75 mm incision was made with a keratome on clear cornea with no manipulation of the conjunctiva. An additional clear cornea side-port incision was also made for the entry of second instrument. Phacoemulsification was then performed in a standard manner (phaco-chop or divide and conquer) following a 360 capsulorrhexis. Cortical matter was removed with an irrigation/aspiration hand piece. A foldable posterior chamber intraocular lens was implanted into all patients. Intracameral cefuroxime of 1 mg/0.1 mL was then given as endophthalmitis prophylaxis. All patients received subconjunctival injection of 5-fluorouracil of 5 mg/0.1 mL at the end of cataract surgery. Postoperatively, antibiotic eye drop (chloramphenicol or ciprofloxacin) was given every two hourly along with topical steroid (dexamethasone). The eye drops were tapered over a minimum period of 6wk and certain patients switched to combined drops (Maxitrol-Dexamethasone + Polymyxin B + Neomycin) along the way during drug tapering. Needling and subconjunctival 5-fluorouracil injections were again done on PRN basis following assessment by treating consultant.
These patients are on regular post-operative follow up and each visit was seen with a glaucoma fellow or consultant.The change in IOP and the number of anti-glaucoma medication usage following the phacoemulsification is our main study parameter. The IOP of every patient was taken using a Goldmann tonometer during each follow up. Visual acuity was checked with Snellen chart and converted into LogMAR value for statistical analysis.
Data was input into Microsoft Excel 2016 for Windows and IBM SPSS version 21.0 for Windows for statistical analysis. IOP before operation was compared with IOP at one- and two-years post phacoemulsification, using Wilcoxon Signed Rank test. Number of anti-glaucoma medications and visual acuity (LogMAR) was compared using pairedt-test.
RESULTS
Fifteen eyes of 13 patients were included for analysis, following the exclusion of six eyes that met our exclusion criteria. All patients who undergone post-trabeculectomy cataract extraction within the time frame were filtered in search of subjects for analysis. The final number is however limited due to multiple reasons which include patient lost on follow up, returned to other centers for continuation of care due to logistic issues, or eyes with however minimal complication during phacoemulsification are excluded. None of our included patients record any post-operative complications to minimize factors that may affect the bleb function. The patients’ basic demographics are listed in Table 1.
The comparison of IOP between pre-phacoemulsification group and post-phacoemulsification group are listed in Tables 2 and 3. The IOP value was the average of readings taken during the patient’s nearest 2-3 follow ups (within 4mo span) at the 1-year and 2-years mark. There is no statistical difference in IOP before and after phacoemulsification. The pre-operative mean IOP was 13.4±2.9 mmHg. At 1y post-phacoemulsification, the mean IOP was 14.2±3.2 mmHg while at 2y post-phacoemulsification, the mean IOP was 15.1±3.3 mmHg. And Table 4 summarizes the success/failure rate comparison between pre-phacomulsification and post-phacoemulsification.
From Table 4 we could see that the number of complete success trabeculectomy is on a decreasing trend. It has dropped by almost half, from 92.9% pre-phacomulsification to 46.7% at 2y post-phacomulsification. One eye has progressed into a failed trabeculectomy, with pressure increment from baseline of 12 mmHg before phacoemulsification, to 21 mmHg on all four classes of anti-glaucoma. These numbers suggest that bleb function is being compromised over time after the cataract surgery.
Table 1 Patient demographics

DemographicResultEyes Right11 Left4Sex M8 F5Age, a Mean57.1±14.4 Range18-79Age, a Mean58.3±14.5 Range19-80Time from trabeculectomy to phaco Mean(mo)14.7±4.3 Range (mo)9-22Type of glaucoma Primary13 Primary open angle glaucoma10 Primary angle-closure glaucoma3 Secondary2Behcet disease1Sclerouveitis1
Table 2 Intraocular pressure comparison (mmHg)

Eyes (no.)Preoperative1y postoperative2y postoperative117211921214123101112413141451112116121321712111282116159131116101416171117121212142118131014141413141315131214Mean±SD13.4±2.914.2±3.215.1±3.3
Table 3 Hypothesis test summary

Null hypothesisPIOP 1y post-phacoemulsification vs pre-phacoemulsification0.357IOP 2y post-phacoemulsification vs pre-phacoemulsification0.212
Using Related-Samples Wilcoxon Signed Rank Test.

