Decreased morphogenetic potential in peach palm stem-like cells in long-term in vitro conditions
2020-05-22rikaMendesGranerGilvanoEblingBrondaniCristinaVieiradeAlmeidaKatherineDerleneBataginPiottoMarcliodeAlmeida
é rika Mendes Graner · Gilvano Ebling Brondani · Cristina Vieira de Almeida ·Katherine Derlene Batagin-Piotto · Marcílio de Almeida
Abstract Peach palm (Bactris gasipaes Kunth) has been micropropagated from pre-procambial cells that provide stem-like cell niches, (i.e., pre-procambial cells), multipotent, pluripotent and totipotent for direct vascularization,adventitious buds and somatic embryogenesis, respectively. The direct induction of adventitious buds and somatic embryogenesis reduces the frequency of mutations when compared to indirect morphogenesis. Long-term in vitro cultivation of perennial species such as peach palm cause the clones to age and deteriorate; however, the consequences for morphogenesis potential are not fully clear. The morphogenic potential of peach palm clones established and in vitro cultivated for 8 years (regeneration of adventitious buds without callus formation) was investigated in leaves, roots and stem bases using histological and histochemical analyses. Data from long-term cultures(8-years-old) was compared to data from short-term cultures (1-year-old). Morphogenic pathways monitoring for direct induction of somatic embryos and adventitious buds revealed a strong morphogenic reduction potential in the pre-procambial cells, parenchyma cells in the proximal region of stem bases, and external cells of leaf sheaths.Initial cells of shoot apical meristems and pre-procambial cells commit cell reprogramming to the undifferentiated state and subsequent acquisition of cellular competence.These results are applicable in the micropropagation of peach palm, with consideration to obtaining clones and their long-term in vitro culture.
Keywords Micropropagation · Multipotency ·Pluripotency · Totipotency · Pre-procambial cells · Peach palm · Bactris gasipaes
Introduction
Micropropagation is an asexual reproduction technique widely used for large-scale propagation of commercial plant species (Akin-Idowu et al. 2009), for superior and disease-free genotypes (Giles and Morgan 1987), and to preserve genetic material in gene banks (Lynch et al.2011). The technique is based on cellular totipotency, the ability of cells to differentiate into any type of cell(Almeida et al. 2015), and in the understanding that morphogenesis is essential to whole plant regeneration (Phillips 2004). Induction, acquisition of competence,determination and cellular differentiation of the organs and tissues of a multicellular organism (Almeida et al. 2015) is the process of morphogenesis (Taylor 1997). A signals network of transcription and plant growth regulators(PGRs) controls morphogenesis, allowing for optimization in two main pathways, (1) somatic embryogenesis, and (2)adventitious organogenesis (Phillips 2004; Almeida et al.2012, 2015). However, considering the principles of cloning (i.e., to produce large quantities of plants from a particular lineage), the indirect standard from callus induction for adventitious organogenesis and embryogenesis should be avoided due to higher somaclonal variation (Bordallo et al. 2004). Furthermore, the consequences of long-term in vitro cultivation on the totipotentiality in cells are still not completely elucidated. The effects of long-term cultivation of species by indirect and direct standards was reported as an inducer of genetic instability in callus culture (Kaeppler et al. 2000), to changes in morphogenic potential (Konan et al. 2010), unfavourable to rooting(Sharma et al. 2007), and to the acclimatization process(Konan et al. 2010). These events have likely been reported as a consequence of clone senescence (Konan et al. 2010;Graner et al. 2015), tissue aging (Valledor et al. 2007;Graner et al. 2015), and the habituation of tissues by PGRs(Akin-Idowu et al. 2009).
