The analgesic,anti-inflammatory,and anti-infection effects of Chai Ge fever relief oral liquid
2020-06-03ShanShanGuoYaXinWangYingJieGaoSaiWeiLuXiaoLanCui
Shan-Shan Guo,Ya-Xin Wang,Ying-Jie Gao,Sai-Wei Lu,Xiao-Lan Cui*
1Institute of Chinese Materia Medica,China Academy of Chinese Medical Sciences,Beijing 100029,China; 2Suzhou Youseen New Drug R&D Co.,Ltd.,Suzhou,Jiangsu 215001,China.
Abstract Backgroud:To explore the analgesic,anti-inflammatory,and anti-infective effects of Chai Ge fever relief oral liquid and provide evidence for clinical application of Chai Ge fever relief oral liquid.Methods:In this study,four groups of experiments were designed:analgesic,anti-inflammatory,antiviral,and antibacterial.In the mouse pain model,the analgesic effect of Chai Ge fever relief oral liquid was studied by the writhing method and pain threshold; the anti-inflammatory effect by measuring the level of capillary permeability in the abdominal cavity of mice in different dose groups and the weight of cotton ball granuloma formation in rats; the antiviral effect by measuring the lung index of a pneumonia model of mouse infected with influenza virus; and the antibacterial effect by comparing the difference in the death protection ratio between each dose group and the model group result.Results:In the analgesic experiment,the high and medium dose of Chai Ge fever relief oral liquid could significantly reduce the number of writhing in mice caused by acetic acid,and the pain threshold of mice in the high-and medium-dose groups was significantly increased for 1–3 hours.In the anti-inflammatory experiment,the medium-dose group could significantly inhibit the increase of capillary permeability in the abdominal cavity of mice caused by acetic acid,and the low-dose group could significantly reduce the weight of rat cotton ball granuloma.In the antiviral experiment,the high-and medium-doses of Chai Ge fever relief oral liquid could significantly reduce the lung index of normal mouse pneumonia model of influenza virus infection and achieve a higher inhibition rate.In the anti-infective experiment,the death protection rate of the high-dose group was significantly different from that of the model control group.All three dose groups could significantly prolong the survival days of infected mice.Conclusion:These experimental results prove that in addition to its antipyretic effect,Chai Ge fever relief oral liquid also has analgesic,anti-inflammatory,antiviral,and antibacterial effects.
Keywords:Chai Ge fever relief oral liquid,Analgesia,Anti-inflammatory,Antiviral,Antibacterial
Background
Chai Ge fever relief oral liquid is derived from the famous traditional Chai Ge muscle pain relief decoction in the ancient Chinese book "Six Volumes of Treatises on Febrile Diseases Caused by Cold”,which is a modified decoction withRadix bupleuri(Chai Hu)andPuerariae lobatae radix(Ge Gen) as monarch medicines andRadix scutellariae(Huang Qin),Rhizoma et radix notopterygii(Qiang Huo),Radix angelicae dahuricae(Bai Zhi),andGypsum fibrosum(Shi Gao)as the minister medicines [1].Chai Ge fever relief oral liquid is composed ofRadix bupleuri(Chai Hu),Puerariae lobatae radix(Ge Gen),Radix scutellariae(Huang Qin),Gypsum fibrosum(Shi Gao),Herba artemisiae annuae(Qing Hao),Raidix paeoniae alba(Bai Shao),andRadix glycyrrhizae uralensis(Zhigancao).It is mainly used for colds caused by exogenous wind and colds that enter the body and transform into heat.The symptoms include fever,aversion to cold,and headache [2].This formula is a compound preparationwhose main pharmacological effect is antipyretic.However,it also has other pharmacological effects such as analgesia,anti-inflammation,antiviral,and antibacterial.We designed pharmacodynamic experiments in line with the requirements of the national guidelines for preclinical evaluation technology of new Chinese medicines and the functional indications of Chai Ge fever relief oral liquid.The purpose of this study is to investigate the analgesic,anti-inflammatory,antiviral,antibacterial pharmacological effects of Chai Ge fever relief oral liquid.Four experimental models,including analgesic,anti-inflammatory,antiviral,and antibacterial,were designed.By comparing the differences in pain threshold,abdominal capillary permeability,lung index,and death protection rate between the experimental animals and the control group,we investigated the pharmacological effects of Chai Ge fever relief oral liquid at different dosages.
