转录中介体复合物的研究进展
2020-05-18王雯刘坤
王雯 刘坤

摘要:转录中介体复合物(Mediator complex)广泛存在于真核生物中,在进化上高度保守,是一个具有模块化组织的大复合物,参与转录因子与RNA聚合酶Ⅱ之间的信息传递。中介体复合物的主要功能是通过其特定亚基与不同信号通路激发的转录因子相互作用,调节下游基因的表达。此外,中介体复合物还与各种辅因子相互作用,参与转录延伸、mRNA输出及选择性剪切等过程,从而调控细胞的各种生理功能。中介体复合物与疾病的密切联系使得将中介体作为靶标研究疾病的治疗方法成为可能。以中介体复合物的结构为基础,重点介绍了中介体复合物在基因表达调控中的分子机制及功能,以期为后续研究提供参考。
关键词:中介体复合物(Mediator complex);结构;分子机制;功能
中图分类号:Q71 文献标识码:A
文章编号:0439-8114(2020)03-0005-08
DOI:10.14088/j.cnki.issn0439-8114.2020.03.001
Resarch progress of transcription mediator complex
WANG Wen,LIU Kun
(School of Life Sciences,Tianjin University,Tianjin 300072,China)
Abstract: The mediator complex, a highly conserved large complex with modular tissue, is widely distributed in eukaryotes and is involved in the transmission of information between transcription factors and RNA polymerase II. The main function of the mediator complex is to regulate the expression of downstream genes by interacting with transcription factors stimulated by different signaling pathways. In addition, the mediator complex interacts with various cofactors to participate in processes such as transcriptional elongation, mRNA export, and selective splicing, thereby controlling various physiological functions of the cell. The close relationship between mediator complexes and disease makes it possible to study the treatment of disease with mediator as a target. Based on the structure of the mediator complex, the molecular mechanism and function of the mediator complex in the regulation of gene expression were introduced, in order to provide reference for the following research.
Key words: mediator complex; structure; molecular mechanism; function
