Response of leaf photosynthetic characteristics of Syringa oblata and Syringa reticulata var. mandshurica to chilling stress
2020-05-22XiaojiaLiuBaiyiAnNaGuCainanGuoXiaogangSunHeWang
Xiaojia Liu · Baiyi An · Na Gu · Cainan Guo · Xiaogang Sun ·He Wang
Abstract Syringa species not only have good ornamental properties but also play an important role in the landscaping and environmental purification of cities. To investigate the chilling stress resistance of Syringa oblata Lindl. and Syringa reticulata var. mandshurica and provide theoretical grounds for the practical cultivation of Syringa species, in vitro leaves were used to study photosynthetic gas exchange parameters and chlorophyll fluorescence parameters. After nine hours of chilling, decreasing rates of net photosynthesis, stomatal conductance, and transpiration in S. reticulata var. mandshurica leaves were significantly greater than that of the S. oblata, while intercellular CO2 concentrations in S. oblata leaves were higher than those in S. reticulata var. mandshurica. The quantum yield of PSII reaction center (ΦPSII) declined in S. reticulata and light capture efficiency () was stable. However, reduction percentages of , ΦPSII, and Fv/Fm in S. oblata were significant higher than those of S. reticulata var. mandshurica. After nine hours of chilling, the relative variable fluorescence of VJ and VI of S. oblata increased and the increasing rate of VJ was greater than VI. In contrast, the change of VJ and VI in S. reticulata var. mandshurica leaves was relatively small. This suggests that chilling primarily damaged the electron transport process of QA to QB at the receptor site of the PSII reaction center. Photosynthetic capacity of S.oblata was more sensitive to chilling stress compared to S. reticulate var. mandshurica,which the limitations were mainly due to non-stomatal factors such as the decrease in electron transport efficiency,activity in the PSII reaction center, and the destruction of the photodamage defense system.
Keywords Chilling stress · Chlorophyll fluorescence characteristics · Photosynthetic capacity · Syringa oblate ·Syringa reticulata var. mandshurica
Introduction
Cold resistance by plants refers to the ability to endure and resist chilling stress, and is closely related with cell membranes, enzyme protection systems, and osmotic regulators (Omran et al. 1971; Lyons 1973). For plants under chilling stress, a series of physiological and biochemical reactions occur to eliminate or reduce damage (Allen and Ort 2001; Ashraf and Harris 2013). Cold resistance of plants is determined by detection of key indicators of the physiological and biochemical reactions (Russell et al.1992; Oguchi et al. 2009). It is more practical to evaluate cold resistance by combining detection of physiological indicators and plant cultivation. Changing global climate,particularly large temperature fluctuation, has been accompanied by persistent extreme weather events.According to China’s National Meteorological Administration, in the past five years, the frequency of a temperature drop of 10 °C in the spring in northeast of China has increased more than 20 times. Agricultural crops and vegetation in cities can be seriously damaged by freezing rain caused by drastic temperature changes in early spring.Therefore, it is important to study physiological responses of greening vegetation to chilling stress.
