rmhTNF-α联合PD-1单抗治疗小鼠Lewis肺癌的研究
2020-05-25朱辽辽张存徐盈李晓菊高源韩俊张英起
朱辽辽 张存 徐盈 李晓菊 高源 韩俊 张英起


[摘要] 目的 研究重組改构人肿瘤坏死因子-α(rmhTNF-α)联合PD-1单抗(anti-PD-1)对小鼠Lewis肺癌的治疗效果及作用机制。 方法 24只6~8周龄C57BL/6雌鼠,依据随机数字表法分为4组,每组6只:生理盐水组、rmhTNF-α组、anti-PD-1单抗组、联合给药组。游标卡尺测瘤体并用活体成像观测。16 d后安乐处死小鼠,剥离肿瘤称重。观察小鼠生存期。免疫组化检测肿瘤CD8+T细胞数目。 结果 与生理盐水组比较,rmhTNF-α组、anti-PD-1组、联合给药组肿瘤体积、瘤重均减小,差异有高度统计学意义(P < 0.01);rmhTNF-α组肿瘤体积大于联合给药组,差异有统计学意义(P < 0.05);而联合给药组与anti-PD-1组肿瘤体积差异无统计学意义(P > 0.05);联合给药组瘤重分别与rmhTNF-α、anti-PD-1组比较,差异均无统计学意义(均P > 0.05)。与生理盐水组比较,rmhTNF-α组、anti-PD-1组、联合给药组生存期增加,差异有统计学意义(P < 0.05或P < 0.01),而联合给药组生存期分别与rmhTNF-α组、anti-PD-1组比较,差异均无统计学意义(均P > 0.05)。rmhTNF-α组与生理盐水组比较,肿瘤CD8+T细胞数目增多(P < 0.05);anti-PD-1组、联合给药组与生理盐水组比较,肿瘤CD8+T细胞数目显著增加(P < 0.01);联合给药组肿瘤CD8+T细胞数目少于rmhTNF-α组(P < 0.01),而与anti-PD-1组比较,肿瘤CD8+T细胞数目差异无统计学意义(P > 0.05)。 结论 rmhTNF-α具有抑制小鼠肺癌的作用,但不具有增强PD-1抗体治疗肺癌的作用。
[关键词] 重组改构人肿瘤坏死因子-α;PD-1单抗;CD8+T细胞;Lewis肺癌
[中图分类号] R730.2 [文献标识码] A [文章编号] 1673-7210(2020)04(a)-0004-04
Study of rmhTNF-α combined with anti-PD-1 in the treatment of Lewis lung cancer in mice
ZHU Liaoliao ZHANG Cun XU Ying LI Xiaoju GAO Yuan HAN Jun ZHANG Yingqi
Biopharmaceutical Teaching and Research Office, Department of Pharmacy, Air Force Medical University, Shaanxi Province, Xi′an 710032, China
[Abstract] Objective To study the therapeutic effect and mechanism of recombinant mutated human tumor necrosis factor-α (rmhTNF-α) combined with PD-1 monoclonal antibody (anti-PD-1) in Lewis lung cancer in mice. Methods A total of 24 female C57BL/6 mice aged 6 to 8 weeks were divided into 4 groups according to the random number table method, with 6 mice in each group: normal saline group, rmhTNF-group, anti-PD-1 group, and combined administration group. The tumor was measured with vernier caliper and observed by in vivo imaging. After 16 days, the mice were euthanized and the tumor was removed and weighed. The survival period of mice were observed. Immunohistochemical was used to detect tumor CD8+T cells. Results Compared with the normal saline group, the tumor volume and tumor weight in the rmhTNF-α group, anti-PD-1 group and the combined administration group all decreased, with highly statistically significant differences (P < 0.01). The tumor volume of rmhTNF-α group was larger than that of the combined administration group, and the difference was statistically significant (P < 0.05). However, there was no significant difference in tumor volume between the combined administration group and the anti-PD-1 group (P > 0.05). There was no statistically significant difference in tumor weight between the combined administration group and the rmhTNF-α group and anti-PD-1 group (all P > 0.05). Compared with the normal saline group, the survival of rmhTNF-α group, anti-PD-1 group and the combined administration group increased, and the differences were statistically significant (P < 0.05 or P < 0.01), while the survival of the combined administration group was not statistically significant compared with the rmhTNF-α group and anti-PD-1 group (all P > 0.05). Compared with the normal saline group, the number of CD8+T cells increased in the rmhTNF-α group (P < 0.05). Compared with the normal saline group, the number of CD8+T cells increased significantly in the anti-PD-1 group and combined administration group (P < 0.01). The number of CD8+T cells in the combined administration group was less than that in the rmhTNF-α group (P < 0.01), while the number of CD8+T cells was not statistically significant compared with that in the anti-PD-1 group (P > 0.05). Conclusion RmhTNF-α can inhibit lung cancer in mice, but it can not enhance the effect of anti-PD-1 to treat lung cancer.