Figure 1 pre and post-phacoemulsification IOP Related Sample Wilcoxon Signed Rank non-parametric statistical test was used to compare between the IOP in view of the small sample size and for the fact that IOP was not normally distributed among the patients. Although the IOP change was statistically insignificant, there was an increased in anti-glaucoma medication usage.
The number of eyes requiring anti-glaucoma to maintain a satisfactory IOP is on a rising trend. There was one eye requiring anti-glaucoma for satisfactory IOP control prior to the cataract surgery. The number of patients requiring anti-glaucoma at 1y post-phacoemulsification increased to four. Interestingly, one eye that needed anti-glaucoma prior to operation no longer needed any after cataract surgery, but another four eyes that did not need any pre-operative required at least one after the surgery.
The average number of anti-glaucoma was 0.07±0.26 prior to cataract surgery. The number of anti-glaucoma medication was increased to 0.73±1.5 at 1y post phacoemulsification. Preoperative: 0.067±0.26; 1y postoperative: 0.733±1.53 (t=-1.625,P=0.126).
More changes were noticed at two years following cataract surgery. The number of eyes requiring anti-glaucoma increased to eight. The average number of anti-glaucoma also increased to 1.7±1.7, which when compared to preoperative numbers of 0.07, is a significant increment (P=0.003, pairedt-test). Preoperative: 0.067±0.26; 2y postoperative: 1.667±1.72 (t=-3.595,P=0.003).
To convince us that the phacoemulsification is the cause of bleb function deterioration in these cases, a random pick of fifteen eyes with uncomplicated trabeculectomy done within the similar period was done. These control cases had to be either success or qualified success at 7mo post-trabeculectomy in order to match our study subjects, where the shortest duration between trabeculectomy and phacoemulsification was also 7mo. There are 13 primary glaucoma and 2 secondary glaucoma among our control cases. We then investigate the pre and post-operative IOP and number of anti-glaucoma usage at 1y and 2y. Table 6 showed the list of IOPs of the 17 controls.
There is a significant IOP reduction before trabeculectomy and 1y after trabeculectomy, from 22.3±8.9 mmHg to 12.5±3.9 mmHg at 1y postoperative. The IOP is well maintained into 2y post-trabeculectomy. This is proven by statistical analysis shown in Table 7.
Table 4 Success/failure summaryn (%)
Table 5 Number of medications

ItemsEyes on anti-glaucoma, nEyes not on anti-glaucoma, nEyes on anti-glaucoma, %Mean number of anti-glaucomaPreoperative1146.7%0.07±0.261y postoperative41126.7%0.73±1.52y postoperative8753.3%1.7±1.7

Table 6 Intraocular pressure comparison of control cases (mmHg)
Table 7 Hypothesis test summary (Control group)

Null hypothesisPIOP 1y post-trabeculectomy vs pre-trabeculectomy 0.001IOP 2y post-trabeculectomy vs 1y post-trabeculectomy 0.551
Using Related-Samples Wilcoxon Signed Rank Test.
Similarly, we also compared the usage of anti-glaucoma medications in the control group before and after trabeculectomy. The summary is listed in Table 8.
Prior to trabeculectomy, all 15 eyes were on anti-glaucoma medications. This number reduced to 0 at 1y post-trabeculectomy. At 2y, only two out of fifteen eyes required at least 1 anti-glaucoma medication to maintain satisfactory IOP control. The medication reduction is significant before and after trabeculectomy (from 4.0±0.0 to 0.00). There is no statistically significant difference between post-operative 1y and 2y in terms of the number of anti-glaucoma usage, (0vs0.2±0.56,t=-1.382,P=0.189), with mean number of anti-glaucoma needed 2y post-trabeculectomy as 0.20±0.56. This reflects that the bleb function in this control group of eyes had no significant deterioration 2y after surgery.
In terms of visual acuity,it was tested with Snellen chart in the clinic at each visit. The Snellen visual acuity was converted into LogMAR for numerical calculation and comparison. Counting finger vision and hand movement vision are regarded as 1.85 and 2.3 respectively[15]. There is no reference on LogMAR value for light perception vision and thus a LogMAR 3.0 was used. The vision of each eye before and at 2y after cataract surgery are listed in Table 9 and Figure 2.
DISCUSSION
Glaucoma is one of the leading causes of blindness in the world. Within the context of Asia continent, the prevalence of glaucoma is 3.54%[16]. In 2013, the number of people (aged 40-80 years) with glaucoma worldwide was estimated to be 64.3 million, increasing to 76.0 million in 2020 and 111.8 million in 2040[16]. IOP is the one most significant risk factor for glaucoma and achieving individualized target IOP is essential in controlling disease progression. Surgical interventions including filtering surgery remains important in achieving the target IOP. Trabeculectomy was known to hasten cataract formation and progression[17-18]. Cataract as a disease of ageing, surgery is thus almost unavoidable for everyone with the increasing average lifespan. Being the two leading causes of blindness, it is extremely likely to have patient having concurrent glaucoma and cataract which might need surgery. It is therefore important to formulate an optimized treatment plan to provide the best quality of life for them.
From the results of current study, it shows that phacoemulsifcation in eyes with previous filtering surgery does affect the bleb function, starting at the 2-year mark after cataract surgery. This is supported by the increased number of eyes requiring anti-glaucoma (pre-operative 1 eye, 2y postoperative 8 eyes), the significant increment of anti-glaucoma needed (from 0.07-1.67,P=0.007). In fact, one subject among the fifteen eyes had progressed into failed trabeculectomy, as the IOP raise from baseline of 12 to 21 on four classes of anti-glaucoma. Our result is similar to the study conducted by Inaletal[8]where the 12.5% of trabeculectomy failed and 45.8% required at least one anti-glaucoma at 2y postoperatve but with no significant change in IOP control. Another study conducted by Robolledaetal[9]also had similar results, where 44.3% of subjects required at least one anti-glaucoma at 2y to control the IOP.
Table 8 Number of medications (Control group)

