Stability and compatibility of common traditional Chinese medicine injections matching with fructose injection
2020-06-03ZheTaoZhangXiaoLiuZeYuanWeiChaoLiangTang
Zhe-Tao Zhang,Xiao Liu,Ze-Yuan Wei,Chao-Liang Tang
1Department of Pharmacy,The First Affiliated Hospital of USTC,Division of Life Sciences and Medicine,University of Science and Technology of China,Hefei 230036,Chinese; 2Department of Anesthesiology,The First Affiliated Hospital of USTC,Division of Life Sciences and Medicine,University of Science and Technology of China,Hefei 230001,Chinese.
Abstract Objective: In recent years,China has attached great importance to the work of traditional Chinese medicine (TCM),more and more Chinese patent medicines are included in the national medical insurance catalogue,and the clinical application of TCM injections is also increasing.This article reviews a total of 20 literature reports on the compatibility of fructose injections with TCM injections from 2005 to the present,and evaluates the stability based on the appearance,pH,insoluble particles and content changes after compatibility,and provides for the rational use of fructose injections.Theoretical evidence provides options for special patients with special solvent limit.
Key words:Fructose injection,Chinese medicine injection,Compatibility,Stability
Background
In recent years,China has gradually attached importance to the development of the Chinese medicine industry.In February 2016,the State Council proposed the “Outline of Strategic Planning for traditional Chinese medicine(TCM)Development (2016–2030)”,which clarified the direction and focus of Chinese medicine development in the next 15 years [1].In the “Healthy China 2030” Planning Outline,the Chinese medicine and related industries market is predicted to be close to 5 trillion yuan in 2030 [2].In 2018 and 2019,the“National Essential Medicines List” has also included several TCM varieties.The proportion of use of traditional medicines is also rising among medical institutions.
As a new dosage form,TCM injection is different from TCM in China,and the current preclinical research is relatively limited.The compatibility information in most drug instructions is limited to 0.9% sodium chloride injection or 5% glucose injection,and the compatibility information is insufficient.Under the circumstances,unreasonable compatibility in the clinical medication process will directly lead to adverse drug reactions [3].With the improvement of living standards,the number of patients who have been admitted to hospital due to other diseases and have a history of diabetes is increasing.It is particularly important for the choice of the vehicle used for infusion therapy for these patients [4].Clinically,fructose injection is often used instead of glucose injection as a drug solvent for patients with insulin resistance such as diabetes,impaired glucose tolerance,and stress hyperglycemia.However,the description of fructose injection only describes that the summary should not be compatible with glycine,ampicillin,furan phenylalanine,hydralazine,pyridazine,thiopental,warfarin and other drugs,and lacks compatibility information with TCM injections.
Stability and compatibility
This review represents a collation of reports in the literature from preclinical studies on the stability and compatibility of admixtures containing fructose injection and one TCM injection.Our goal is to provide a resource relevant to the stability and compatibility of commonly used TCM injection delivered in fructose injection (Table 1).
Experiment time range
The time range of investigation should cover the time required for the mixture be dispensed in the popularization of intravenous drug preparation centers (PIVAS) and delivered to the infusion station and infusion time used clinically in patients with normal infusion speed of the liquid droplets 40–60·min-1.According to this calculation,considering the influence of some factors such as drug delivery time,the experiment time range normally set up to 0–4 h.In this review,maximum time range expanded to 0–24 h.
Appearance and characters
The appearance and character of the mixed injection can be observed through the colorimetric tube to observe whether its color,clarity has changed and whether there is precipitation or turbidity.
The color character is the comprehensive expression of the inherent quality of the TCM injection.Real macromolecular substances are an important material basis for the color of TCM injections.That is,after the TCM injection is filtered by molecular sieve (ultrafiltration tube),the color is obviously lighter,the fingerprint pattern after ultrafiltration has no obvious change,and the total peak area is close to the peak area of the original solution [20].
TCM injections are mostly plant extracts,often containing phenol category ingredients.The discoloration experiments of pyrogallic acid and tannic acid suggest that the colored substances produced by TCM injections are mainly macromolecular substances,because the color becomes significantly lighter after ultrafiltration,and the smaller the molecular weight cutoff,the lighter the color.
In this review,most of the TCM mixtures had good clarity,no insoluble foreign bodies were seen with the naked eye,and the appearance was stable.The clarity of the mixed solution of Tanreqing and fructose changed after being left for 180 minutes.In the injection test,the insoluble particles are often checked after the clarity check meets the regulations.
Insoluble particles
Due to the complex composition and different preparation process of medicine injection,some pigments,tannin,starch or protein will be present in the liquid in colloidal form during the extraction and purification process.After the injection is compatible with other drugs,it can undergo oxidation,condensation,hydrolysis and other reactions [21].The number and form of insoluble particles will change to varying degrees.Insoluble particles will not enter the body selectively,which can cause granuloma,pulmonary edema,phlebitis,pyrogen reactions,allergies,etc.,can also be deposited in the liver,spleen and bone marrow to cause harm to the human body.