中介體复合物(Mediator complex)最早在酵母中被发现,此后陆续在果蝇和小鼠的细胞中发现了酵母中介体复合物的同源蛋白[1,2]。2004年,科学家对已发现的中介体复合物各亚基采用了统一的命名法,突出了所有真核生物中中介体复合物的保守性。中介体复合物作为最大的衔接蛋白之一,由30个不同亚基组成,不同模块之间可以发生各种构象变化,可以与3 000个潜在的转录因子相互作用,几乎参与细胞中的所有基因转录,因此理解这种大分子机制是分子生物学中的巨大挑战之一。中介体复合物在转录中具有突出的作用,不仅稳定起始复合物,还参与DNA折叠和染色质相关的DNA重塑等[3]。近年来中介体复合物的功能及对疾病的影响备受关注,它在酵母、植物和人体的多种信号转导途径中发挥重要作用。本文重点论述中介体复合物的亚基组成和结构,试图归纳其参与的转录调节过程及功能,以期为研究中介体复合物提供理论依据。
1 中介体的组成和结构
中介体复合物是一个由多亚基组成的生物大分子,在哺乳动物中大小约2 MDa。由于其具有异质性、内在灵活性,并且尺寸较大,存在亚基多,纯化产率较低,中介体复合物的研究一度受到技术和方法限制[4]。近年来,通过体外生化试验和冷冻电镜技术等先进手段,中介体的研究取得了较大的突破,中介体复合物的组成结构得到了体外重建[4-7]。
1.1 酵母中介体复合物的组成和结构
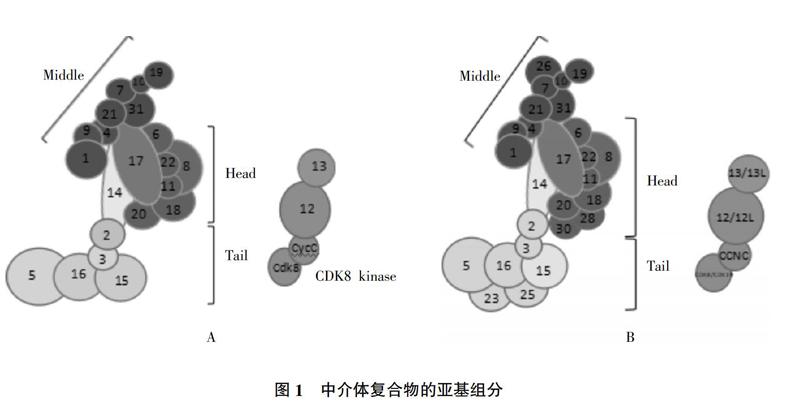
酵母中介体复合物(Mediator of RNA polymerase II transcription subunit,MED)由25个亚基组成(图1A),大小约1.4 MDa,其中MED1、MED4、MED7、MED9、MED10、MED19、MED21和MED31构成中介体的头部模块,MED6、MED8、MED11、MED14、MED17、MED18、MED20及MED22构成中部模块,头部模块和中部模块是中介体复合物的核心,与RNA聚合酶Ⅱ(RNA polymerase Ⅱ,Pol Ⅱ)相互作用参与转录过程[8]。酵母中介体15个核心亚基复合物的晶体结构已被报道(不含MED1)[5]。MED2、MED3、MED5、MED15和MED16组成尾部模块,主要通过与转录因子相互作用以调控转录,MED12、MED13、CycC同CDK8构成细胞周期素依赖性激酶8(Cyclin-dependent kinase 8,CDK8)模块,该模块与中介体的其余部分可逆性结合以调节转录过程[4,9,10]。MED14连接中介体头部、中部和尾部模块[11,12]。在体外对头部和中部模块进行重组时发现,头部和中部模块可以彼此稳定结合,但得到的复合物在转录中是无活性的,在此基础上加入MED14可恢复转录活性并显著增强复合物与Pol Ⅱ的相互作用。同时还发现MED14添加到复合物中显著增强了与Pol Ⅱ的相互作用[4]。
1.2 哺乳动物中介体复合物的组成和结构
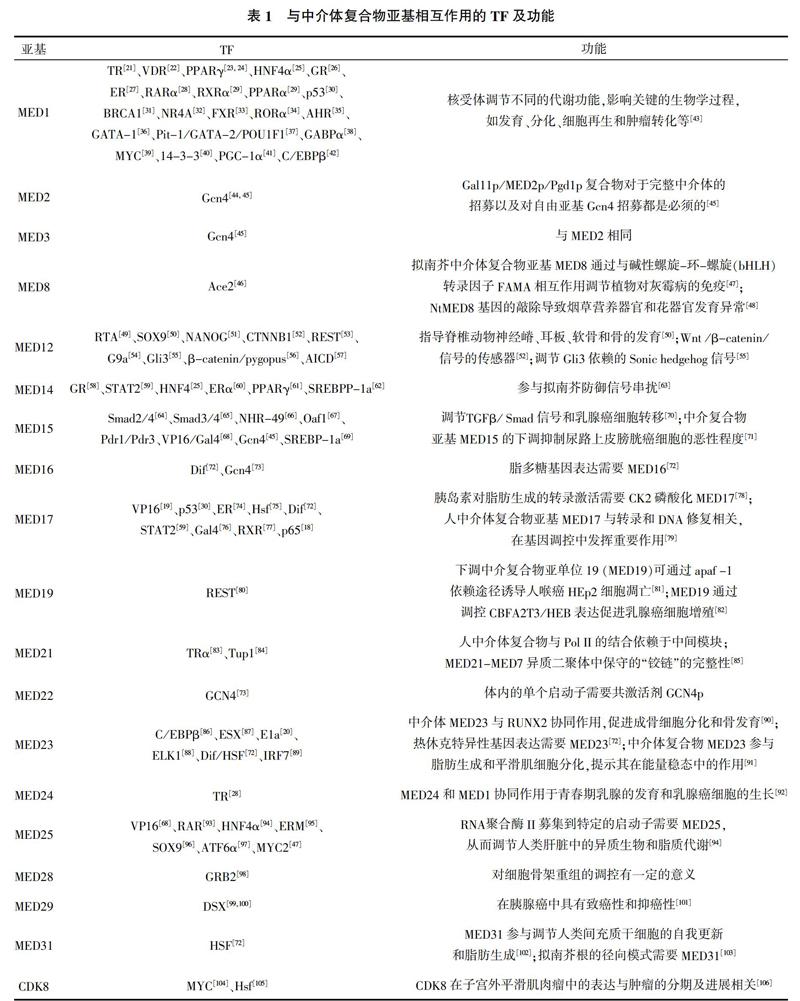
哺乳动物中的中介体复合物由30个蛋白亚基组成,与酵母中介体类似可以分成不同的模块。从单细胞生物到后生动物的进化过程中,中介体的组成和结构高度保守[4](图1B),酵母中介体复合物的亚基模块与哺乳动物的亚基模块存在广泛同源性。在后生动物中介体中,MED24、MED27和MED29分别与酵母中的MED5、MED3和MED2直系同源[10]。但随着生物的进化,转录的基因数目和复杂性不断增加,导致中介体复合物的亚基数也在增加。例如人中介体亚基MED23、MED25、MED26、MED28和MED30在酵母中介体复合物中不存在,本文中这5个组分的模块分配方式是预测的结果。
1.3 中介体复合物的构象变化及三维结构