Micropropagation is widely used with various species of palms, such as Phoenix dactylifera L. (Al-Khayri and Naik 2017), Areca catechu L. (Karun et al. 2004), Euterpe oleracea Mart. (Ledo et al. 2002) and Elaies guineensis Jack (Konan et al. 2010). This tissue culture technique has also been used for Bactris gasipaes (Almeida and Kerbauy 1996; Almeida and Almeida 2006; Steinmacher et al.2007a, b, 2011; Graner 2009; Almeida et al. 2012; Graner et al. 2013, 2015), but few studies have reported on the regeneration through organogenesis and/or somatic embryogenesis by the direct morphogenic standard(Almeida and Kerbauy 1996; Almeida and Almeida 2006;Graner 2009; Almeida et al. 2012; Graner et al.2013, 2015), and with the simultaneous occurrence of both morphogenic events (Graner 2009; Almeida et al. 2012;Graner et al. 2015). The pre-procambial cells (PPCs) of B. gasipaes act like stem cells (i.e., target cells) to the application of NAA and BAP, providing niches for multipotent, pluripotent and totipotent stem-like cells for vascular differentiation, organogenesis and somatic embryogenesis (Almeida et al. 2012). However, long-term in vitro propagation of B. gasipaes can lead the PPCs of clones to the loss of morphogenetic potential for propagule induction (Graner et al. 2015).
Therefore, to identify and monitor the morphogenic reduction in stem cell niches such as PPCs, histological and histochemical analyses were performed in stem bases containing apical meristems of 1 year and 8-year-old B.gasipaes clones.
Materials and methods
Plant material
Seeds from Uruc¸uca (Bahia State, Brazil, Inaceres Company) and from plants cultivated in the experimental area at the Vegetable Production Department of ESALQ/USP in Piracicaba, Sa~o Paulo State, were used for the establishment and in vitro maintenance of long- and short-term cultures. Old and young clones from in vitro germination of excised embryos were established in 2002 and in 2010 and cultivated in vitro for 8 and 1 years, respectively (Figs. 1,2a, b). Samples from the stem base containing the apical meristem were collected from the long-term cultures(Fig. 2a). These were developed from adventitious buds induced by direct organogenesis, (i.e., without callus development), in clones cultured on a medium supplemented periodically with PGRs during the 8 years of culture (Figs. 1, 2a). The same sampling was performed on plants of the short- and long-term cultures, established and maintained in vitro for 1 year (Figs. 1, 2b). In both cultures in this study, roots and branches developed (Fig. 2).
Culture medium and culture conditions
The culture medium consisted of Murashige and Skoog(1962) MS salts supplemented with sucrose (30 g L-1),myoinositol (100 mg L-1) and thiamine (5 ml L-1), at pH 5.8 (Fig. 1). The long-term cultures were maintained for 8 years (Fig. 2a). These plants were subcultured every 3 months, for a total of four subcultures per year over 8 years. Among the subcultures, alterations included MS medium (without PGRs) and MS plus 12.9 μM α-naphthaleneacetic acid (NAA) and 3.55 μM 6-benzylaminopurine (BAP). For each subculture, the medium was renewed every 30 days to maintain freshness and quality(Fig. 1). Subcultures on free MS medium encouraged the full development of the root system in clones from longterm cultures (8-years-old), as well as the maintenance of the induction of adventitious buds (i.e., roots and shoots)by habituation to plant growth regulators (Meins 1989).Subsequently, these complete clones of long-term cultures were again exposed to the same PGRs at the same concentrations for maintaining the adventitious buds by alternating the culture medium with and without PGRs until 8 years of culture, during which time samples were collected.
Short-term cultures were established and cultivated in vitro for 1 year under the same conditions and medium as the long-term cultures (8-years-old) (Figs. 1, 2b), alternating culture medium with and without PGRs every3 months and renewing freshness and quality of the culture medium every 30 days for 1 year.

Fig. 1 In vitro culture and analyses of stem bases flowchart from peach palm(Bactris gasipaes). Adapted from Graner et al. (2015)
Test tubes containing 10 ml of culture medium were utilized. The medium was prepared with deionized water and 3.5 g L-1agar. The pH was adjusted to 5.8 with 0.1 N HCl and 0.1 N NaOH before adding the agar to the culture medium, and the mixture autoclaved at 121 °C (≈1.0 kgf cm-2) for 20 min. The cultures were maintained in a growth room at 25 ± 2 °C under a light intensity of 42 μmol m-2s-1for a 16 h photoperiod.