Previous studies have reported that chilling stress causes closure of stomata, and the increase in stomatal resistance of CO2diffusion results in decreased photosynthesis and inhibition of photosynthate transportation which leads to the premature decline of leaves (Satoh and Fork 1982; Shu et al. 2011). Photosynthesis is the foundation for plants to acquire materials and energy. About 95% of plant dry matter comes directly from photosynthesis (Silvaa et al.2010). Chilling stress could damage photosynthetic apparatus predominantly photosystem II (PSII). As the photochemical reaction center, PSII includes over 20 core protein subgroups such as chlorophyll a and b light-harvesting antenna complexes (LHC), oxygen-evolving complexes (OEC), and D1 proteins, which involves a series of photosynthetic processes, including the absorption of light energy, water photolysis, and electron transport (Allahverdiyeva et al. 2013; Chen et al. 2017; Zhang et al.2017). Under chilling stress conditions, PSII electron transport may decline, severe stress resulted in photoinhibition even photo-oxidation or photobleaching (Haldimann and Strasser 1999; Sharma et al. 2015). Chlorophyll fluorescence kinetics, a non-destructive probe, plays an important role in studying light energy absorption, electron transport, and light energy utilization under stress (Ushimaru et al. 1995; Müller et al. 2001; Zhang et al.2012a,b, 2016; Mathur et al. 2014). Under chilling stress,the inhibition of chlorophyll synthesis limits light energy absorption; stomata closure causes the decrease of CO2supply (Malik et al. 2001; Ashraf and Arfan 2005; Falk and Munné-Bosch 2010; Duan et al. 2012), and photosynthate transport slows down (Ushimaru et al. 1995; Foyer and Shigeoka 2011). Photosynthetic enzyme activity and the PSII reaction center is decreased (Du et al. 2010; Cazzaniga et al. 2012) and photosynthetic carbon assimilation is significantly reduced (Ahmed et al. 2002; Johnson 2011).In recent years, the physiological response of different plants to chilling has been intensively studied worldwide(Guo et al. 2007; Li and Ma 2012; Ravi et al. 2013; Nichols et al. 2015). A number of studies have evaluated the physiological processes and status of plants using indicators such as net photosynthetic rates, water use efficiency,and PSII photochemical efficiency (Gombos et al. 2010;Chen et al. 2015; Gameiro et al. 2016). Therefore, it is important to study plant photosynthetic efficiency under chilling stress based on photosynthetic physiological processes in order to screen for species that can tolerate chilling or to improve plant resistance to chilling.
With continued climate changes, extreme weather events will occur more frequently, especially persistent chilling and freezing in northern China (Gosling and Arnell 2016). Chilling could cause major decreases in grain production in cities. Therefore, it is urgent to consider problems caused by early spring chilling. Syringa oblata Lindl.,a common species for urban planting, has many advantages, including rapid growth, beautiful form, easy of cultivation and management, and high resistance to a variety of stresses. However, S. Oblata is vulnerable to chilling stress. There are few studies of the response of S.Oblata and Syringa reticulata var. Mandshurica, another potential urban species to chilling stress. In this study,leaves of the two Syringa species were used to compare morphological features, gas exchange and chlorophyll fluorescence parameters under chilling stress. We investigated whether the species with greater resistance to chilling would maintain higher photosynthetic ability, and if chilling caused damage or inhibition such as stomatal and nonstomatal limitation to photosynthesis and/or damage to the donor and acceptor sides of PSII. Clarifying the issues will increase understanding of the mechanism of chilling resistance in Syringa.
Materials and methods
The experiment was conducted at the Horticulture College of Jilin Agricultural University, Jilin Province in July 2016.Testing materials were one-year-old S. oblata and S.reticulata var. mandshurica seedlings provided by the Forest Botanical Garden of Jilin. Seedlings were transplanted into 20-cm high plastic pots, cultured with peat moss, irrigated and weeded regularly. Chilling treatment was applied in August, a period when the plants of both species were at a vigorous growing stage. A 4-cm cutting ring was used to take leaf fragments from each plant. The fragments were floated in a 4 °C water bath and the relevant parameters measured at 3, 6, and 9 h after chilling.
Determination of gas exchange parameters
In each treatment of the two species, the second last fully expanded leaves on new branches were collected. Net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and intercellular CO2concentration (Ci)was measured by using a Li-6400 photosynthesis system(Licor Corporation, US) under the photon flux density of 1000 μmol m-2s-1, and CO2concentration of 390-410 μl L-1. Water use efficiency (WUE) was calculated according to WUE = Pn/Tr. Each treatment had 10 replicates. Relative water content (RWC) of a leaf was calculated as: RWC = (FW - DW)/FW × 100%. FW and DW mean fresh weight and dry weight, respectively.
Determination of chlorophyll fluorescence parameters
Top fully expanded leaves of both species were dark adapted for 30 min. PSII maximum photochemical efficiency (Fv/Fm), initial fluorescence (Fo), actual photochemical efficiency of PSII (ΦPSII), and the light capture efficiency of the PSII reaction center under light adaptation(Fv′/Fm′) were measured as described by Hu et al. (2007).The measurement for each treatment was repeated five times.
