Clinical control effect analysis of intravenous infusion of lidocaine and dexmedetomidine for prevention and treatment of CRBD
2020-06-06BangJianZhangXiaoYanYangQiangXueJuanShen
Bang-Jian Zhang, Xiao-Yan Yang, Qiang Xue, Juan Shen
Department of Anesthesiology, Panzhihua Central Hospital, Sichuan Province 617067
Keywords:
ABSTRACT
1. Introduction
Giving indwelling catheters to male patients after general anesthesia can eあectively improve patient comfort [1]. But patients with general anesthesia, with no memory and cognition for catheter cerebral cortex, combined with the mechanical stimulation of catheter this could trigger a bladder discomfort, called catheter clinical source sex bladder discomfort cath-eter-related bladder discomfort,CRBD) [2]. CRBD is one of the causes of anesthesia restlessness, which can aggravate postoperative pain, aあect patients' quality of life, and reduce surgical satisfaction [3]. At present, the treatment methods of CRBD include dexmedetomidine, sufentanil, lidocaine and so on. Among them, dexmedetomidine is α2-adrenal receptor agonist, which is a commonly used sedative for intubation and ventilator in severe patients [4]. Sufentanil is an opioid receptor agent that has a significant analgesic eあect. However, it has side eあects such as sphincter and skeletal muscle spasm, which may lead to patients complicated with CRBD. Lidocaine is a kind of amide local anesthetics with fast onset, high bioavailability and few side eあects. However, there is still no clinical conclusion on the eきcacy of the treatment of CRBD. Therefore, 210 male patients undergoing elective general anesthesia in our hospital were selected for the following study.
2. Materials and methods
2.1 Clinical data
A total of 210 male patients who underwent elective general anesthesia in our hospital from May 2017 to April 2018 were selected and divided into lidocaine group, dexmedetomidine group and control group, 70 cases each.
Inclusion criteria :(1) patients undergoing elective general anesthesia;(2) the patients were between 19 and 65 years old;(3) ASA sizing: Ⅰ level ~ Ⅱ level;(4) all patients are male, and catheterization is required;(5) this study was approved by the medical ethics committee of our hospital, and the informed consent for surgery was signed with the patient.
Exclusion criteria: (1) malignant tumor causing urinary obstruction and urinary calculi; (2) patients with urinary tract and primary renal diseases; (3) neurogenic bladder and precedent gland tumors; (4) severe diabetes and hypertension; (5) mental illness; (6) severe bleeding or serious surgical complications; (7) previous CNS disease.
2.2 Operation method
The catheter indwelling operation was carried out in the three groups without consciousness after general anesthesia, and the comfortable 16F catheter was lubricated with paraきn oil and then placed into the bladder. All the operations were completed by the same group of routine physicians, and the patients' vital signs were closely monitored during the operation. Lidocaine group: at the time of anesthesia induction, 1.5mg/kg lidocaine (Jiangsu Jichuan Pharmaceutical co., LTD., national drug approval: H20059049) was dropped stably. After intubation, patients were given 0.5~1mg/kg·h bupivacaine +2 mg/kg·h lidocaine anesthesia was maintained. Dexmedetomidine group: 0.5 μg/kg dexmedetomidine (Jiangsu Hendrui Pharmaceutical co., LTD., national drug approval: H20090248) was dropped at the time of anesthesia induction. After intubation, patients were given 0.5~1mg/kg·h bupivacaine +0.4 μg/kg·h dexmedetomidine for anesthesia maintenance.Control group: same volume of normal saline was dropped statically during anesthesia induction, and 0.5~1mg/kg·h bupivacaine + same volume of normal saline was continuously injected intravenously after intubation. Three groups of postoperative analgesia scheme are: 2~3μg/kg sufentanil (Yichang Renfu Pharmaceutical co., LTD., national drug approval:H20054172) + 10mg dexamethasone (Shiyao group Ouyi Pharmaceutical co. LTD, national drug approval:H20052358) + 2.0 mg droperidol (Beijing yongkang pharmaceutical co., LTD., national drug approval: H11020578) + 100 ml saline, lock time is 5 ~ 6 min, within 1h dose limit of 30 ml, 4h dose limit for 50 ml [5]. Extubation was performed without mechanical ventilation support.
2.3 Observation indicators and evaluation methods
The operation time, intraoperative sufentanil dosage and postoperative sufentanil dosage of the three groups were compared. CRBD score and visual analogue pain score (VAS) at the time points immediately after extubation (T0), 30min after extubation (T1), 2h after extubation (T2), 6h after extubation (T3), and 12h after extubation (T4) were compared. At the same time, the German Philips monitor was used to detect the patients' heart rate (HR) and systolic blood pressure (SBP), and the incidence of various adverse reactions was recorded.