Histological analyses

Fig. 2 Stem bases (bounded by dashed lines) of peach palm (Bactris gasipaes) used to obtain histological and histochemical samples.a Plants of long-term in vitro cultures and b plants of short-term in vitro cultures. Bar = 0.5 cm
Longitudinal histological sections were made on stem bases containing the shoot apex in ten old peach palm clones (long-term cultures) and ten young peach palm clones (short-term cultures) (Figs. 1, 2a, b). The sections were fixed in a glutaraldehyde/formaldehyde solution(Karnovsky 1965) and dehydrated through a graded alcohol series to 100% (v v-1). Finally, the samples were embedded in hydroxyethyl methacrylate resin (Leica, Heidelberg,Germany), in accordance with the manufacturer’s recommendations and were cut using a rotary microtome into 5 μm longitudinal sections. The sections were stained in 0.05% (v v-1) toluidine blue in phosphate-buffered saline and citric acid (Sakai 1973) and mounted on histological slides using synthetic resin. The histological sections were analyzed and photomicrographed under a light microscope equipped with a Samsung camera (SDC-313 Series).
Histochemical analyses
Tissue samples from the peach palm clones were subjected to specific histochemical tests with periodic acid-Schiff stain and naphthol blue black (Fisher 1968) as described by Almeida et al. (2012) to verify the presence of polyphenols,proteins and polysaccharides during morphogenesis.Polysaccharides in the cell walls, cytoplasm and amyloplasts were identified by their pink colour, while phenolic compounds were orange from the periodic acid-Schiff stain. Proteins were stained blue by naphthol blue black.The sections were photomicrographed under a light microscope equipped with a Samsung camera (SDC-313 Series).
Results and discussion
In this study, histological analysis of long-term in vitro cultivation identified exclusive competence for pre-procambial cells (PPCs) for differentiation in procambial strands and thereafter in vascular bundles in the proximal region with the absence of a meristematic centre inductors of somatic embryos, and the development of only one adventitious bud (Fig. 3a-e).
In the distal region, the central stem cells or corpus derived from inner layers of the tunica (Dickison 2000) of apical meristems of short-term cultures showed a higher nuclear-cytoplasmic ratio when compared to cells of the same tissue in long-term cultures (Fig. 3c, h). In this section, the PPCs of the short-term-cultures and long-term cultures were from cells originating from the shoot apical meristem, here defined as the PPCs initial cells (Fig. 3b, dg, j). They showed an isodiametric form with slender cell walls and high nuclear-cytoplasmic ratios (Almeida et al.2012, 2015) (Fig. 3d, i). The process of predominantly periclinal cell division of these young PPCs cultures originated as meristemoids (Fig. 3g, j, k) that presented larger cells with lower nuclear-cytoplasmic ratios surrounding these meristemoids (Fig. 3j, k). These meristems were observed in the unipolarization meristematic centres with only one meristematic pole, establishing the meristematic pole of the stem apex (Fig. 3i) and simultaneously establishing poles of shoots and roots (bipolarized meristematic centres) (Fig. 3g, j, k). This is characteristic of the direct processes (i.e., without callus induction), adventitious organogenesis and somatic embryogenesis with multicellular origins (Almeida et al. 2012). The authors (also studying Bactris gasipaes) observed that the combination of NAA (α-naphthaleneacetic acid) and BAP (6-benzylaminopurine) added to the MS medium developed PPCs from shoot apices from 10-month in vitro cultures to act as target cells in three morphogenic routes: (1) multipotent,forming vascular bundles, (2) pluripotent, forming adventitious buds (i.e., adventitious organogenesis), and (3)totipotent cells, developing embryos (i.e., somatic embryogenesis). These results were also observed in this study for the short-term cultures (Fig. 3f-k), since NAA and BAP induced PPCs through the same morphogenic routes, acting as multipotent cells that develop vascular bundles (Fig. 3a, b), as pluripotent cells developing adventitious buds (Fig. 3i), and finally as totipotent cells developing somatic embryos, highlighting the multicellular origin, due to the frequent incidence of traces of meristematic centres PPCs with two poles (bipolarized PPCs)(Fig. 3g, j, k).