Determination of a fast-phase chlorophyll fluorescence OJIP curve
Fast chlorophyll fluorescence induction kinetics (OJIP fluorescence induction curves) and 820 nm light reflection curves were measured by M-PEA continuous excitation fluorescence instrument (Handy, UK). The last fully expanded leaf was dark adapted for 30 min before testing.The four characteristic points: O, J, I and P on the OJIP curve corresponded to time points of 0, 2, 30, and 1000 ms;the corresponding relative fluorescence intensities were expressed as FO, FJ, FI, and Fm. The OJIP curve was analyzed using the JIP-test to obtain maximum photochemical efficiency of the PSII (Fv/Fm), photosynthetic performance index based on the absorbed light energy(PIABS), ratio of absorbed energy used for electron transfer after QA-(φEo), maximum quantum yield of non-photochemical quenching (φDo), absorbed light energy per unit reaction center (ABS/RC), absorbed light energy used for reduction of QAper unit reaction center (TRo/RC), absorbed light energy used for electron transfer per unit reaction center (ETo/RC), and dissipated energy per unit reaction center (DIo/RC). The calculation followed the methods described by Strasser et al. (1995).
Data analysis
Excel (2007) and SPSS (22.0) software were used for statistical analyses. One-way analysis of variance(ANOVA) and the Tukey test were used to compare the results of the two species. Differences were considered significant if p ≤0.05 and very significant if p ≤0.01.
Results
Gas exchange parameters
Under normal growth conditions, Pn, Gs, and Trof S. oblata(Hereafter abbreviated as SO) were lower than those of S.reticulata var. mandshurica (Hereafter abbreviated as SR)while its Ciwas significantly higher than that of SR. With increased duration of chilling, Pn, Gs, and Trvalues of two species displayed a significant decreasing trend, while their Civalue increased over time (Fig. 1). The decrease of Pn,Gs, and Trin the SO leaves was greater than leaves of SR.After 9 h of chilling, the values of Pn, Gs, and Trin the SO leaves were lower than SR leaves by 57.4% (p <0.01),68.7% (p <0.01), and 67.3% (p <0.01), respectively, but its Civalue was higher by 40.1% (p <0.01).
Photochemical efficiency
Fv/Fmof the two Syringa species showed a declining trend with increased duration of stress, however, decreased rate of Fv/Fmwas greater in SO leaves than SR leaves (Fig. 2).Fv/Fm, Fv′/Fm′, and ΦPSIIof SO leaves declined with longer chilling periods, and the decrease rates of SO leaves were greater than SR leaves. After 9 h of chilling, the increasing rate of Foin SO leaves (14.4%) was higher than SR leaves (12.6%), however, the decreasing rate of Fv/Fmin SO leaves was significantly higher than in SR leaves.
Fast chlorophyll fluorescence induction kinetic curve
With increased duration of chilling, the OJIP curve of the two Syringa species changed. The fluorescence intensity of points J (2 ms), I (30 ms), and P (1000 ms) on the OJIP curve significantly declined over time (Fig. 3). The decreasing rate of relative fluorescence intensity reached the greatest at point P. With the increased duration of chilling, the decreasing rate increased; the decreasing rate of SO was greater than SR.
Effects of chilling on ABS/RC, TRo/RC, ETo/RC,and DIo/RC
During 9 h of chilling, ABS/RC of the two Syringa species showed a decreasing trend, and between 3 and 9 h, the ABS/RC of SO leaves was significantly higher than that of SR leaves. TRo/RC decreased under chilling for both species. Throughout the treatment, there was no significant difference in TRo/RC of the two species; however, DIo/RC showed an increasing trend with prolonged chilling(Fig. 4b, d), and between 3 and 9 h of treatment, ETo/RC of SR leaves became significantly higher than that of SO leaves. After 9 h of chilling, ETo/RC of SR leaves was higher by 39.3% than SO leaves (p <0.01), and DIo/RC was lower by 24.2% than that of SO leaves (p <0.01).