中介体复合物在转录中的作用涉及多种机制,其中构象改变在功能调节中发挥关键作用[13]。中介体的较大体积和表面积有助于与多种分子及蛋白发生相互作用,使其构象发生变化,已经证明中介体复合物能够与多种转录因子结合,并与转录起始复合物(Preinitiation complex,PIC)相互作用形成前延伸复合物(Pre-elongation complex,PEC)[14],中介体复合物的结构变化可能导致PEC的结构和功能发生改变。研究发现中介体复合物的不同亚基与转录因子p53相互作用会导致构象发生不同的变化,并显著影响PIC的活性[15]。此外,在其他转录因子结合中介体时也观察到类似的构象变化,说明转录因子可能通过改变中介体复合物的构象去调节PIC活性,从而调节下游基因表达[16]。中介体复合物与Pol Ⅱ相互作用时,核心模块(core Mediator,cMED)发挥主要作用,cMED的结构(图2)包含15个亚基,不含MED1,原因可能是在结晶过程中发生了解离[5]。该结构揭示了头部模块和中部模块的亚基结构,也显示了两个模块间的相互作用方式,为功能的研究提供了分子机制和理论基础。
2 中介体复合物在基因表达调控中的分子机制及功能
中介体复合物几乎参与了所有的基因转录,而基因的转录调控是一个非常复杂的过程,先前研究的转录控制模型对基因调控原理提供了重要的见解,揭示了中介体如何将上游众多信号通路的信息正确地传递至基因启动子上的转录装置,从而精确地控制下游基因的表达[17],以下结合近年来对中介体的研究,对中介体复合物在基因表达调控中的分子机制及功能作详细描述。
2.1 增强子招募中介体
中介体在转录激活中起作用的关键是被增强子招募,然后与不同启动子相对应的转录起始复合物(Preinitiation complex,PIC)相互作用,以调节转录。中介体复合物并不与DNA直接结合,而是与转录因子(Transcription factors,TFs)相互作用招募至增强子处。不同亚基与对应TF的这种相互作用是靶基因活化的必要条件,所以特定亚基的缺失可以在不同程度上阻止对应TF调节的基因表达,酵母和低等后生动物的遗传研究已经证明了这一点,并在哺乳动物中也有发现[18]。例如,亚基MED1是核受体的共同靶标,研究发现敲除小鼠胚胎成纤维细胞中的MED1会导致核受体依赖性基因表达缺陷,而与其他亚基相互作用的TF的激活未受到负面影响[19],敲除小鼠细胞中MED23会致使转录因子ELK-1或E1A的失活,但并不影响转录因子VP16和p53的活化[20]。中介体复合物主要通过尾部模块与IF相互作用[8],其他的亚基也有参与,中介体亚基与不同转录因子相互作用及相关功能详见表1。
2.2 中介体复合物与PIC相互作用促进PIC的形成
中介体被招募到增强子区域后,为协助PIC装配,被传送至在核启动子形成的PIC处[107,108]。增强子结合IF后招募共转录激活因子以修饰和重塑染色质,用于改变染色质结构并使其更易获得其他因子,其中中介体就是共转录激活因子之一,然后招募更多的共激活因子组装PIC,包括Pol Ⅱ(含12个亚基)、通用转录因子(General transcription factors,GTFs)、轉录起始因子(Transcription initiation factor,TIF)ⅡA、TIFⅡB、TIFⅡD、TIFⅡE、TIFⅡF和TIFⅡH[109]。中介体复合物能够与PIC直接相互作用,不同于被增强子招募时中介体包含所有模块,与核心启动子相互作用时不含Cdk8激酶模块,头部和中部模块发挥主要作用。招募后的Pol Ⅱ、TFs和中介体复合物参与构成转录起始的全酶,Pol Ⅱ的羧基末端结构域(Carboxyl terminal domain,CTD)区域是中介体复合物与之结合的重要位点,包括解旋酶活性和激酶活性等多种酶活性的TF IIH,能够帮助打开DNA双链,磷酸化Pol Ⅱ CTD,以促使转录起始。经典理论认为中介体复合物在全酶中的作用是短暂的,在Pol Ⅱ完成转录起始并即将转录延伸时,中介体复合物将与Pol Ⅱ分离,分离的过程与CTD的磷酸化有关。有报道称CTD的磷酸化与中介体复合物的Cdk8激酶模块有关,并认为Cdk8激酶模块通过磷酸化CTD抑制转录过程[110]。这一定程度上与中介体复合物和PIC相互作用时不包含Cdk8激酶模块的结论一致。