[Key words] Recombinant mutated human tumor necrosis factor-α; PD-1 monoclonal antibody; CD8+T cell; Lewis lung cancer
肿瘤坏死因子-α(TNF-α)由巨噬细胞产生,介导内毒素诱导的肿瘤坏死[1],在免疫中发挥关键作用[2]。TNF-α可以改变血管通透性,使抗肿瘤药物更易到达肿瘤组织周围[3]。免疫检查点抑制剂PD-1单抗(anti-PD-1)治疗晚期肺癌有效率为20%,与细胞因子的联合治疗成为新策略[4-5]。因此,本研究利用优化的重组改构人肿瘤坏死因子-α(recombinant mutated human tumor necrosis factor-α,rmhTNF-α)联合anti-PD-1治疗小鼠Lewis肺癌,探索rmhTNF-α是否具有提高anti-PD-1治疗效果的作用及作用机制。
1 材料与方法
1.1 实验材料
rmhTNF-α(上海赛达生物药业有限公司,批号:SC20180401);anti-PD-1(西安壮志生物科技有限公司,批号:BE0146);Luciferase慢病毒(上海吉凯基因化学技术有限公司,批号:LPK001);Lewis肺癌细胞(中国科学院上海细胞库,目录号:TCM7);小鼠CD8分子免疫组化试剂盒(北京中杉金桥有限公司,批号:SP9000)。
1.2 实验动物
24只C57BL/6雌鼠,6~8周龄,体重(18±2)g,购自空军军医大学动物中心,生产许可证号:SCXK(军)2017-0021。饲养和实验遵循实验动物护理及使用指南[6]。
1.3 分组与给药
luciferase慢病毒转染Lewis肺癌细胞,皮下接種2×106。由随机数字表法将小鼠分为4组,每组6只:生理盐水组、rmhTNF-α组、anti-PD-1组、联合给药组。rmhTNF-α依据前期实验的有效剂量[7]。rmhTNF-α肌肉注射10 000 U/只,anti-PD-1腹腔注射125 μg/只,3 d/次,共4次。抑瘤率(%)=(1-给药组平均瘤重/对照组平均瘤重)×100%。肿瘤体积(mm3)=长径×短径2/2。
1.4 免疫组化
参考侯志超等[8]步骤:标本石蜡包埋切片后脱蜡水化,抗原修复,室温冷却,H2O2封闭,一抗孵育过夜,磷酸盐缓冲溶液(PBS)冲洗,孵育二抗,PBS冲洗,滴加链霉卵白素,PBS冲洗,DAB显色,苏木精复染,分化,脱水,透明,中性树脂封片。应用Image-Pro Plus 6.0软件处理数据。
1.5 统计学方法
用SPSS 25.0软件进行娄据分析,计量资料以均数±标准差(x±s)表示,两组比较采用LSD-t检验,四组比较采用One-way ANOVA分析,生存期用log-rank分析,以P < 0.05为差异有统计学意义。
2 结果
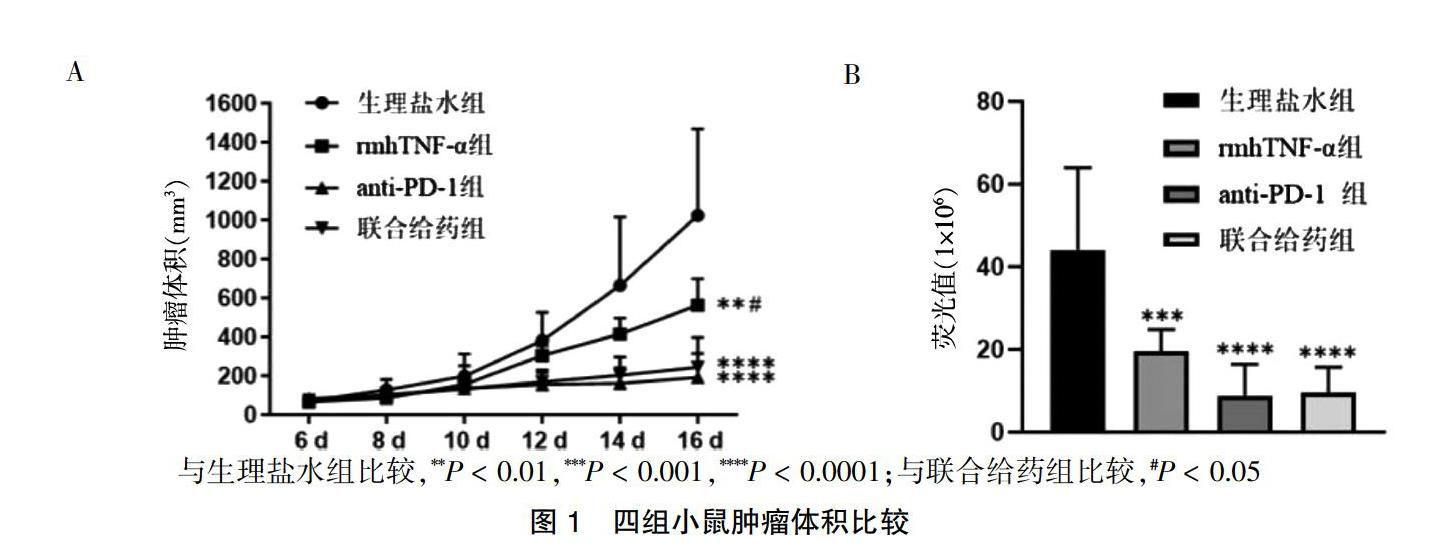
2.1 四组小鼠肿瘤体积比较
四组肿瘤体积比较,差异有高度统计学意义(F = 13.824,P < 0.01)。与生理盐水组比较,rmhTNF-α组、anti-PD-1组、联合给药组肿瘤体积减小,差异有高度统计学意义(P < 0.01);rmhTNF-α组肿瘤体积大于联合给药组,差异有统计学意义(P < 0.05);而联合给药组与anti-PD-1组肿瘤体积差异无统计学意义(P > 0.05)。四组活体成像平均荧光值比较,差异有高度统计学意义(F = 12.548,P < 0.01)。rmhTNF-α组平均荧光值低于生理盐水组(P < 0.01),anti-PD-1组、联合给药组平均荧光值显著低于生理盐水组(P < 0.01),联合给药组平均荧光值与rmhTNF-α组、anti-PD-1组比较,差异无统计学意义(P > 0.05)。见图1。