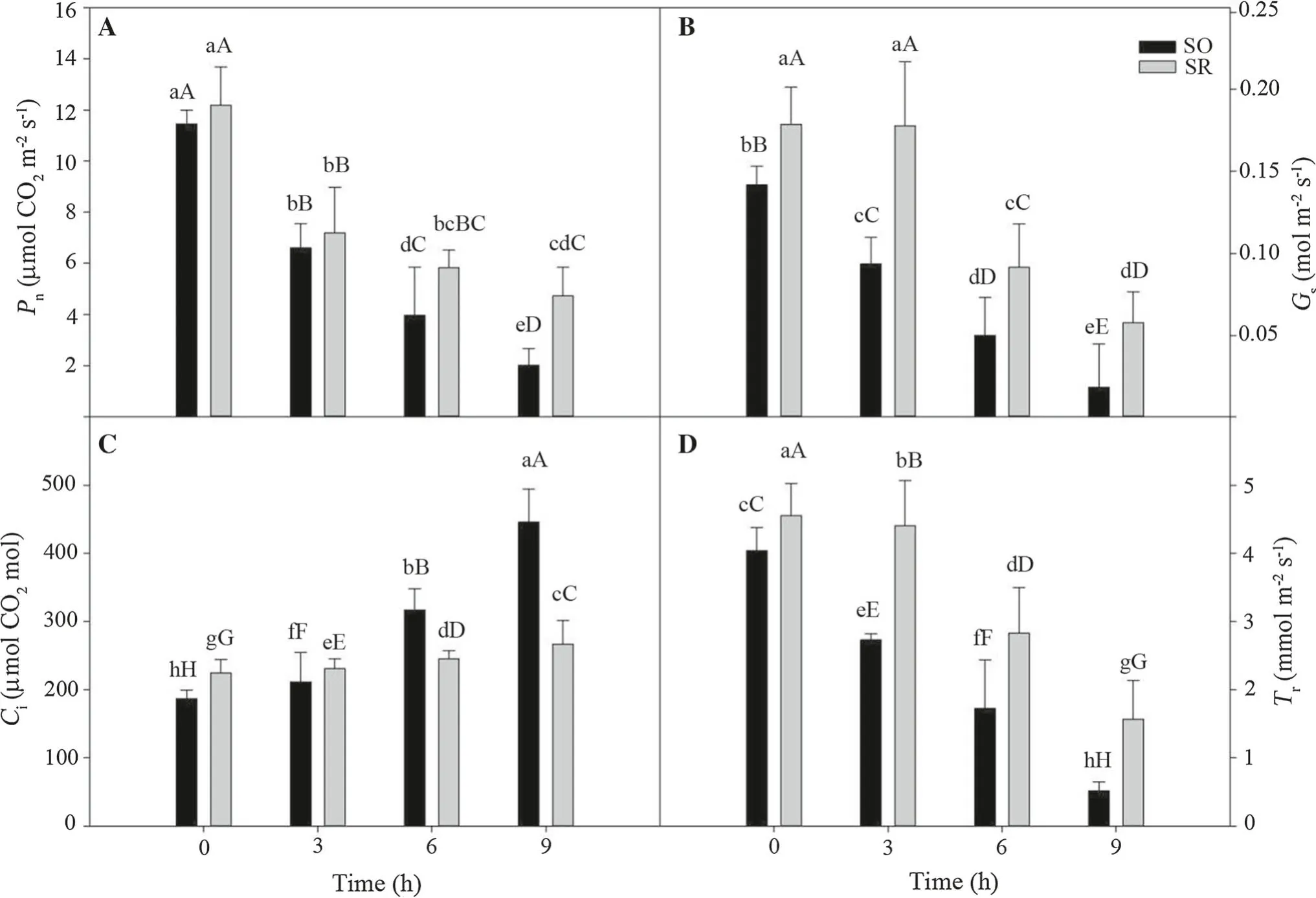
Fig. 1 Gas exchange parameters in leaves of S. oblata (SO) and S.d intercellular CO2 concentration (Ci). Data are mean ± SE; lowerreticulate var. mandshurica (SR) under chilling stress. a net photo-case and uppercase letters indicate significant differences at p <0.05 synthesis (Pn); b stomatal conductance (Gs); c transpiration rate (Tr);and p <0.01, respectively
JIP-test radar plot
The chlorophyll fluorescence parameters of the two species after 1 h of chilling were defined as 1. After 9 h of chilling,the Fo, FJ, FI, and Fmof both species were lower than 1 and displayed a declining trend; however, the Fo, FJ, FI, and Fmof SR leaves were higher than SO leaves. VJ, VI, and Fv/Foof SR leaves were all close to 1, which indicated that there were no differences after 1 h of treatment. However, after 9 h of chilling, VJand VIof SO leaves were 1.36 and 1.11,respectively, and Fv/Fowas 0.72. The PIABSof SR leaves decreased by 22.8% compared to that after 1 h of chilling,while that of SO leaves declined by 78.7%, higher than that of SR. The Ψo, φEo, and φDoof SR leaves after 9 h did not change significantly, nevertheless the Ψoand φEoof SO leaves decreased by 25.4% and 52.1%, respectively, and its φDoincreased by 113.5% (Fig. 5).
Discussion
Photosynthesis is one of more sensitive processes to chilling. Under chilling conditions, stomata of SO leaves gradually shut down and CO2supply was reduced with the decline of stomatal conductance, namely, the limitation of photosynthetic stomatal factor. Under severe stress, a decline of photosynthetic capacity was the result of synergistic limitations of stomatal and non-stomatal factors and the decline was often dominated by non-stomatal factors (Huppe and Turpin 1994; Gong et al. 2013). In this study, stomatal