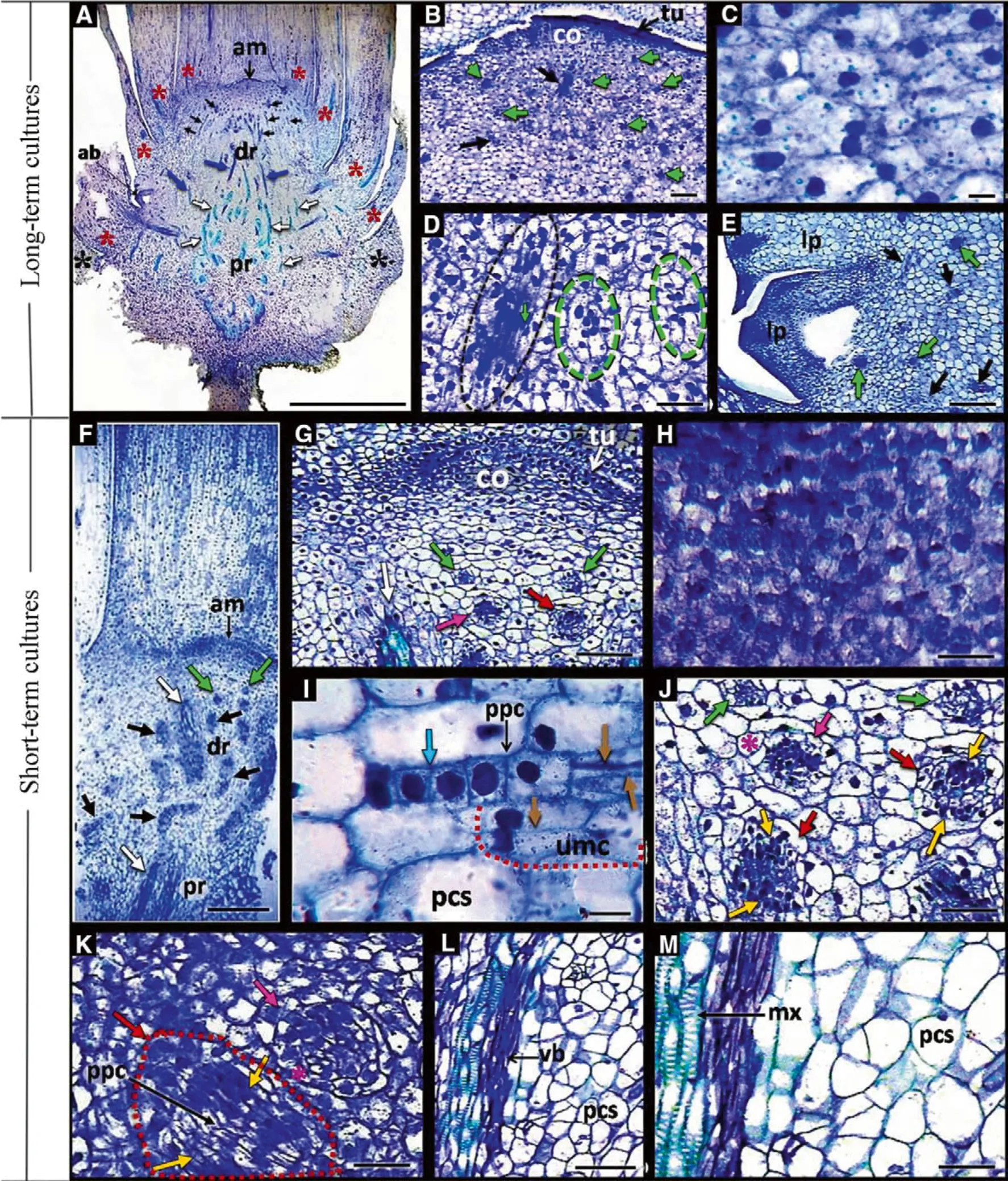
◀Fig. 3 Longitudinal sections of stem bases from the long-term and short-term cultures stained with toluidine blue. a Overview of the stem base over long-term cultivation showing the PPCs and traces of procambial cells in the distal region and traces of procambial cells during differentiation into vascular bundles in the proximal region.Highlight the development of adventitious. b General overview of the meristematic region of the shoot apex in the distal region in plants over long-term cultivation. c Detail of the shoot apical meristem containing the central stem cells (corpus) in the plants of the longterm cultures. d Detail of initial pre-procambial cells and PPCs in the distal region of the plants of old cultures. e Detail of the adventitious bud observed in a. f Overview of the stem base over short-term cultivation, exhibiting the PPCs traces and procambial cells differentiating conductive xylem elements. g Detail of the stem apical meristem region in the distal region of the stem base of plants over short-term cultivation. h Shoot apical meristem containing the central stem cells (corpus) in the plants of the short-term cultures, present high nuclear/cytoplasmic ratio. i Adventitious organogenesis in plants over short-term cultures. j, k Details of morphogenic events evidenced in f, and g highlighting the development of somatic embryos in the globular stage. l, m Proximal region of the short-term cultures with traces of procambial cells originating the elements of vascularization. am apical meristem, tu tunica, co corpus, ab adventitious buds, dr distal region; pr proximal region, lp leaf primordium, pc procambial cells, ppc pre-procambial cells, umc and red dashed line meristematic centre unipolarized, vb vascular bundle,mx metaxylem, pcs parenchyma cells, white arrows vascular bundles,green arrows and dashed line initial pre-procambial cells, black arrows and dashed line PPCs, light blue arrow, anticlinal division,brown arrows periclinal divisions, pink arrows meristemoids, red arrows bipolarization of meristemoids, yellow arrows shoot and root poles, red asterisks inner leave primodium, pink asterisks outer cell layers of the meristems, black asterisks external leave primodium.Bar: A = 2 mm; B, E, G, L, N, P, Q, R, S and T = 100 μm; C, H and I = 10 μm; F = 0.25 mm; D, J, K and M = 50 μm