2.2 四组小鼠肺癌抑瘤率比较
四组瘤重比较,差异有高度统计学意义(F = 9.903,P < 0.01)。与生理盐水组比较,rmhTNF-α组、anti-PD-1组及联合给药组瘤重均显著减轻(P < 0.01),且anti-PD-1组有1只小鼠肿瘤消失。联合给药组瘤重分别与rmhTNF-α组、anti-PD-1组比较,差异均无统计学意义(均P > 0.05)。见图2。抑瘤率比较见表1。
2.3 四组小鼠生存期比较
与生理盐水组比较,rmhTNF-α组生存期增加,差异有统计学意义(P < 0.05),anti-PD-1组、联合给药组生存期也增加,差异有高度统计学意义(P < 0.01),而联合给药组生存期分别与rmhTNF-α组、anti-PD-1组比较,差异均无统计学意义(均P > 0.05)。见图3。
与生理盐水组比较,*P < 0.05,**P < 0.01
图3 四组小鼠生存曲线(n = 6)
2.4 四组小鼠肿瘤CD8+T细胞数目比较
四组小鼠肿瘤CD8+T细胞数目比较,差异有高度统计学意义(F = 25.587,P < 0.01)。rmhTNF-α组与生理盐水组比较,肿瘤CD8+T细胞数目增加(P < 0.05);anti-PD-1组、联合给药组与生理盐水组比较,肿瘤CD8+T细胞数目显著增加(P < 0.01);联合给药组肿瘤CD8+T细胞数目少于rmhTNF-α组(P < 0.01),而与anti-PD-1组比较,肿瘤CD8+T细胞数目差异无统计学意义(P > 0.05)。见图4。
3 讨论
TNF-α调节免疫系统[9],rmhTNF-α有高活性和低毒性[10]。临床结果显示,癌症患者对rmhTNF-α联合化疗的响应率高于单独化疗(P < 0.05)[11-12]。Siurala等[13]研究结果显示mTNF-α显著增强过继性T细胞的抗瘤效应。Elia等[14]发现低剂量TNF能增强免疫检查点阻断剂联合过继细胞的治疗效果。近年来,免疫检查点阻断剂的研究成为热点。Gettinger等[15]研究显示纳武单抗治疗晚期肺癌,患者5年总生存率仅为16%。Wrangle等[16]的实验发现纳武单抗能阻止肿瘤细胞免疫逃逸。Asano等[17]报道低剂量白细胞介素-2联合PD-1阻断剂具有抗肿瘤免疫的协同作用。
本研究中rmhTNF-α不具有促进anti-PD-1抑制肺癌的作用。据文献报道[18-19],TNF可增强肿瘤细胞PD-L1表达,诱发免疫抑制,这可能抵消了TNF-α对免疫系统的激活作用,导致与anti-PD-1无协同抑瘤作用。Perez等[20]也发现,TNF抑制剂联合CTLA-4和PD-1抗体可提高抗肿瘤效果。
综上,rmhTNF-α与anti-PD-1无协同抑瘤作用。这也提示细胞因子在体内作用的复杂性。免疫治疗方法的联合,应用在不同肿瘤、不同动物模型,可能作用都是不一致的。本研究对未来肺癌免疫治疗方式的研究奠定基础。
[参考文献]
[1] Locksley RM,Killeen N,Lenardo MJ. The TNF and TNF receptor superfamilies:integrating mammalian biology [J]. Cell,2001,104(4):487-501.