conductance and transpiration rates of the two Syringa species decreased after 3 h of chilling.Decreasing Pn, Gs, and Trvalues in SO leaves were greater than those of SR, indicating that the gas exchange parameters of SO leaves were more sensitive to chilling stress than that of SR.

Fig. 2 Effects of chilling stress on Fo,Fv/Fm, , and ΦPSII.a PSII maximum photochemical efficiency (Fv/Fm); b the initial fluorescence (Fo); c the actual photochemical efficiency of PSII(ΦPSII); d the light capture efficiency of PSII reaction center under light adaptation (); SO: S. oblata; SR: S. reticulata var.mandshurica. Data are mean ± SE values; lower-case letters denote significant differences (p <0.05), and upper-case letters larger differences (p <0.01)
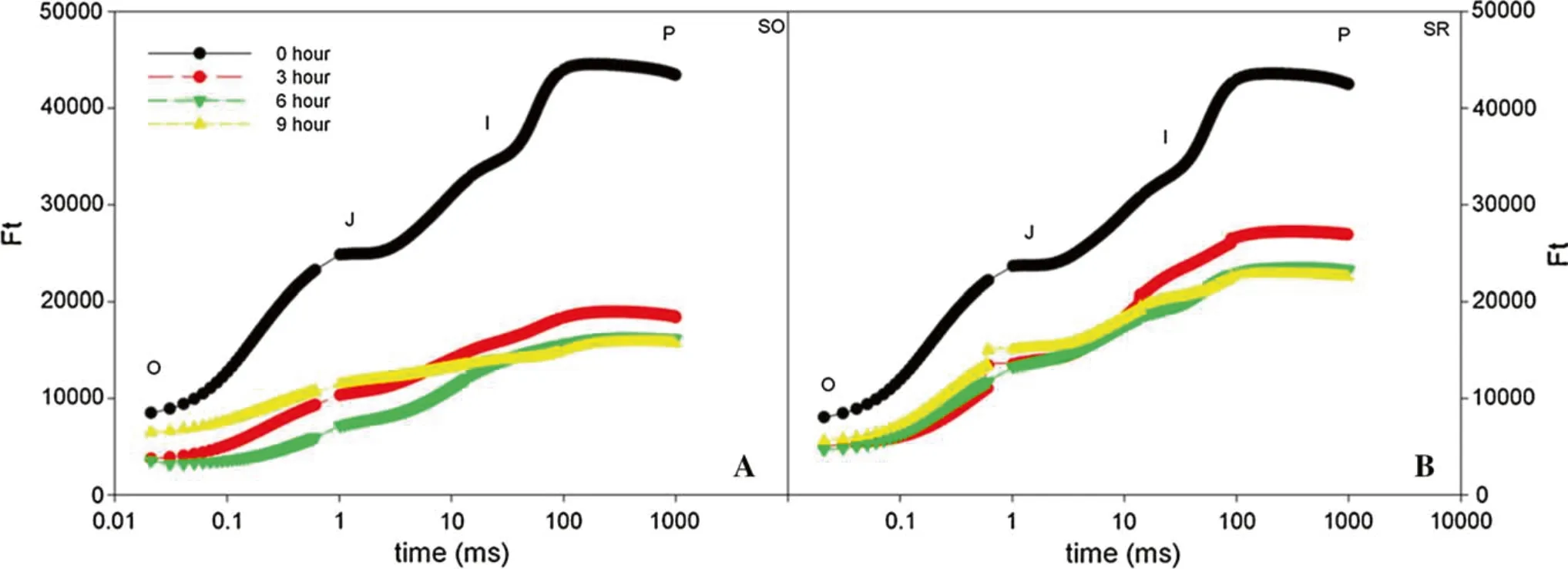
Fig. 3 Fast chlorophyll fluorescence induction curve in leaves under chilling stress. Ft: Relative fluorescence intensity. a S. oblate., b S.reticulata var. mandshurica
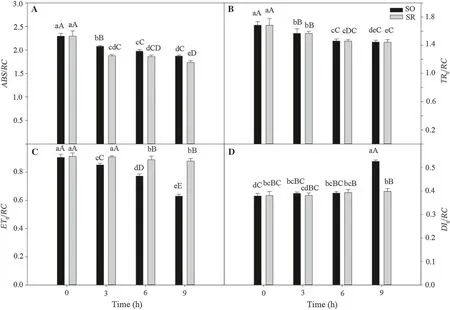
Fig. 4 Effects of chilling stress on ABS/RC, TRo/RC, ETo/RC, and DIo/RC.. a absorption flux per reaction centers (ABS/RC); b trapped energy flux per reaction centers (t = 0) (TRo/RC); c electron transport flux per reaction centers (t = 0) (ETo/RC); d dissipated energy flux per RC (at t = 0) (DIo/RC); SO: S. oblata; SR: S. reticulata var.mandshurica. Data in the figure are mean ± SE values; different lower-case letters denote significant differences (p <0.05), and upper-case letters denote larger differences (p <0.01)
Several studies have demonstrated that under adverse conditions, most damage sites of the photosynthetic apparatus were located in the PSII, Therefore, keeping high photochemical activity of PSII is critical to a strong cold resistance of plants. Chilling stress not only damages cell