The PPCs also originated traces of procambium cells which differentiated into vascular bundles in the proximal region of the stem bases of long-term cultures (Fig. 3a) and short-term cultures (Fig. 3f, l, m). The formation of vascular bundles in the distal region was a common observation (Fig. 3f, g), indicating that PGRs, particularly NAA,stimulated periclinal divisions of PPCs in the regions of procambial differentiation and establishment of vascular elements, as suggested by Almeida et al. (2012). Vascular cells usually differentiate at a predictable time and position to form a specific vascular model, and the arrangement of this vascular network can be altered by local signals or in response to environmental stimuli (Dettmer et al. 2009;Almeida et al. 2012, 2015). The most important correlative signal is provided by auxin, which coordinates vascular differentiation (Almeida et al. 2012, 2015). Both short and long-term cultures were maintained under the same in vitro conditions (Fig. 1) as used in peach palm shoot apices(Almeida et al. 2012); however, vascular differentiation was not observed in the long-term cultures in the distal region of stem bases (Fig. 3a-d), i.e., cellular competence of PPC differentiation was observed only in the proximal region (Fig. 3a). These observations corroborate results of histochemical tests with periodic acid-Schiff stain and naphthol blue-black in the distal region of stem bases(Fig. 4). PPCs competence differentiate into procambial cells was detected in short-term cultures where the presence of elevated proteins and polysaccharides was found but showed higher elongation than by PPCs (Fig. 4b, c).The PPCs over short-term cultivation provide niches for stem-like cells for vascular differentiation (Fig. 3f, g),optimizing the vascularization system during in vitro establishment, considering that the histochemical analyses showed proteins and phenolic contents inside the cells during xylem differentiation (Fig. 4f, i) (Almeida et al.2012). The same observations were detected in the vascular bundles of the proximal stem bases plants of long-term and short-term cultures (Fig. 5), whereas parenchyma cells in this region showed moderate protein content, and high content of polysaccharides and starch (Fig. 5a, g), indicating these ergastic substances are energy resources during vascular system development (Almeida et al. 2012).
Histochemical tests of stem bases (Figs. 4, 5) identified that the initial cells of the shoot apical meristem of the long-term cultures only showed protein contents, whereas initial cells from the same tissue of short-term cultures showed higher protein levels and the presence of starch and polysaccharides (Fig. 4a, d). This suggests metabolic changes related to the function activity and maintenance of meristematic cells of the long-term cultures, although the cytoplasm density, characterized only by the presence of proteins by naphtol blue-black (Fig. 4a) further indicated the occurrence of RNA synthesis and some metabolic activity (Stein et al. 2010).