Materials and methods
Experimental site
Antiviral and antibacterial experiments were performed in the ABSL-2 Biosafety Laboratory of the Institute of Chinese Materia Medica,China Academy of Chinese Medical Sciences,while the experiments on rats were conducted in the Experimental Animal Center of the same institute.Rabbit experiments were performed at Beijing Jinmuyang Experimental Animal Breeding Co.,Ltd.
Drugs,animals,reagents and equipment
Experimental drug.Chai Ge fever relief oral liquid(dry extract):Product of Suzhou Yusen New Drug Development Co.,Ltd.Properties:brown powder.Storage conditions:cool and dry place.Human clinical dosage:9.6 g dry cream per person per day,with 1.3%baicalin content.Chai Ge fever relief oral liquid dry cream:The human clinical dosage is 9.6 g dry cream/60 kg/day,with 1.3% baicalin content.Correspondingly,the high,medium,and low doses for mice are 3.52 g dry cream/kg/day,1.76 g dry cream/kg/day,and 0.88 g dry cream/kg/day,respectively.The low dose is equivalent to twice of the clinical dosage for humans.
Positive control drug.Ribavirin granules:products of Sichuan Baili Pharmaceutical Group Co.,Ltd.Indications:for the treatment and prevention of viral respiratory infections.Properties:white particles.Specifications:50 mg/bag.Dosage:150 mg orally,three times a day.The clinical dosage is 450 mg/60 kg/day for humans; the equivalent dosage for mice is 82.5 mg/kg.
Amoxicillin capsule:product of Zhongshan Branch of Zhuhai Federal Pharmaceutical Co.,Ltd.Indications:broad-spectrum antibacterial.Specification:0.5 g/capsule.Dosage:1 capsule/time for adults,three times per day.The clinical dosage for human is 2.0 g/60 kg; the equivalent dosage for mice is 370 mg/kg/day.
Aspirin enteric-coated tablets:product of Bayer Schering Pharma.Indications:for fever caused by common cold or influenza,as well as mild to moderate pain such as headache,joint pain,migraine,toothache,muscle pain,neuralgia,and dysmenorrhea.Specification:0.1 g/tablet.Properties:enteric-coated tablets,which is white after removing the coating.The regular dosage in this laboratory is 300 mg/kg/day for mice,150 mg/kg/day for rats,and 100 mg/kg/day for rabbits.
Qingkailing oral liquid:product of Yabao Pharmaceutical Factory of Beijing University of Traditional Chinese Medicine Co.,Ltd.Indications:clearing heat and detoxifying,resolving phlegm and clearing collaterals,refreshing the brain and resuscitating,and treatment of fever and upper respiratory tract infection.Specification:10 ml/ampoule.Dosage:3 ampoule per time,3 times per day.The clinical dosage for human is 60 ml/60kg/day,the equivalent dose for mice is 11 ml/kg/day,the equivalent dose for rats is 5.6 ml/kg/d,and the equivalent dose for rabbits is 3 ml/kg/day.During the experiments,each of the aforementioned drugs was administered by gavage at equal volumes and different concentrations; the volume for mice is 0.2 ml/10 g each time.
Experimental animals.ICR mice (SPF/VAF grade)from Beijing Hua Fukang Biotechnology Co.,Ltd.,animal license SCXK (Jing) 2009-0004 and Peking University Health Science Center,animal license SCXK (Jing) 2011-0012.Weights:14 ± 1 g for in vivo antiviral experiments,18 ± 1 g for in vivo antibacterial experiments,and 19 ± 1 g for other experiment.SD rats (SPF/VAF grade) were provided by the Experimental Animal Center of the Academy of Military Medical Sciences of the People’s Liberation Army.Animal license:SCXK (Army) 2007-0004.Rats for cotton ball granulomatosis weighed 165 ± 15g.