membranes but also inhibits photosynthetic carbon assimilation and further aggravates photoinhibition. Currently,the damage of chilling to PSII is considered an important factor for inhibiting photosynthesis (Zhang et al. 2012a,b Sharma et al. 2015). Chilling can influence physiological and biochemical processes such as photosynthetic electron transport and photophosphorylation, and may cause degradation or damage to the PSII reaction center. Chilling significantly increased Foin leaves but reduced Fv/Fmand ΦPSIIand the primary light energy conversion efficiency and electron transport efficiency in PSII. The stress also inhibited the primary processes of photosynthesis, reduced the transformation of carbon assimilation and the accumulation of organic matter, and led to the damage of the photosynthetic apparatus, which resulted in the decrease of photosynthetic capacity (Jahnke et al. 1991; Govindjee 1995; Wu et al. 2012). In this study, with the increased duration of chilling, Fv/Fmof the two Syringa species showed a decreasing trend and the decreasing rate of Fv/Fmin SO leaves was greater than SR. High light intensity is an important factor resulting in photoinhibition under stress(Jung et al. 1998; Wang et al. 2001; Takahashi et al. 2017).Under persistent chilling,of SR leaves did not change significantly. However,and ΦPSIIof (SO)leaves decreased and Forose. Under consistent chilling, the changing rates ofand ΦPSIIin SO leaves were greater than that of SR leaves, which indicates that the decrease of photosynthetic carbon assimilation capacity in the two species was induced by persistent chilling related to the decrease of photosynthetic PSII functions. The main processes included the decline of photochemical activity in the PSII reaction center and the inhibition of electron transport.