Morphogenesis related to organogenesis and/or somatic embryogenesis in the distal stem bases was not found(Figs. 3b, d, 4b, c) when subjected to histochemical treatment. This decrease in the morphogenic potential, particularly of PPCs (Fig. 3a-e), indicates that the maintenance of shoot apical meristems and their ability for cell division and differentiation are committed and results in changes in cellular reprogramming by exposure to PGRs. This may be due to the fact that in woody plants the lifetime is determined by the amplitude of persistent meristems and by maintaining their capacity to divide and differentiate into new shoots over the years (Munné-Bosch 2007). This is related to the complex interactions of external and internal factors that can lead to mutagenesis during mitosis. During in vitro culture, the internal factor is the micro-environment within the culture flask, whereas temperature and luminosity (external factors) are controlled during the whole in vitro maintenance. Therefore, the development of new propagules, adventitious buds and somatic embryos,would be compromised in plants exposed to stimuli from their own in vitro culture conditions, particularly by the exogenous application of PGRs during long-term in vitro cultivation.

Fig. 4 Longitudinal sections of the distal region of stem bases frompolysaccharides. h Bipolarization of meristematic centres (somatic the long-term and short-term cultures, double staining with naphtolembryogenesis) during periclinal divisions. i Unipolarization of blue-black and periodic acid-Schiff. a Shoot apical meristem withmeristematic centres (adventitious buds) during the periclinal division initial cells of long-term cultures showing only protein content;of PPCs. Highlighting the presence of the vascular bundle in cross b High protein content in PPCs, reduced proteins and polysaccharidessection with high phenolic content in xylem conducting cells. ppc prein long-term cultures parenchyma cells; c PPCs in procambial cellsprocambial cells, vb vascular bundle, umc meristematic centre showing high protein and discreet polysaccharide contents; d Shootunipolarizade, red arrows protein, yellow arrows starch, white arrows apical meristem with the initial cells of the plants over short-termpolysaccharides, blue arrows polyphenols found on cellular walls,cultivation showing. e High protein content in PPCs and reducedblack arrows periclinal divisions, red dashed line meristematic protein content and reduced polysaccharides in parenchyma cells.centre, bipolarizade pink arrows initial shoot and root poles, black f Initial stages of PPCs during differentiation in meristematic centre.dashed line meristematic centre unipolarizade, black asterisks Highlighting the adjacent vascular bundle in a longitudinal sec-parenchyma cells, red asterisk outer layer meristemoid cells.tion. g Meristemoid surrounded by cells with a higher content ofBar = 50 μm
The PPCs of the long-term and short-term cultures displayed identical characteristics to the initial cells of the shoot apical meristems. However, the presence of proteins was more intense in PPCs of short-term cultures compared to those of long-term cultures (Fig. 4b, e), indicating higher RNA synthesis and metabolic activities (Stein et al. 2010).This agrees with the observations of Almeida et al. (2012)who detected high protein contents in PPCs. They also reported reduced concentrations of starch and polysaccharides, especially in unpolarized meristematic centres(organogenesis) and bipolarized meristematic centres (somatic embryogenesis); similar results were obtained in this study for these structures and the adjacent parenchyma cells of short-term cultures (Fig. 4h-i).
The presence of starch has been reported during the acquisition of embryogenic competence (Verdeil et al.2007; Rocha et al. 2012). This study identified an elevated presence of polysaccharides and reduced presence of starch in short-term cultures in the initial stages in established meristematic centres (Fig. 4f, g), suggesting that these substances are intensely consumed and mobilized in the initial stages of meristematic centre unipolarization and bipolarization (Almeida et al. 2012). In Picea glauca Moench Voss, the detection of polysaccharides and starch during somatic embryogenesis was possible only in more advanced stages of development (Joy et al. 1991) than observed in this study. The authors suggested that factors that may influence the pattern of macromolecule deposition during embryo development in vitro are the lack of maternal influences, culture conditions and embryo positioning which may alter the pattern of storage product deposition. Generally, starch also provides energy for organogenesis and the supply of osmotic agents in the form of soluble sugars (Lemoine et al. 2013; Fortes and Pais 2000).