[2] Van Horssen R,Ten Hagen TL,Eggermont AM. TNF-alpha in cancer treatment:molecular insights,antitumor effects,and clinical utility [J]. Oncologist,2006,11(4):397-408.
[3] Folli S,Pèlegrin A,Chalandon Y,et al. Tumor necrosis fector can enhance radio-antibody uptake in human colon carcinoma xenografts by increasing vascular permeability [J]. Int Cancer,1993,53(5): 829-836.
[4] Garris CS,Arlauckas SP,Kohler RH,et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-γ and IL-12 [J]. Immunity,2018,49(6):1148-1161.e7.
[5] Van den Bergh JMJ,Smits ELJM,Berneman ZN,et al. Monocyte-derived dendritic cells with silenced PD-1 ligands and transpresenting interleukin-15 stimulate strong tumor-reactive T-cell expansion [J]. Cancer Immunol Res,2017,5(8):710-715.
[6] Liu Y,Zhang Y,Zheng X,et al. Galantamine improves cognition,hippocampal inflammation,and synaptic plasticity impairments induced by lipopolysaccharide in mice [J]. J Neuroinflammation,2018,15(1):112-116.
[7] Yan Z,Zhao N,Wang Z,et al. A mutated human tumor necrosis factor-alpha improves the therapeutic index in vitro and in vivo [J]. Cytotherapy,2006,8(4): 415-423.
[8] 侯志超,伊力亞尔·夏合丁.实体肿瘤组织石蜡切片的免疫组化实验方法总结[J].世界最新医学信息文摘,2017, 17(41): 56.
[9] Ruegg C,Yimaz A,Bieler G,et al. Evidence for the involvement of endothelial cell integrin αVβ3 in the disruption of the tumor vasculature induced by TNF and IFN-γ [J]. Nat Med,1998,4(4):408-414.
[10] Li M,Qin X,Xue X,et al. Safety evaluation and pharmacokinetics of a novel human tumor necrosis factor-alpha exhibited a higher antitumor activity and a lower systemic toxicity [J]. Anticancer Drugs,2010,21(3):243-251.
[11] Li M,Xu T,Zhang Z,et al. Phase Ⅱ multicenter,randomized,double-blind study of recombinant mutated human tumor necrosis factor-a in combination with che-motherapies in cancer patients [J]. Cancer Sci,2012, 103(2):288-295.
[12] Ma X,Song Y,Zhang K,et al. Recombinant mutated human TNF in combination with chemotherapy for stage ⅢB/Ⅳ non-small cell lung cancer:a randomized,phase Ⅲ study [J]. Sci Rep,2015,4:9918.
[13] Siurala M,Havunen R,Saha D,et al. Adenoviral delivery of tumor necrosis factor-α and interleukin-2 enables successful adoptive cell therapy of immunosuppressive melanoma [J]. Mol Ther,2016,24(8):1435-1443.
[14] Elia AR,Grioni M,Basso V,et al. Targeting tumor vasculature with TNF leads effector T cells to the tumor and enhances therapeutic efficacy of immune checkpoint blockers in combination with adoptive cell therapy [J]. Clin Cancer Res,2018,24(9): 2171-2181.
[15] Gettinger S,Horn L,Jackman D,et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer:results from the CA209-003 study [J]. J Clin Oncol,2018,36(17):1675-1684.
[16] Wrangle JM,Velcheti V,Patel MR,et al. ALT-803,an IL-15 superagonist,in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised,open-label,phase 1b trial [J]. Lancet Oncol,2018,19(5): 694-704.
[17] Asano T,Meguri Y,Yoshioka T,et al. PD-1 modulates regulatory T-cell homeostasis during low-dose interleukin-2 therapy [J]. Blood,2017,129(15): 2186-2197.
[18] Lim SO,Li CW,Xia W,et al. Deubiquitination and stabilization of PD-L1 by CSN5 [J]. Cancer Cell,2016,30(6):925-939.
[19] Bertrand F,Montfort A,Marcheteau E,et al. TNF-α blockade overcomes resistance to anti-PD-1 in experimental melanoma [J]. Nat Commun,2017,8(1): 2256.
[20] Perez-Ruiz E,Minute L,Otano I,et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy [J]. Nature,2019,569(7756):428-432.
(收稿日期:2019-12-04 本文編辑:刘永巧)