Fig. 5 JIP-test radar plot of two Syringa species under chilling stress SO: S. oblata; SR: S. reticulata var. mandshurica
Changes of light energy distribution parameters in PSII reaction center was greater in SO leaves than in SR leaves,especially the ΦPSIIof SO leaves decreased by 21.9%,while SO leaves only by 6.0%. This indicates that SR leaves can maintain sufficient supply of absorbed light energy for photochemical reactions under persistent chilling stress. In addition, PIABScan reflect not only light energy captured by the PSII reaction center but also the transport capacity of photosynthetic electrons between the two photosystems (Sun et al. 2008; Huang et al. 2010).Therefore, PIABSwas more representative parameter than Fv/Fmin reflecting photochemical activity (Strauss et al.2006; Salmela et al. 2011). In this study, after 9 h of chilling treatment, Fv/Fmand PIABSof SR leaves were both significantly higher than that of SO leaves. This indicated that photochemical activity of PSII in SR leaves was more cold tolerant. To maintain the normal physiological functions in PSII, energy absorption and distribution were the most crucial factors. During chilling, the ABS/RC of the two species showed a decreasing trend, which might be related to the degradation or inactivation of the reaction center under prolonged chilling. With increased duration of chilling, the charge separation capacity and electron transport capacity of PSII decreased, leading to the decline of TRo/RC and ETo/RC and an increase of DIo/RC. The decrease of φEoand increase of φDoin SO leaves were greater than that of SR leaves, indicating that under chilling, the proportion of absorbed light energy in the unit reaction center declined while the proportion of light energy dissipated as heat increased. Excessive heat dissipation is competitive with energy in the electron transport chain and results in a lack of energy. It causes a decrease in the accumulation of assimilation capacity of ATP, NADPH and photosynthetic carbon, and consequently, a decline in cold tolerance. Therefore, excessive heat dissipation indicates that, under chilling stress, the light energy utilization capacity of the PSII reaction center in SO leaves was lower than in SR leaves. The energy flow parameters in the reaction center reflect light energy absorption and utilization by plants and also indirectly reflect the activity and amount of reaction centers (Taranishi et al. 1974; Spichalla and Desborogh 1990). However, the proportion of light energy used for photosynthetic electron transport in SR leaves was greater than that in SO leaves under chilling stress, and supplies relatively more energy to maintain its cold tolerance.