The experiment was conducted following the protocol approved by the Ethics Committee of Institute of Chinese Medicine,China Academy of Chinese Medical Sciences (Permit Number:20120705).And this research was conducted in accordance with the internationally accepted principles for laboratory animal use and care as found in the European Community guidelines (EEC Directive of 1986;86/609/EEC).
Bacterial strains.Staphylococcus aureus(standard strain 26003) was purchased from the Chinese National Institute for the Control of Pharmaceutical and Biological Products,provided by the New Drug Research and Development Center of the Chinese Medicine Institute,and stored in a -80°C refrigerator in our laboratory.
Virus strains.The H1N1 influenza virus FM1 strain was purchased from the Influenza Room of the Institute for Viral Disease Control and Prevention of the Chinese Center for Disease Control and Prevention.The passage of the virus was in conventional chicken embryos in this laboratory and stored in a refrigerator at -80°C.
Reagents.Nutrient broth culture medium:product of China National Institute of Pharmaceuticals and Biological Products.Acetic acid:product of Beijing Chemical Plant.Ether:product of Beijing Reagent Company,batch number 20090707.Evans blue:product of Beijing Supply Station of China National Pharmaceutical Group Corporation,batch number 871226.
Equipment.CO2incubator:product of Yamato Scientific Co.,Ltd.,of Japan.Type A2 biological safety cabinet,model MSC1.8:Product of Thermo,Germany.HZQ-F160 shock incubator:product of Harbin Donglian Electronic Technology Development Co.,Ltd.Eppendorf centrifuge 5810R:product of Germany.Balance for weighing mouse and animals,model 02376:product of Shanghai Yueping Scientific Instrument Co.,Ltd.Electronic balance model AL204:product of METTLER TOLEDO Instrument Co.,Ltd.Electrothermal thermostatic sink DK-600B:product of Shanghai Senxin Instrument Co.,Ltd.IVC mouse breeding cage:product of Suzhou Teaching Cage Factory.Portable thermometer TH-212,model TH09829:product of Beijing Haichuang Hi-Tech Technology Co.,Ltd.
Analgesia equipment
Effect on acetic acid-induced pain model in mouse(writhing method)[3].Mice were randomly divided into model control group,aspirin control group,Qingkailing oral liquid control group,and three dose groups of Chai Ge fever relief oral liquid.There were 10 rats in each group.The drugs were administered by gavage at 0.2 ml/10 g/per mouse each time,and the control group mice were administered distilled water under the same conditions.One hour after the last administration,each mouse was injected intraperitoneally with 1% acetic acid at 0.2 ml per mouse,and the number of typical writhing in 20 min was observed for each mouse.
Pain inhibition rate (%) of acetic acid-induced pain in mice=(pain threshold after administration-pain threshold before administration)/pain threshold before administration × 100%
Effect on hot plate-induced pain in mice (pain threshold)[4].Adjust the thermostatic water bath by filling the water bath with water so that the water surface is in contact with the hot plate.Adjust the thermostatic device to control the water temperature between 55 ± 5°C,prewarm for 10 minutes,and keep the temperature constant.
Animal screening:Take female mice weighing 20 ±2 g,and put one mouse on a hot plate at a time.The time (seconds) required from placing the mouse on the plate to foot licking is counted as the normal pain threshold.The animals that licked their feet for less than 5 seconds or greater than 30 seconds and those that jumped were discarded.Repeat the measurement of normal pain threshold and take the average of the two pain thresholds as the pain threshold of the mouse before drug administration.
Experimental method:a total of 60 qualified mice were randomly divided into blank control group,aspirin control group,Qingkailing control group,and three dose groups of Chai Ge fever relief oral liquid according to the normal pain threshold,with 10 mice in each group.Each group was administered by gavage with 0.2 ml/10 g/per animal each time,and the control group was administered distilled water under the same conditions.The pain threshold of each mouse was measured at 1 h and 2 h after administration.If there was no response for 60 sec,the mice were removed,and the pain threshold was calculated for 60 sec.