ItemsEyes on anti-glaucoma (no.)Eyes not on anti-glaucoma (no.)Eyes on anti-glaucoma (%)Mean number of anti-glaucomaPreoperative1515100%4.0±0.01y postoperative 0150.00%0.0±0.02y postoperative21513.34%0.2±0.6
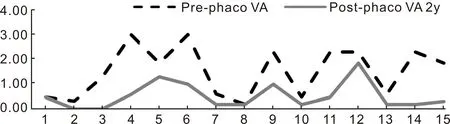
Figure 2 Pre and post-phacoemulsification visual acuity The mean visual acuity pre-phacoemulsification was 1.52±1.00 while the 2y post-phacoemulsification visual acuity the mean improved to 0.53±0.54.
Table 9 Preoperative and postoperative visual acuity

Eye(no.)Preoperative VALogMARSnellen2y postoperative VALogMARSnellen10.486/180.486/1820.306/120.006/631.303/600.006/643.00PL0.606/2451.85CF1.303/6063.00PL1.006/6070.606/240.186/980.186/90.186/992.30HM1.006/60100.486/180.186/9112.30HM0.486/18122.30HM1.85CF130.606/240.186/9142.30HM0.186/9151.85CF0.306/12Mean±SD1.52±1.006/1800.53±0.546/21
VA:Visual acuity.
Most of the recent studies do record a significant IOP increment following cataract surgery, starting from as early as 3mo post phacoemulsification[2-3,6-7,9-13]. This implies that a certain degree of bleb function impairment is present following cataract surgery. Our study do not show such significant increment in IOP at 2y post phacoemulsification (preoperative: 13.4 mmHg and 2y postoperative: 15.1 mmHg,P=0.21). The IOP control was however achieved by PRN needling and 5-FU injections and increasing number of anti-glaucoma required. There was however one study conducted in Korea which concluded that temporal clear cornea phacoemulsification in eyes with filtering bleb don’t have adverse effect on long-term IOP control, but it was a projected result based on Kaplan-Meier survival analysis[19]. Approximately 83% of previous successful trabeculectomy could last for 5y[20]. This percentage is greatly reduced when a cataract surgery is performed on an eye with filtering bleb. It is thus logical to formulate a treatment plan that could improve the survival of a filtering bleb.
Following the publication of EAGLE study conducted by Azuara-Blancoetal[21]that suggests early cataract or clear lens extraction gives significant IOP reduction in primary angle closure patients, a lens surgery should be performed in an attempt to control the IOP prior to a trabeculectomy. This could also be true even in the case of primary open angle glaucoma, where a study conducted by Mathaloneetal[22]showed a significant decrease in the number of anti-glaucoma required. Studies also shows that trabeculectomy in a pseudophakic eye does not have an increased risk of failure; it is probably more beneficial to perform a cataract surgery prior to a trabeculectomy[23-24]. Phacoemulsification should be the cataract surgery of choice as it causes less postoperative inflammation compared to extracapsular cataract extraction[25-26]. In cases where an extracapsular cataract extraction is the safer choice for patient, it should still be performed for patient safety. A study done by Halikiopoulosetal[5]pointed that phacoemulsifcation and ECCE make no difference in terms of affecting bleb function.
In conclusion, phacoemulsification does cause significant impairment on bleb function over time. A control group of 15 eyes (to achieve ratio of 1∶1 with our study subjects) were included to support our conclusion. Even though the IOP control might still be adequate after phacoemulsification by restarting or adding anti-glaucoma medications, this could lead to problems such as patient adherence, medication intolerance or patient frustration, which could very well be the initial indications why trabeculectomy was done in the first place. Nonetheless, the aim of vision improvement following a cataract surgery is well achieved in our study subjects, which definitely would have improved their quality of life[27]. The timing and sequence of cataract and glaucoma surgery is therefore of utmost importance and should be planned properly for best patient outcome in terms of both vision and glaucoma control. At least 6mo should be given after trabeculectomy before a cataract surgery is planned[28], with the longer the duration will theoretically give the bleb a better chance of survival.
There are a few limitations that need acknowledgement in this study. Firstly, the sample size is limited. A bigger sample size would have given a better power on the results. However, as this is a retrospective study in one single center, with combined surgeries and trabeculectomy in pseudophakic eyes are more common, samples fulfilling our criteria were limited. A multi-center study with longer period may be a stronger evidence to support the finding. As for all retrospective studies, the quality of data may be flawed, and thus may affect the result as well.