CRBD score [6] : 0 point (the patient did not feel any discomfort of bladder and urethra);1 point (complained of mild discomfort when questioned, tolerable); 2 points (the patient feels urgent urination, painful urination and lower abdominal distension, but the patient can barely endure); 3 points (the patient felt urgent urination, painful urination, and obvious feeling of abdominal distension, with obvious physical and behavioral responses, limbs flailing and trying to pull out the catheter).
VAS pain score [7] : a swimming scale with a length of 10cm was used, and 0 point and 10 points were marked at both ends of one side. 0 point indicated no pain at all, and 10 points indicated severe pain. Patients were asked to mark the position representing their own pain on the other side of the scale, and the data were read by the same physician. All patients and the anesthesiologist responsible for evaluating the patient's sensory experience were not aware of the medication and grouping in this study.
2.4 Statistical methods
Measurement data were expressed as (±s), one-way anova was used for the comparison between non-repeated measurement data groups, and anova was used for the comparison between repeatedmeasurement data groups. χ2 test was used for counting data. P value <0.05 indicated that the diあerence was statistically significant, and SPSS16.0 was used for the statistical software.
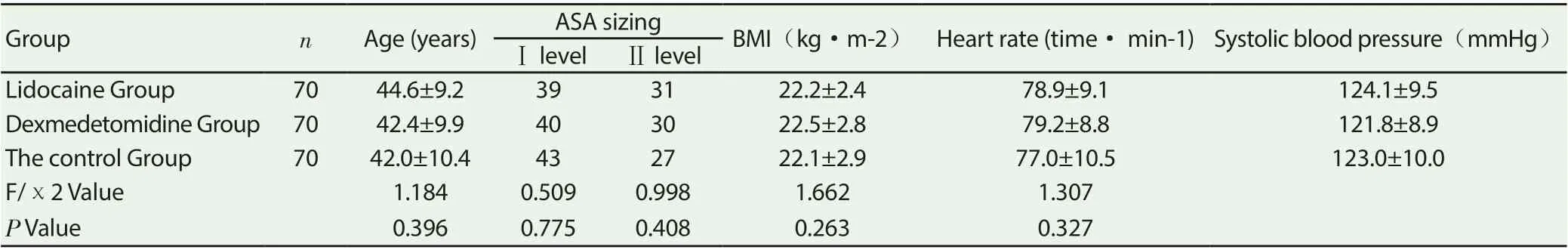
Table 1 comparison of baseline data among the three groups
Table 2 comparison of operation time and sufentanil dosage among the three groups (±s)

Table 2 comparison of operation time and sufentanil dosage among the three groups (±s)
GroupnThe operation time(min) Intraoperative sufentanil dosage(μg) Postoperative sufentanil dosage(μg)Lidocaine Group70167.9±21.84.24±0.4933.11±5.20 Dexmedetomidine Group70173.0±25.14.31±0.6034.08±6.14 The control Group70170.5±27.44.40±0.5736.29±5.75 F Value1.1980.8842.481 P Value 0.5350.6370.098
Table 3 comparison of CRBD score and VAS score at diあerent postoperative time points of the three groups (±s)

Table 3 comparison of CRBD score and VAS score at diあerent postoperative time points of the three groups (±s)
Note: compared with the control group at the same time point*P<0.05, compared with the dexmedetomidine group at the same time point #P<0.05