Fig. 5 Longitudinal sections of proximal regions of stem bases fromepidermis of the leaf sheath of short-term cultures in cell death.peach palm from plants of the long-term and short-term culturesj Highlighting the process of cell death in the parenchyma cells of the double staining with naphtol blue-black and periodic acid-Schiff.leaf sheath of short-term cultures. k, l Parenchyma cells with a Showing high protein content and sparse amiloplasts in the vascularembryogenic competence and intact pro-embryos originated from bundle cells and parenchyma cells with a high content of protein,asymmetric divisions of the cells adjacent to the abaxial epidermis of starch and polysaccharides in the long-term cultures. b Unviablethe leaf sheath in the plants over short-term cultivation. vb vascular cluster of pro-embryos in the proximal region of the long-termbundle, es external sheath, is internal sheath, n nucleus, i.e.cultures. c Detail of cell and a pro-embryo in cell death process,intercellular space, ade adaxial epidermis, abe abaxial epidermis,highlighting the absence of ergastic substances inside these cells andred arrows protein, yellow arrows starch, green arrows starch the malformed nucleus. d External and internal leaf sheaths of thedegradation, white arrows and orange asterisks = polysaccharides,plants over long-term cultivation. e, f Parenchyma and epidermal cellsblack arrows polyphenols, pink arrows plasmatic membrane retracof the external leaf sheath in cell death and evidencing intensetion, yellow dashed line pro-embryos undergoing the PCD, black deposition of phenols in cell walls. g vascular bundles anddashed line viable pro-embryos, black asterisks parenchyma cells.parenchyma cells in short-term cultures. h External leaf sheath inBar: A, C, E, F, G, I, J, K and L = 50 μm; B and D = 100 μm;old cultures. i Showing parenchyma cells adjacent to the adaxialH = 200 μm
Histochemical analyses also identified pro-embryos originating from asymmetric divisions of sub-epidermal parenchyma cells via dedifferentiation and transdifferentiation and isolated from adjacent cells by polysaccharides(Fig. 5b, c). Nevertheless, the absence of ergastic substances (non-protoplasmic materials), deformed nuclei (i.e.,in degenerative processes) and retraction of the plasma membrane, evidenced cell death and the unviable cluster of all pro-embryos (Fig. 5b, c). Almeida et al. (2012),studying the same species, detected in subepidermal parenchyma cells of adventitious buds a similar morphogenic route in the presence of NAA and TDZ (thidiazuron),optimizing in vitro regeneration of peach palm. However,Almeida et al. (2012) showed, by double staining with naphtol blue-black and periodic acid-Schiff that some proembryos clusters were probably limited and full development was due to physical factors and competition for nutrients. In this study, the subepidermal parenchyma cells in the proximal region of long-term cultures acted as totipotent for the induction of somatic embryos of unicellular origin. However, further pro-embryo development was not possible, likely due to the long-term culture of peach palm clones during in vitro maintenance.
In the leaf sheath tissues located more externally in the proximal region of the stem bases of the long-term cultures, double staining with naphtol blue-black and periodic acid-Schiff identified the occurrence of cell death with severe oxidation and the degeneration of cell walls due to the presence of polyphenols, polysaccharides and the retraction of the plasma membrane (Fig. 5d-f) (Almeida et al. 2012; Graner et al. 2015). In the leaf sheaths of the short-term cultures, parenchyma cells were detected adjacent to the abaxial epidermis, showing embryogenic competence and pro-embryos from asymmetric divisions of these subepidermal cells (Fig. 5h-l). The presence of phenols in pro-embryo cells is directly related to somatic embryogenesis induction (Alemanno et al. 2003; Almeida et al. 2012). Although this was not observed in the cells of pro-embryos in this study, histochemical staining revealed starch contents and polysaccharides in intercellular spaces during this morphogenesis, and this was higher in the adaxial to abaxial surface direction; starch degeneration was observed in adjacent cell layers (Fig. 5h-l). These observations clearly demonstrate that starch is related to the acquisition of embryogenic competence (Verdeil et al.2007; Rocha et al. 2012) and is mobilized and used in the initial stages of somatic embryogenesis.