Anti-inflammation experiment [5]
Effect on peritoneal capillary permeability in mice.Sixty mice were randomly divided by weight into the blank control group,model control group,aspirin control group,Qingkailing oral liquid control group,and three dose groups of Chai Ge fever relief oral liquid,with 10 mice in each group.Drugs were administered by gavage at 0.2 ml/10 g/per mouse for once,and the control group was administered distilled water under the same conditions.One hour after the last administration,each mouse was injected with 0.5%Evans blue liquid at 0.1 ml/10 g in the tail vein.Except for the normal control group,the other groups were then intraperitoneally injected with 1% acetic acid at 0.2 ml/each,and the animals were sacrificed by dislocation 20 minutes after.Wash the abdominal cavity with 6 ml of normal saline,repeatedly rub the abdomen gently for 20 times,suck 4 ml of washing solution,centrifuge at 3000 rpm for 15 minutes,and take the supernatant to measure the optical density at 630 nm.
Effects on the formation of cotton ball granuloma in rats.Take rats and anesthetize them with urethane and make an abdominal incision of about 1 cm in length under sterile conditions.Expand the subcutaneous tissue with hemostatic forceps,implant sterilized cotton balls of 20 mg into both the left and right groin subcutaneously,and suture the skin.Randomly divide the animals into three control groups(aspirin control group,Qingkailing oral liquid control group,and Chai Ge fever relief oral liquid group),with 10 mice in each group.Start drug administration on the day of surgery at 1 ml/100 g,once a day for 7 consecutive days.On the 8th day,the rats were weighed at first and then were decapitated and sacrificed at 1 hour after to remove the cotton ball granulation tissue.After drying in an oven at 70°C for 12 hours,the tissues were weighed,and the weight of the cotton ball was subtracted to obtain the dry granuloma weight.At the same time,calculate the granulomatous coefficient.
Granulomatous weight coefficient=(dry weight of cotton ball granulomas-original weight of cotton balls 40 mg)/body weight (g).
Effects on mouse influenza virus pneumonia model.ICR mice were randomly divided into seven groups by weight:normal control group,model control group,ribavirin control group,Qingkailing oral liquid control group,and three dose groups of Chai Ge fever relief oral liquid,with 10 animals in each group and equal numbers of males and females.Except for the normal control group,mice were lightly anesthetized with diethyl ether and infected with 15 LD50 influenza viruses (FM1) by nasal drops,35 ul each.Start drug administration on the day of infection at 0.2 ml/10 g/per animal,once a day for 4 consecutive days.Normal control group and model control group were administered with distilled water under the same conditions.On the fifth day,one hour after drug administration,weigh the animals,take blood from the orbits,separate the serum,dissect the animal,and weigh the lungs to calculate the lung index and lung index inhibition rate.
Inhibition rate of mouse influenza virus infection pneumonia model (%)=(model group lung index-drug group lung index)/(model group lung index-normal group lung index) × 100%.
Death protection effect on S.aureus-infected mice
Preparation of infectious bacterial liquid.Take Staphylococcus aureus and inoculate in nutrient broth culture medium,and culture at 37°C for 16 hours.The turbidity concentration was 9 × 108 bacteria/ml.
Infection and grouping of animals.ICR mice were randomly divided into seven groups by body weight,which were model control group,amoxicillin control group,Qingkailing control group,and three dose groups of Chai Ge fever relief oral liquid,with 20 animals in each group that were half males and half females.After grouping,drugs were administered orally to each animal at 0.2 ml/10 g,once/day for 3 consecutive days,and the model control group was given distilled water under the same conditions.On the 3rd day,before drug administration,the animals of each group were intraperitoneally injected with bacterial solution at a concentration of 9 × 108 bacteria/ml,0.2 ml per animal for modeling.The death of the animals was observed daily after infection,and the number of deaths was recorded for 14 consecutive days.Calculate the mortality,protection rate,average survival days,and life extension rate.
Life extension rate ofS.aureus-infected mice (%)=(survival time of the drug group-survival time of the model control group)/survival time of the model control group×100%.
Statistical analysis
All quantitative data are shown as mean ± standard deviation.Statistical significance was analyzed by chi-square test or t-test using IBM SPSS Statistics 25.0 software.Student's t-test was used to analyze the signifcance of the two single-agent groups.The rate comparison was used chi-square test.Comparisons withP< 0.05 were considered to be statistically significant.