2.3 中介体复合物的“招募后”功能
越来越多的证据表明,中介体不仅在招募Pol Ⅱ装配PIC中起关键作用,也参与招募后的诸多功能,以使靶基因的转录从多方面得到调控,达到精确的效果。
中介体复合物参与转录延伸。构成核心正相关因子(Positive transcriptional elongation factor b,P-TEFb)的cyclinT1/Cdk9异二聚体通常被认为是刺激RNA聚合酶Ⅱ延伸的转录活性形式,大约一半的细胞P-TEFb存在于具有7SK snRNA和HEXIM1蛋白的无活性复合物中,剩余的一半与溴结构域蛋白Brd4相互作用,与Brd4的结合使P-TEFb 具有转录活性。哺乳动物Brd4属于BET家族,基于其他BET蛋白在转录中的作用,Brd4被认为可能也参与了转录,人转录介体复合物被报道含有Brd4或Brd4类似蛋白[111,112]。除此之外,有研究发现中介体复合物与P-TEFb有直接的物理相互作用[113]。在酵母中还发现中介体复合物能与延伸因子TFIIS相互作用,进一步影响延伸因子DSlF,从而调节转录延伸过程[114]。
中介體复合物参与mRNA的输出。成熟的mRNA形成之后,需要通过核孔复合物(Nuclear pore complexes,NPCs)输出到细胞质中,保守的转录偶联输出(Conserved transcription-coupled export,TREX2)复合物与NPC结合并参与转录和mRNA输出[115,116]。最近的一项研究发现在酵母中中介体与TREX2复合物之间有直接相互作用,这表明由中介体协调的转录调节与NPC介导的mRNA输出有关[117]。通过一系列试验进一步揭示了TREX2复合物直接与中介体的中间模块相互作用。TREX2复合物与中介体的相互作用被认为参与转录和mRNA转运的复杂调节[118]。
中介体复合物在选择性剪切过程中起重要作用[119];中介体是酵母基因组中负责高级染色质折叠的关键复合物之一;中介体复合物与转录记忆有关,已在酵母和人体中证实了这种功能[118]。中介体参与转录绝大部分过程,上述只是其中小部分,更多的功能需要被发现和补充。
3 小结与展望
中介体复合物在酵母中的组成和结构研究已经相对成熟,但核心结构中不包括MED1,这个结果并未得到清晰的解释。随着生物的不断进化,中介体复合物的复杂性也提高,在高级的物种例如人体中,中介体的结构尚未解析,组成成分及分布需要进一步确定。中介体复合物几乎参与真核细胞的所有转录过程,中介体功能的异常与许多癌症有关,例如MED29调节胰腺癌的关键细胞功能,包括致癌和抑制肿瘤的功能[101],MED15在睾丸生殖细胞肿瘤中差异表达[120]等。中介体亚基已经被作为治疗癌症的靶标,其中中介体复合物亚基MED15的敲低抑制了泌尿道上皮膀胱癌细胞的恶性肿瘤;MED1作为ER关键的共激活因子,在ER依赖的基因表达和雌激素依赖的乳腺癌生长过程中起到关键性作用,而且参与了另一类内分泌治疗药物Tamoxifen的耐药形成过程,与乳腺癌患者的不良预后有着高度相关;siRNA介导的CDK8沉默抑制乳腺癌细胞的增殖和生长[121]。还有研究表明中介体复合物与真菌感染等有关,有望成为更多疾病的治疗靶标。目前对它与癌症等疾病的研究大多数处于理论阶段,将其应用于临床研究更加迫切。
参考文献:
[1] BOUBE M,FAUCHER C,JOULIA L,et al. Drosophila homologs of transcriptional mediator complex subunits are required for adult cell and segment identity specification[J].Genes Dev,2000, 14(22):2906-2917.
[2] JIANG Y W,VESCHAMBRE P,ERDJUMENT-BROMAGE H,et al. Mammalian mediator of transcriptional regulation and its possible role as an end-point of signal transduction pathways[J].Proc Natl Acad Sci USA,1998,95(15):8538-8543.
[3] SIERECKI E. The Mediator complex and the role of protein-protein interactions in the gene regulation machinery[J].Semin Cell Dev Biol,2018,(in press).
[4] CEVHER M A,SHI Y,LI D,et al. Reconstitution of active human core Mediator complex reveals a critical role of the MED14 subunit[J].Nature structural & molecular biology,2014,21(12):1028-1034.
[5] NOZAWA K,SCHNEIDER T R,CRAMER P. Core Mediator structure at 3.4 ?魡 extends model of transcription initiation complex[J].Nature,2017,545(7653):248-251.
[6] WANG X,SUN Q,DING Z,et al. Redefining the modular organization of the core Mediator complex[J].Cell Res,2014,24(7):796-808.
[7] TSAI K L,SATO C T,SATO S,et al. Subunit architecture and functional modular rearrangements of the transcriptional mediator complex[J].Cell,2014,157(2):1430-1444.
[8] SOUTOURINA J. Transcription regulation by the Mediator complex[J].Nature reviews molecular cell biology,2017,19(4):262-274.
[9] PLASCHKA C,LARIVI?魬RE L,WENZECK L, et al. Architecture of the RNA polymerase II-Mediator core initiation complex[J].Nature,2015,518(7539):376-380.
[10] SOUTOURINA J. Transcription regulation by the Mediator complex[J].Nature reviews molecular cell biology,2017,19(4):262-274.