In subepidermal parenchyma and in centres of the leaf sheaths of short-term cultures, cell death was detected(Fig. 5h-i). This suggests that the presence of large quantities of polysaccharides in the layers adjacent to the embryogenic cells is due to the degeneration of cell walls during cell death (Fig. 5h-k). These together with starch are important energy resources for the induction and development of pro-embryo competence (Verdeil et al.2007; Rocha et al. 2012). Direct somatic embryogenesis,with a multicellular origin from a meristematic centre or polarization from leaf primordia parenchyma cells were reported for peach palm (Almeida and Almeida 2006),demonstrating a high morphogenic potential of the leaf sheaths to the acquisition of embryogenic competence in short-term cultures. Despite that quiescent cells may return to cell division through appropriate treatments (Roche et al.2017), our observations show that the return to cell proliferation of the initial pre-procambial cells and PPCs was not possible in long-term cultures (Figs. 3d, 4a-d). Even with appropriate stimuli such as the exogenous application of NAA and BAP to the medium (Graner 2009; Almeida and Almeida 2006; Almeida et al. 2012; Graner et al.2015), the return to cell proliferation did not occur,although TDZ (thidiazuron) makes possible organogenesis and induction of somatic embryos of unicellular origin(Graner 2009; Almeida et al. 2012; Devi et al. 2014).
Histone modification and DNA methylation are epigenetic mechanisms that have a key role in the regulation of gene silencing and activation, and changes in DNA methylation may occur when plants are exposed to in vitro culture conditions (Valledor et al. 2007; Wang and Bayles 2012; Us-Camas et al. 2014). In unarmed peach palm microplants, TDZ added to the MS medium activated a silenced gene (or a group of genes) (Graner et al. 2013). Therefore, it is likely that the absence of a response by the long-term cultures to PGRs can be attributed to habituation (Akin-Idowu et al. 2009), as NAA and BAP, possibly aggravated by aging or senescence of cells or tissues, particularly of PPCs (Graner et al. 2015), considering that both processes promote the methylation of nuclear DNA (Kim et al. 2010). Furthermore, the parenchyma cells in the leaf sheaths of external leaf primordia are more susceptible to environmental factors (e.g., the micro-environment inside the culture flask), particularly in relation to contact of the leaf sheaths with the medium, which may result in cells being incapable of acting as totipotent in the induction of somatic embryos in long-term cultures. An increase in the endogenous levels of auxins and/or cytokinins may have led to leaf sheath cell death and loss of competence of parenchyma cells to induce somatic embryos (Fig. 5d-f),compared to the results of the one-year-old clones(Fig. 5j-l).
This study provides evidence that the pre-procambial cells or PPCs between the initial cells of the shoot meristem and the area of cell differentiation of procambial cells,in the distal region of the stem base plants of long-term cultures (Figs. 3a, b, d, 4b, c) ceased to provide niches for multipotent, pluripotent and totipotent stem-like cells for direct vascularization, adventitious buds and somatic embryogenesis, respectively, compared with the short-term cultures (1-year-old) (Fig. 3f, g, i-k, 4h, i).
Although the potential for development of adventitious buds has been observed in only one of 10 long-term cultures, the origin was uncertain by the advanced stage of development of the adventitious buds (Fig. 3e). However,the adventitious buds possibly originated from meristematic activity of unipolarized procambial strands. This also shows the reduction of PPC pluripotency due to prolonged in vitro maintenance, considering for the species that the incidence of adventitious buds is elevated in shoot apices in vitro cultured for 8 months with the combination of NAA and BAP that originate from the most inner of the shoot apices PPCs (Almeida et al. 2012).
Based on our results, the prolonged in vitro maintenance of B. gasipaes caused a reduction in the morphogenic potential of clones, particularly of the initial cells of shoot apical meristems and PPCs, committing cell reprogramming to the return to the undifferentiated state and subsequent acquisition of competence for multipotency,pluripotency and totipotency. These observations suggest the need to add caveats to the principle of totipotentiality credited by Haberlandt (1902) that each plant cell has the genetic potential to develop as a complete organism. Furthermore, keeping in mind that long-term culture can lead to changes in morphogenic potential in stem-like cells of peach palm clones, the periodic monitoring by histological and histochemical analyses could help to prevent the loss of micropropagated plants.
杂志排行
Journal of Forestry Research的其它文章
- Past, present and future of industrial plantation forestry and implication on future timber harvesting technology
- Effects of climate changes on distribution of Eremanthus erythropappus and E. incanus (Asteraceae) in Brazil
- Effects of climate and forest age on the ecosystem carbon exchange of afforestation
- Effect of gap size and forest type on mineral nitrogen forms under different soil properties
- Effect of forest thinning on hydrologic nitrate exports from a Nsaturated plantation
- Floristic analysis and dominance pattern of sal (Shorea robusta)forests in Ranchi, Jharkhand, eastern India