Results
Analgesic test
Effects on acetic acid-induced pain in mice(writhing method).By calculating the number of writhing in each group of mice and the pain suppression rate of each drug group,it could be seen that the high and medium doses of Chai Ge fever relief oral liquid could significantly reduce the number of writhing caused by acetic acid in mice.The pain suppression rate reached 31.43% and 26.86%,respectively,which was significantly different from that of the model group (P< 0.05) (Table 1).
Effects on hot plate-induced pain mouse model(pain threshold).By calculating the threshold of hot plate-induced pain in the mouse models in each group,it could be seen that the pain threshold of the mice in the high-and medium-dose groups of Chai Ge fever relief oral liquid increased significantly at 1-3 hours after drug administration,which was significantly different from the model group (P< 0.05) (Table 2).
Anti-inflammatory test
Effects on peritoneal capillary permeability in mice.By calculating the OD values of the test animals in each group,it could be seen that the medium dose of Chai Ge fever relief oral liquid could significantly inhibit the increase of capillary permeability in mice caused by acetic acid,and the difference was statistically significant compared with the model control group (P< 0.05) (Table 3).
Effects on the formation of cotton ball granuloma in rats.By comparing the dry weight of granuloma in each experimental group,it could be seen that the low dose of Chai Ge fever relief oral liquid could significantly reduce the weight of cotton ball granuloma in rats,which was significantly different from the model group (P< 0.05) (Table 4).
Effects on flu virus-infected mouse pneumonia model
The following is the known lung index formula:lung index=lung wet weight (g)/body weight (g).By calculating the lung index and inhibition rate of each experimental group,it could be seen that the high and medium dose of Chai Ge fever relief oral liquid could significantly reduce the lung index of flu virus-infected mouse pneumonia model,which was significantly different from the model control group.The inhibition rate was 29.29% and 23.36% in the high-and medium-dose groups,respectively (Table 5).Lung index inhibition rate=(lung weight in virus control group-lung weight in drug group)/(lung weight in viral control group-lung weight in normal control group) ×100%.
Death protection effect on S.aureus-infected mice