IndicatorsGroupT0T1T2T3T4 CRBD ScoreLidocaine Group0.73±0.141.05±0.28*#1.39±0.37*#1.45±0.22*#1.13±0.30*#Dexmedetomidine Group0.70±0.121.22±0.26*1.55±0.48*1.65±0.32*1.39±0.27*The control Group0.68±0.161.44±0.311.72±0.531.86±0.301.57±0.31 F ValueF Between groups=15.517、F Time=26.309、F Interaction=9.552 P ValueP Between groups=0.000、P Time=0.000、P Interaction=0.000 VAS ScoreLidocaine Group2.26±0.852.71±0.93*3.17±0.95*3.30±0.88*2.52±0.81*Dexmedetomidine Group2.16±0.822.69±1.00*3.23±0.89*3.27±0.92*2.58±0.79*The control Group2.19±0.793.31±1.043.86±1.203.79±0.952.98±0.74 F ValueF Between groups=12.003、F Time=16.784、F Interaction=6.398 PValueP Between groups=0.000、P Time=0.000、P Interaction=0.000
Table 4 comparison of HR and SBP values at diあerent postoperative time points between the two groups (±s)
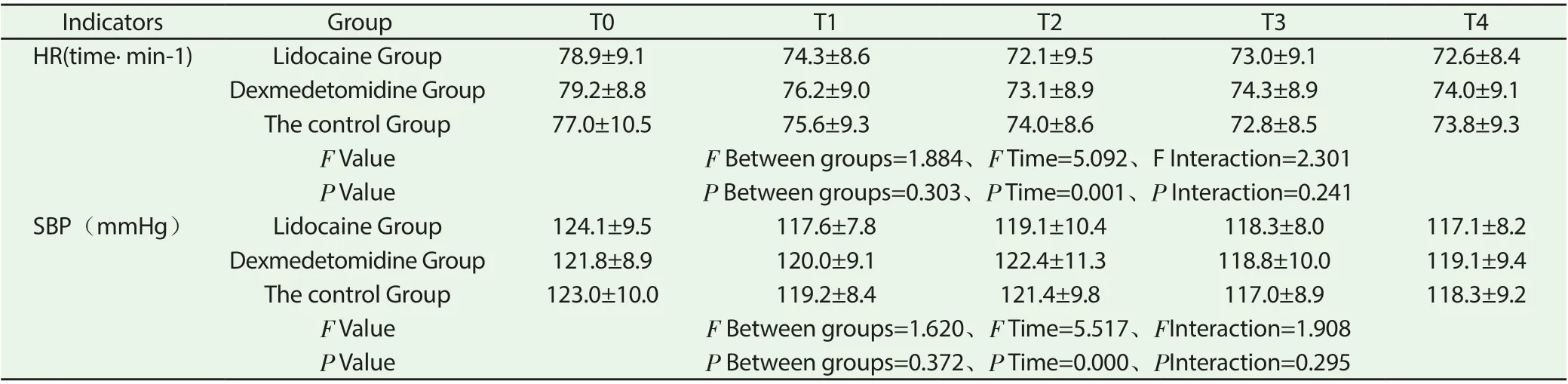
Table 4 comparison of HR and SBP values at diあerent postoperative time points between the two groups (±s)
Indicators GroupT0T1T2T3T4 HR(time· min-1)Lidocaine Group78.9±9.174.3±8.672.1±9.573.0±9.172.6±8.4 Dexmedetomidine Group79.2±8.876.2±9.073.1±8.974.3±8.974.0±9.1 The control Group77.0±10.575.6±9.374.0±8.672.8±8.573.8±9.3 F ValueF Between groups=1.884、F Time=5.092、F Interaction=2.301 P ValueP Between groups=0.303、P Time=0.001、P Interaction=0.241 SBP(mmHg)Lidocaine Group124.1±9.5117.6±7.8119.1±10.4118.3±8.0117.1±8.2 Dexmedetomidine Group121.8±8.9120.0±9.1122.4±11.3118.8±10.0119.1±9.4 The control Group123.0±10.0119.2±8.4121.4±9.8117.0±8.9118.3±9.2 F ValueF Between groups=1.620、F Time=5.517、FInteraction=1.908 P ValueP Between groups=0.372、P Time=0.000、PInteraction=0.295
3. Results
3.1 Baseline data of the three groups of subjects
The baseline levels of age, ASA grade, BMI, heart rate and systolic blood pressure of lidocaine group, dexmedetomidine group and control group were not significantly diあerent (P> 0.05); Table 1.
3.2 Comparison of Operation Time and Sufentanil Dosage among the Three Groups
There were no statistically significant diあerences between lidocaine group, dexmedetomidine group and control group in operation time, intraoperative and postoperative sufentanil dosage (P>0.05); (table 2).
3.3 Comparison of CRBD Score and VAS Score at Different Postoperative Time Points among the Three Groups
At the time of T0, there was no significant difference in CRBD score and VAS score among the three groups (P> 0.05). From T1 to T4, the CRBD score of the lidocaine group was lower than that of the dexmedetomidine group and the control group (P<0.05), the VAS score of the lidocaine group was lower than that of the control group (P<0.05), and the CRBD score and VAS score of the dexmedetomidine group were lower than that of the control group (P<0.05); (table 3).
3.4 Comparison of HR and SBP Values Measured at Different Time Points after Surgery among the Three Groups of Patients
At the time of T0 ~ T4, the diあerence of HR and SBP values among the three groups was not statistically significant (P>0.05); (table 4).