According to the 2015 edition of theChinese Pharmacopoeia,the volume of intravenous injection with a labeled volume of 100 ml or more,unless otherwise specified,contains 1 μm and more than 10 μm particles per 1 ml.The number of particles of 25 μm and above 25 μm shall not exceed 3 particles; the volume of intravenous injection,sterile powder for intravenous injection,concentrated solution for injection and sterile bulk drug for injection,unless otherwise specified In addition,the number of particles containing 10 μm and more than 10 μm in each sample container shall not exceed 6,000 particles,and the number of particles containing 25 μm and more than 25 μm shall not exceed 600 particles.
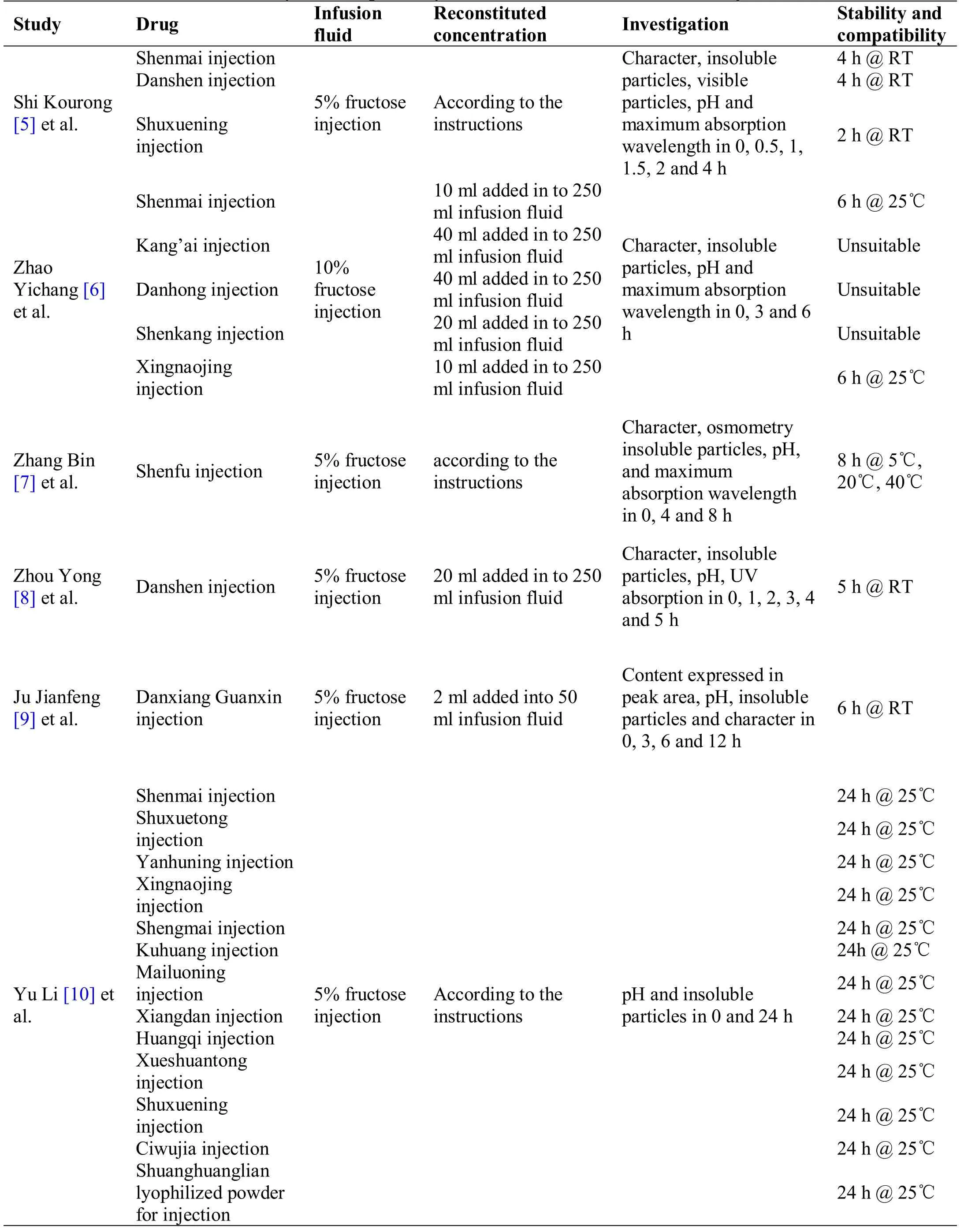
Table 1 Stability and compatibility of individual reconstituted TCM injections

Table 1 Stability and compatibility of individual reconstituted TCM injections (continued)
After the combination of Shenmai,Danshen and Shuxuening,the particles larger than 10μm and 25μm in some samples increased to varying degrees,but not significantly.The combination of Shuxuetong,Reduning and Fructose injection produces more insoluble particles,and Tanreqing compatibility solution will precipitate more particles with the prolonged storage time.After the combination of Danhong injection,Shenkang injection,Kangai injection and 10% fructose injection,the insoluble particles are outside the scope ofChinese Pharmacopoeia.