Within two weeks after normal mice were infected withS.aureus,the mortality of the model group was 90%,and the average survival time was 2.55 days.The administration of Chai Ge fever relief oral liquid started on the day of the infection for 4 consecutive days,which significantly reduced the animal deaths in all three dose groups.The death protection rate in the high-dose group was significantly different from that in the model control group; all three doses significantly extended the survival days of infected mice,with significant difference compared to the model control group (P< 0.01) (Table 6).

Table 1 Effects of Chai Ge fever relief oral liquid on acetic acid-induced pain in mice

Table 2 Effects of Chai Ge fever relief oral liquid on hot plate-induced pain in mice

Table 3 Effects of Chai Ge fever relief oral liquid on capillary permeability in mice
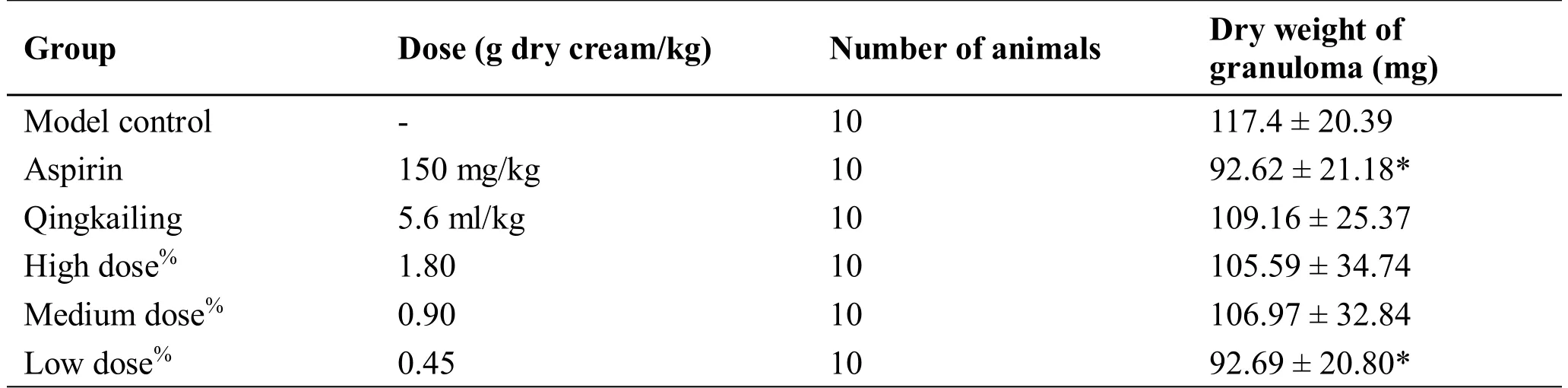
Table 4 Effects of Chai Ge fever relief oral liquid on the formation of cotton ball granuloma in rats

Table 5 Effects of Chai Ge fever relief oral liquid on flu virus-inflected mouse pneumonia model
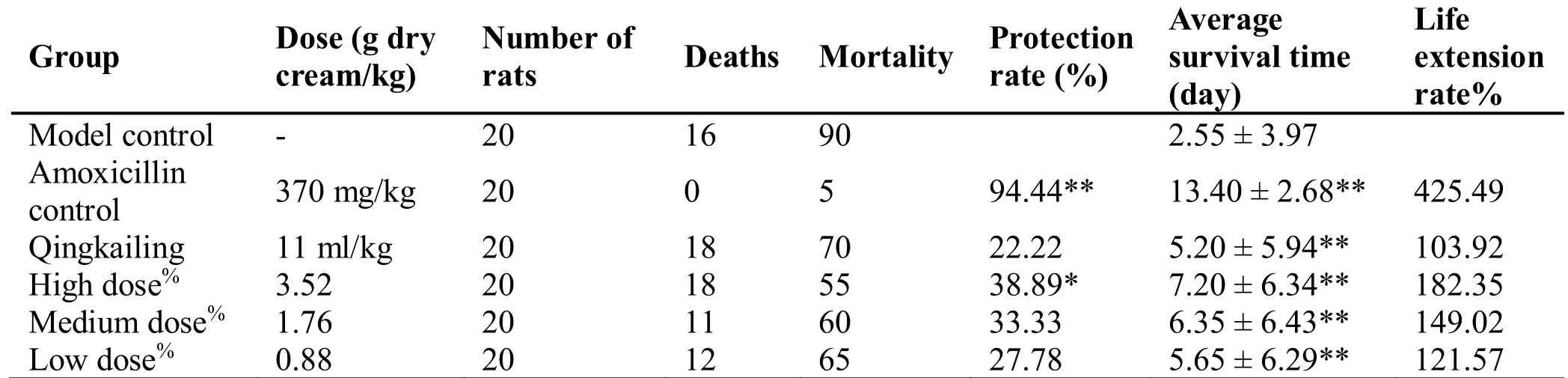
Table 6 Death protection effect of Chai Ge fever relief oral liquid on S.aureus-infected mice
Discussion
Chai Ge fever relief oral liquid is a compound preparation derived from Chai Ge muscle pain relief decoction.It mainly consists ofRadix bupleuri(Chai Hu) andPuerariae lobatae Radix(Ge Gen) as the monarch drugs andRadix scutellariae (Huang Qin),Gypsum fibrosum(Shi Gao),andHerba artemisiae annuae(Qing Hao) as the minister drugs.It belongs to pungent-cool antipyretics and is mainly used to treat the symptoms of exogenous wind-cold with exterior asthenia,stagnation,and heat transformation [6].
Radix bupleuri(Chai Hu) has the effects of soothing liver and relieving stagnation,dispersing and antipyretics,enhancing Yang and elevating Qi [7].Studies have shown that in addition to its antipyretic effect,the herb also has other pharmacological effects such as anti-inflammatory and antiviral [8].It can significantly reduce the lung index of virus-infected mice and thus reduce their mortality rate [9] and can also significantly inhibit the pain response of tested mice [10].Radix puerariae(Ge Gen) is rich in puerarin [11]; hence,it has good anti-inflammatory and analgesic effects [12].These two herbs are the monarch drugs of the compound.In addition,among the minister drugs,Radix scutellariae(Huang Qin) has the pharmacological antipyretic,anti-inflammatory,and antiviral effects [13],andRadix scutellariae(Shi Gao) also has pharmacological antipyretic,muscle pain relief,and antiviral effects [14].Therefore,the combination of drugs of different nature in the decoction can enhance analgesic,anti-inflammatory,antiviral,and antibacterial therapeutic effects of Chai Ge fever relief oral liquid.