4. Discussion
The pathogenesis of CRBD may be as follows : (1) bladder spasm drives detrusor activity to induce CRBD[8]; (2) abundant sympathetic and parasympathetic catheters in the urethra and inflammatory stimulation induce the disease [9]; (3) anesthetic drugs can inhibit the function of the nerve center. If the recovery rate of the subcortical central function is faster than that of the cerebral cortex, patients will have central focal sensitization symptoms, and thus experience sensory responses and abnormal processing. If the area of action is urinary-tract related nerves, CRBD[10-11] is easily triggered.
Previous studies have shown [12] that catheters inserted after induction of general anesthesia have a high incidence of CRBD, which is also prone to wake restlessness and hemodynamic fluctuations during anesthesia. If not actively dealt with, it is highly likely to cause drainage tube prolapse, incision rupture, cardiovascular accidents and other adverse consequences.Lidocaine is a local anaesthetic drug of amide group, which has significant central nervous system excitation and inhibition eあect [13-14]. Recent studies have found that intravenous lidocaine infusion can reduce the dose of anesthetic drugs required for inhalation before surgery and enhance its sedative eあect [15]. At the same time, it can also reduce the expression level of blood catecholamine in postoperative patients, improve the symptoms of nausea and vomiting, and accelerate postoperative intestinal peristalsis and exhaust. In order to improve the adverse consequences of CRBD, this group intends to give lidocaine intravenous drip treatment to the patients.
This study suggests that lidocaine intravenous drip can eあectively improve CRBD symptoms in patients with general anesthesia. The mechanism may be as follows :(1) lidocaine can reduce the body's inflammatory response and relieve local stress in the urinary tract by blocking sodium channels and inhibiting polynuclear protein synthesis [16]. (2) muscarinic receptors are the main mediators of bladder smooth muscle contraction, and lidocaine can eあectively block the activation of muscarinic receptors and thus inhibit bladder spasm [17]. (3) after entering the bladder, lidocaine can increase the threshold of potential conduction of the bladder vagus nerve, reduce the release and transmission of reflex arc, and reduce the rate of bladder spasm [18]. This study shows that dexmedetomidine can also alleviate CRBD to some extent. Dexmedetomidine is α2-adrenergic agonist with a molecular weight of 200.28000. It has strong sedative, analgesic and anti-anxiety eあects [19-20]. It should be noted that this product is mainly excreted through the kidney, so patients with renal insuきciency should be used with caution.
This study shows that lidocaine and dexmedetomidine are feasible in the control of CRBD and have high safety. Sufentanil is μopioid receptors, is one of the potent analgesic drugs, but sufentanil respiratory depression, bronchospasm, adverse reactions such as arrhythmia, can also cause Mr DE muscle spasm, skeletal muscle stiあness, this will increase the risk of CRBD, so clinical needs in the protection of analgesic eきcacy, reduce the dosage of sufentanil. By comparing the blood pressure and heart rate of the three groups of patients at the time T0 ~ T4, it was found that the diあerences in the measured values of HR and SBP of the three groups of patients were not statistically significant, which supported the above conclusion, suggesting that lidocaine has the characteristics of high eきcacy and safety in the prevention and treatment of CRBD.
In this study, through randomized, controlled and double-blind studies, it was found that both the lidocaine group and dexmethylene could prevent and treat CRBD, and the lidocaine group had more advantages in preventing and treating CRBD at the time between T1 and T4. In conclusion, intravenous lidocaine infusion can significantly reduce the incidence of CRBD in male patients who need catheterization after general anesthesia.
杂志排行
Journal of Hainan Medical College的其它文章
- Meta-analysis of clinical efficacy of Duhuo Jisheng Decoction in the treatment of lumbar intervertebral disc herniation
- Analysis of effect of terbutaline sulfate combined with shunning on acute attack of asthma complicated with lobar pneumonia in children
- Study on the relationship between knee injury recovery and traditional Chinese medicine constitution of young male amateur cyclist in Hainan island cycling league
- Changes and clinical value of serum HBV RNA level on HBeAg positive CHB patients treated with the treatment of Peg IFN-α
- The effect of patients with chronic hepatitis B HBeAg(+)with poor response to treatment by converting to sequential treatment with tenofovir dipivoxil
- Clinical efficacy of angina pectoris after pci in patients with coronary heart disease complicated with type 2 diabetes by Yiqi Yangyin and Huatan Tongluo recipe