Some studies show that the number of insoluble particles decreases in varying degrees from 0 to 1 h.The reason for the analysis may be that a large number of bubbles were generated when the compatibility was completed.The tester mistakenly thought that the generated bubbles were particles and counted,resulting in a high-count result [22].
pH
According to the 2015 edition of theChinese Pharmacopoeia,the pH value of intravenous injection should be 5 to 6.The solubility of certain ingredients in TCM is related to the pH of the solution.If the pH is not appropriate,the stability of the injection is likely to decrease and produce precipitation.The active ingredients are alkaloids,organic acids,phenols,glycosides,which are relatively stable under certain pH conditions.If the pH value changes,their solubility also changes.If the pH value is not adjusted properly,the alkaloid is easy to precipitate when the alkalinity of the liquid is strong; otherwise,when the acidity is strong,the acidic components and some glycosides are easy to precipitate.
After the combination of Shenmai,Danshen and Shuxuening with fructose injection,the pH fluctuations were all small,and the pH value was the most stable within 0–2 h.After the combination of Shengmai,Reduning,Fufang Kushen,Danhong with fructose injection,the pH fluctuated at 4–8 h.After the combination of Shuxuetong and fructose injection,the average pH change range was relatively large.Different studies have found that the compatibility of the same TCM injection and fructose solution is different.This may be due to the different manufacturers and the different medicinal materials and auxiliary materials [23].
Discussion
Diabetes patients in our country are increasing and often accompanied by other diseases and complications.Therefore,the use of Chinese medicine injections in diabetic patients is increasingly widespread.Due to the salting-out effect,sodium chloride injection will greatly increase the probability of adverse reactions in TCM injections.
As an isomer of glucose,fructose is directly metabolized to fructose 1,6-diphosphate in the liver by fructokinase and hexokinase and bypasses the rate-limiting enzyme for direct glycolysis.The activity of fructose kinase does not depend on insulin regulation.It can be metabolized into glycogen without insulin,and unmetabolized drugs are excreted by the kidney as a prototype.The pH range of fructose injection is 3.0 to 5.5,which is similar to that of glucose injection.Therefore,compared with the solution of glucose injection combined with insulin,in theory,diabetics can choose to have higher safety and more blood sugar stable fructose injection as a vehicle [24].
However,most of the instructions for TCM injections lack the compatibility information of fructose injection as a solvent.Although various reasons such as administration methods,dosages,and individual differences among patients will affect the safety of TCM injections,the use of TCM injections and infusions is influential.An important reason is for its safety.Studies have shown that the adverse reactions caused by combined medication account for about 50% of the adverse reactions of TCM injections [25].Therefore,the compatibility of fructose injection as the solvent of TCM injections is stable.The inspection is an important link to ensure the safety of diabetic patients using Chinese medicine injections.
In recent years,many medical institutions,government departments,pharmaceutical companies,and higher medical schools have conducted studies on the stability of Chinese medicine injection related compatibility.The main indicators investigated include:solution appearance,pH,insoluble particles,ultraviolet spectrum,and maximum absorption wavelength,and the content of main ingredients.The setting range of the inspection time also refers to the actual clinical situation,covering the time taken by the static dispensing center to dispense the pharmaceutical solution and deliver it to the infusion station and the patient’s infusion.
However,there are still some problems in this type of research at this stage.For example,most of the infusion stability studies are only for one batch of a manufacturer.The data is insufficiently represented,the composition of TCM is complex,and different manufacturers or different batches of the same manufacturer,Because of the inconsistency of the origin of the medicinal materials or the harvest season,the impurities of the injections are different,which will affect the compatibility of TCM injections[26].The stability of the TCM solution after the experiment was tested at room temperature,and the solution was prepared according to the conventional dosage.The acceleration conditions and the lowest dosage have not been investigated [27].Chinese medicine the composition of the injection is complex,and some components may undergo oxidation,condensation,hydrolysis and other reactions with time.This may be one of the important reasons for its clinical adverse reactions.The current experimental conditions cannot detect the stability of all components.
With the PIVAS,pharmacists have managed the infusion safety of patients more fully and conveniently than ever before.This also requires PIVAS pharmacists to strengthen their professional knowledge and business level in the daily work process.Compatibility taboos should be rejected,and the relevant staff should be informed; for drugs that have no clear compatibility taboos,they should also pay close attention to the abnormalities that may occur in the preparation process and clinical application stage,and promptly handle and analyze them to reduce infusion risk,improve the safety of medication.
杂志排行
Drug Combination Therapy的其它文章
- The analgesic,anti-inflammatory,and anti-infection effects of Chai Ge fever relief oral liquid
- Mechanism of Qingre Yangyin Chushi Pill in the treatment of gout based on NLRP3/GSDMD pyroptosis pathway
- Study on the therapeutic effect of external application of Shounian powder combined with acupuncture at Zhitong acupoint on intercostobrachial nerve syndrome after breast cancer operation
- Analysis on the compatibility rules and mechanism of formulae treatment for COVID-2019 based on the TCM inheritance assistance system and network pharmacology