[11] TSAI K,YU X,GOPALAN S,et al. Mediator structure and rearrangements required for holoenzyme formation[J].Nature,2017,544(7649):196-201.
[12] ROBINSON P J,TRNKA M J,PELLARIN R,et al. Molecular architecture of the yeast Mediator complex[J].eLife,2015,4.e08719.
[13] ROBINSON P J,TRNKA M J,BUSHNELL D A,et al. Structure of a complete Mediator-RNA polymerase II Pre-Initiation complex[J].Cell,2016,166(6):1411-1422.
[14] LARIVIERE L,SEIZL M,CRAMER P. A structural perspective on Mediator function[J].Curr Opin Cell Biol,2012,24(3):305-313.
[15] MEYER K D,LIN S C,BERNECKY C,et al. p53 activates transcription by directing structural shifts in Mediator[J].Nat Struct Mol Biol,2010,17(6):753-760.
[16] LIN J J,LEHMANN L W,BONORA G,et al. Mediator coordinates PIC assembly with recruitment of CHD1[J].Genes Dev,2011,25(20):2198-2209.
[17] POSS Z C,EBMEIER C C,TAATJES D J. The Mediator complex and transcription regulation[J].Crit Rev Biochem Mol Biol,2013,48(6):575-608.
[18] VAN ESSEN D,ENGIST B,NATOLI G,et al. Two modes of transcriptional activation at native promoters by NF-kappaB p65[J].PLoS Biol,2009,7(3):e73.
[19] ITO M,YUAN C X,MALIK S,et al. Identity between TRAP and SMCC complexes indicates novel pathways for the function of nuclear receptors and diverse mammalian activators[J].Mol Cell,1999,3(3):361-370.
[20] STEVENS J L,CANTIN G T,WANG G,et al. Transcription control by E1A and MAP kinase pathway via Sur2 mediator subunit[J].Science,2002,296(5568):755-758.
[21] FONDELL J D,GE H,ROEDER R G. Ligand induction of a transcriptionally active thyroid hormone receptor coactivator complex[J].Proc Natl Acad Sci USA,1996,93(16):8329-8333.
[22] RACHEZ C,LEMON B D,SULDAN Z,et al. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex[J].Nature,1999,398-824.
[23] GE K,CHO Y W,GUO H,et al. Alternative mechanisms by which mediator subunit MED1/TRAP220 regulates peroxisome proliferator-activated receptor gamma-stimulated adipogenesis and target gene expression[J].Mol Cell Biol,2008,28(3):1081-1091.
[24] GE K,GUERMAH M,YUAN C,et al. Transcription coactivator TRAP220 is required for PPARγ2-stimulated adipogenesis[J]. Nature,2002,417-563.
[25] MALIK S,WALLBERG A E,KANG Y K,et al. TRAP/SMCC/mediator-dependent transcriptional activation from DNA and chromatin templates by orphan nuclear receptor hepatocyte nuclear factor 4[J].Mol Cell Biol,2002,22(15):5626-5637.
[26] HITTELMAN A B,BURAKOV D,INIGUEZ-LLUHI J A,et al. Differential regulation of glucocorticoid receptor transcriptional activation via AF-1-associated proteins[J].EMBO J,1999,18(19):5380-5388.
[27] KANG Y K,GUERMAH M,YUAN C X,et al. The TRAP/Mediator coactivator complex interacts directly with estrogen receptors alpha and beta through the TRAP220 subunit and directly enhances estrogen receptor function in vitro[J].Proc Natl Acad Sci USA,2002,99(5):2642-2647.
[28] YUAN C X,ITO M,FONDELL J D,et al. The TRAP220 component of a thyroid hormone receptor-associated protein (TRAP) coactivator complex interacts directly with nuclear receptors in a ligand-dependent fashion[J].Proc Natl Acad Sci USA,1998, 95(14):7939-7944.
[29] ZHU Y,QI C,JAIN S,et al. Isolation and characterization of PBP,a protein that interacts with peroxisome proliferator-activated receptor[J].J Biol Chem,1997,272(41):25500-25506.