The activity difference in PSII reaction center of the two Syringa species under chilling stress was analyzed using the fast-phase chlorophyll fluorescence kinetic technique.Our study determined that, under normal conditions, the relative fluorescence intensity at each point of the OJIP curve in SO leaves was lower than in SR leaves, which might be related to the existence of large amounts of anthocyanins in SO leaves. The absorption spectrum of anthocyanin is similar to the absorption spectrum of photosynthetic pigment and can regulate light absorption by photosynthetic pigment of leaves. On the other hand,anthocyanins have strong antioxidant capacity and can quench oxygen free radicals. Under chilling stress, the OJIP curve of SR leaves did not change significantly, while the Ftat each point of SO leaves declined significantly compared to controls. After 9 h of chilling, the Ftat each point on the OJIP curves was lower than that at 0 h (start).However, since the variability of the original OJIP curve was greatly affected by external factors (Qu et al. 2012),the OJIP curves were standardized. The relative fluorescence intensity at point O of all OJIP curves was defined as zero and point P defined as 1, which determined that all the OJIP curves would have the same O and P points. In this experiment, after 9 h of chilling, the standardized OJIP curve of SR leaves changed only slightly compared with that at 0 h, while the relative variable fluorescence of VJand VIat points J and I, respectively, on the standardized OJIP curve of SO leaves increased, and the rate of increase of VJwas greater than that of VI. Under stress, the accumulation of excess electrons in the photosynthetic electron transport chain is mainly concentrated on the receptor side of PSII, and takes more time for electrons to flow from(primary quinone electron acceptor of PSII) to QB(secondary quinone electron acceptor of PSII) than to transport to QAvia pheophytin (Pheo) to produce.This results in the obstruction of electron transport from QAto QBand the accumulation of. The change of relative fluorescence intensity at point J (2 ms) of the OJIP curve reflect the accumulation of, which indirectly reflects the electron transport capacity from QAto QBat the receptor side of the PSII reaction center (Scandalios 1993;Troll and Lindsey 1955; Strasser et al. 1997; Li et al.2009). After 9 h of chilling, the relative variable fluorescence intensity at 2 ms (VJ) of the OJIP curve of SO leaves increased. At 2 ms, the openness of the active reaction center (Ψo) significantly decreased, indicating that at 9 h of chilling, electrons were transported from QAto QBon the receptor side of PSII in SO leaves, causing an over-reduction of QAand accumulation of. However, after 9 h of chilling, the changes of VJand Ψoin SR leaves were insignificantly, indicating that SR leaves maintained relatively high electron transport efficiencies under persistent chilling. This is primarily because electron transport from QAto QBat the receptor side of PSII was not significantly inhibited.
Some studies suggest that the synthesis of D1 proteins in plants is inhibited and their degradation is accelerated under stress. The electron transport complex QBis mainly combined with the D1 proteins within chloroplasts, and the inhibited synthesis and accelerated degradation of D1 proteins can cause the separation of QBand D1 and may result in the inability to accept electrons (Jiang et al. 2006;Nadia et al. 2006). In this study, decreasing electron transport efficiency from QAto QBat the receptor side of PSII in SO leaves might be related to inhibited synthesis or accelerated degradation of D1 proteins within chloroplasts.However, the D1 proteins of SR leaves were less affected by chilling. This warrants further study. In addition, large amounts of reactive oxygen species (ROS) were produced in photosynthesis under adverse conditions, a product of an increase of excess excitation energy of PSII. The energy exists in the electron transport chain as electrons, and the ROS increases when the chain is blocked, resulting in oxidative damage to proteins (Asada 2006; Kreslavski et al.2007). In this study, SR leaves under chilling stress had relatively high electron transport efficiency, which is beneficial to reduce the production of ROS in photosynthesis and maintain normal physiological functioning.
Conclusion
The photosynthetic capacity of S. oblata leaves was more sensitive to chilling stress than leaves of S. reticulata var.mandshurica; the limitations on photosynthesis were more likely due to non-stomatal factors such as a decrease in electron transport efficiency and activity in the PSII reaction center, a disorder in light energy distribution, and/or the destruction of the photodamage defense system. The inhibition of electron transport in S. oblata leaves was mainly due to the resistance of electron transport from QAto QBon the PSII receptor side. Consequently, S. reticulata is a better choice for planting in cold areas of northern China.
杂志排行
Journal of Forestry Research的其它文章
- Past, present and future of industrial plantation forestry and implication on future timber harvesting technology
- Effects of climate changes on distribution of Eremanthus erythropappus and E. incanus (Asteraceae) in Brazil
- Effects of climate and forest age on the ecosystem carbon exchange of afforestation
- Effect of gap size and forest type on mineral nitrogen forms under different soil properties
- Effect of forest thinning on hydrologic nitrate exports from a Nsaturated plantation
- Floristic analysis and dominance pattern of sal (Shorea robusta)forests in Ranchi, Jharkhand, eastern India