To further validate the pharmacological effects of Chai Ge fever relief oral liquid,we designed four similar experimental models in this study.First,in the acetic acid-induced mouse pain model,the high-and medium-dose groups could significantly reduce the number of painful writhing caused by acetic acid in mice and could also augment the threshold of hot place-induced pain in mice,with significant difference compared to the model control group.This confirms that the drug has a significant analgesic effect in both pain models.In the anti-inflammation experiment,the medium dose of the drug significantly inhibited the increase of acetic acid-caused peritoneal capillary permeability in mice,and the low dose significantly reduced the weight of cotton ball granuloma in rats,which were both significantly different from the model group.It is known that all drugs have their upper limits,the dose-effect relationship of traditional Chinese medicines is complicated,and the addition and subtraction of the doses of various medicines in the same decoction can be used to treat different diseases.The results of this study confirmed that the medium and low doses of the drug were effective,while the high dose group showed no obvious effect.The reason for this phenomenon is speculated to be the negative feedback effect caused by the dose-effect relationship of the drugs that leads to competitive inhibition.In addition,in the flu virus-infected mouse pneumonia model,the high and medium doses of the drug can also significantly reduce the lung index of the pneumonia mice compared to the model control group.This validates that the drug can significantly reduce lung inflammation in flu virus-infected mice.Yang et al.[15]found that some compound preparations compatible withRadix bupleuri(Chai Hu) could also inhibit hemolytic S.aureus,Streptococcus,and other bacteria.Therefore,we also designed the experimental model ofS.aureus-infected mice.The results showed that the number of deaths in the three dose groups was significantly reduced,and the death protection rate in the high-dose group was significantly different from that of the model control group.In addition,the survival days of infected mice were prolonged in all three dose groups,with significant differences compared to the model control group.This verifies that Chai Ge fever relief oral liquid can significantly reduce the mortality ofS.aureus-infected mice,can prolong its average survival days,and has a good antibacterial effect.
Second,the positive control drugs selected in this study have been proved in clinical research to have good therapeutic effects.For example,aspirin enteric-coated tablets and Qingkailing oral solution have good analgesic and anti-inflammatory effects[16–17]; ribavirin granules,amoxicillin capsules,and Qingkailing oral solution [18] also have good anti-infective effects.However,many drugs also produce some adverse reactions while they are effective in the treatment process.For example,the aspirin enteric-coated tablets can cause upper abdominal discomfort,nausea,poor appetite,and other degrees of gastrointestinal mucosal damage in patients[19].Although Qingkailing oral liquid is a proprietary Chinese medicine,it can also cause adverse reactions such as allergy and rashes [20].Given the relatively less adverse effects of Chinese medicines,it is therefore important to continuously seek for Chinese medicines that are effective and safe,with minimal or even no adverse reactions.
In summary,the results of the four model groups designed in this study confirmed that,in addition to its antipyretic effect,this drug also has other pharmacological effects such as analgesic,anti-inflammatory,antiviral,and antibacterial.The data also validates to a certain degree that the individual medicinal components in Chai Ge fever relief oral liquid coordinates with each other to jointly achieve good therapeutic effects,which is in line with the holistic concept of traditional Chinese medicine and is of certain scientific significance [21],providing a direction of thinking for future studies.
杂志排行
Drug Combination Therapy的其它文章
- Stability and compatibility of common traditional Chinese medicine injections matching with fructose injection
- Analysis on the compatibility rules and mechanism of formulae treatment for COVID-2019 based on the TCM inheritance assistance system and network pharmacology
- Study on the therapeutic effect of external application of Shounian powder combined with acupuncture at Zhitong acupoint on intercostobrachial nerve syndrome after breast cancer operation
- Mechanism of Qingre Yangyin Chushi Pill in the treatment of gout based on NLRP3/GSDMD pyroptosis pathway