[30] MEYER K D,LIN S C,BERNECKY C,et al. p53 activates transcription by directing structural shifts in Mediator[J].Nat Struct Mol Biol,2010,17(6):753-760.
[31] WADA O,OISHI H,TAKADA I,et al. BRCA1 function mediates a TRAP/DRIP complex through direct interaction with TRAP220[J].Oncogene,2004,23(35):6000-6005.
[32] WANSA K D,MUSCAT G E. TRAP220 is modulated by the antineoplastic agent 6-Mercaptopurine,and mediates the activation of the NR4A subgroup of nuclear receptors[J].J Mol Endocrinol,2005,34(3):835-848.
[33] PINEDA T I,FREEDMAN L P,GARABEDIAN M J. Identification of DRIP205 as a coactivator for the Farnesoid X receptor[J].J Biol Chem,2004,279(35):36184-36191.
[34] ATKINS G B,HU X,GUENTHER M G,et al. Coactivators for the orphan nuclear receptor RORalpha[J].Mol Endocrinol,1999, 13(9):1550-1557.
[35] WANG S,GE K,ROEDER R G,et al. Role of mediator in transcriptional activation by the aryl hydrocarbon receptor[J].J Biol Chem,2004,279(14):13593-13600.
[36] STUMPF M,WASKOW C,KROTSCHEL M,et al. The mediator complex functions as a coactivator for GATA-1 in erythropoiesis via subunit Med1/TRAP220[J].Proc Natl Acad Sci USA,2006,103(49):18504-18509.
[37] GORDON D F,TUCKER E A,TUNDWAL K,et al. MED220/thyroid receptor-associated protein 220 functions as a transcriptional coactivator with Pit-1 and GATA-2 on the thyrotropin-beta promoter in thyrotropes[J].Mol Endocrinol,2006, 20(5):1073-1089.
[38] UDAYAKUMAR T S,BELAKAVADI M,CHOI K H,et al. Regulation of Aurora-A kinase gene expression via GABP recruitment of TRAP220/MED1[J].J Biol Chem,2006,281(21):14691-14699.
[39] LIU X,VORONTCHIKHINA M,WANG Y L,et al. STAGA recruits Mediator to the MYC oncoprotein to stimulate transcription and cell proliferation[J].Mol Cell Biol,2008,28(1):108-121.
[40] ZILLIACUS J,HOLTER E,WAKUI H,et al. Regulation of glucocorticoid receptor activity by 14--3-3-dependent intracellular relocalization of the corepressor RIP140[J].Mol Endocrinol,2001, 15(4):501-511.
[41] WALLBERG A E,YAMAMURA S,MALIK S,et al. Coordination of p300-mediated chromatin remodeling and TRAP/mediator function through coactivator PGC-1alpha[J].Mol Cell,2003, 12(5):1137-1149.
[42] LI H,GADE P,NALLAR S C,et al. The Med1 subunit of transcriptional mediator plays a central role in regulating CCAAT/enhancer-binding protein-beta-driven transcription in response to interferon-gamma[J].J Biol Chem,2008,283(19):13077-13086.
[43] JIA Y,VISWAKARMA N,REDDY J K. Med1 subunit of the mediator complex in nuclear receptor-regulated energy metabolism,liver regeneration,and hepatocarcinogenesis[J].Gene Expr,2014,16(2):63-75.
[44] NATARAJAN K,JACKSON B M,ZHOU H,et al. Transcriptional activation by Gcn4p involves independent interactions with the SWI/SNF complex and the SRB/mediator[J].Mol Cell,1999,4(4):657-664.
[45] ZHANG F,SUMIBCAY L,HINNEBUSCH A G,et al. A triad of subunits from the Gal11/tail domain of Srb mediator is an in vivo target of transcriptional activator Gcn4p[J].Mol Cell Biol,2004,24(15):6871-6886.
[46] MEHTA S,MIKLOS I,SIPICZKI M,et al. The Med8 mediator subunit interacts with the Rpb4 subunit of RNA polymerase II and Ace2 transcriptional activator in Schizosaccharomyces pombe[J].FEBS Lett,2009,583(19):3115-3120.
[47] LI X,YANG R,CHEN H. The Arabidopsis thaliana Mediator subunit MED8 regulates plant immunity to Botrytis Cinerea through interacting with the basic helix-loop-helix (bHLH) transcription factor FAMA[J].PLoS One,2018,13(3):e193458.
[48] WANG F,WEI H,TONG Z,et al. Knockdown of NtMed8, a Med8-like gene,causes abnormal development of vegetative and floral organs in tobacco (Nicotiana tabacum L.)[J].Plant Cell Rep,2011,30(11):2117-2129.
[49] GWACK Y,BAEK H J,NAKAMURA H,et al. Principal role of TRAP/mediator and SWI/SNF complexes in Kaposi's sarcoma-associated herpesvirus RTA-mediated lytic reactivation[J].Mol Cell Biol,2003,23(6):2055-2067.
[50] RAU M J,FISCHER S,NEUMANN C J. Zebrafish Trap230/Med12 is required as a coactivator for Sox9-dependent neural crest,cartilage and ear development[J].Dev Biol,2006,296(1):83-93.
[51] TUTTER A V,KOWALSKI M P,BALTUS G A,et al. Role for Med12 in Regulation of Nanog and Nanog Target Genes[J].Journal of biological chemistry,2009,284(6):3709-3718.
[52] KIM S,XU X,HECHT A,et al. Mediator is a transducer of Wnt/beta-catenin signaling[J].J Biol Chem,2006,281(20):14066-14075.
[53] DING N,ZHOU H,ESTEVE P,et al. Mediator Links Epigenetic Silencing of Neuronal Gene Expression with X-Linked Mental Retardation[J].Molecular cell,2008,31(3):347-359.
[54] DING N,ZHOU H,ESTEVE P O,et al. Mediator links epigenetic silencing of neuronal gene expression with x-linked mental retardation[J].Mol Cell,2008,31(3):347-359.
[55] ZHOU H,KIM S,ISHII S,et al. Mediator modulates Gli3-dependent Sonic hedgehog signaling[J].Mol Cell Biol,2006,26(23):8667-8682.
[56] CARRERA I,JANODY F,LEEDS N,et al. Pygopus activates Wingless target gene transcription through the mediator complex subunits Med12 and Med13[J].Proc Natl Acad Sci USA,2008,105(18):6644-6649.
[57] XU X,ZHOU H,BOYER T G. Mediator is a transducer of amyloid-precursor-protein-dependent nuclear signalling[J].EMBO Rep,2011,12(3):216-222.
[58] CHEN W,ROGATSKY I,GARABEDIAN M J. MED14 and MED1 differentially regulate target-specific gene activation by the glucocorticoid receptor[J].Mol Endocrinol,2006,20(3):560-572.
[59] LAU J F,NUSINZON I,BURAKOV D,et al. Role of metazoan mediator proteins in interferon-responsive transcription[J].Mol Cell Biol,2003,23(2):620-628.
[60] LEE J E,KIM K,SACCHETTINI J C,et al. DRIP150 coactivation of estrogen receptor alpha in ZR-75 breast cancer cells is independent of LXXLL motifs[J].J Biol Chem,2005,280(10):8819-8830.
[61] GRONTVED L,MADSEN M S,BOERGESEN M,et al. MED14 tethers mediator to the N-terminal domain of peroxisome proliferator-activated receptor gamma and is required for full transcriptional activity and adipogenesis[J].Mol Cell Biol,2010,30(9):2155-2169.
[62] TOTH J I,DATTA S,ATHANIKAR J N,et al. Selective coactivator interactions in gene activation by SREBP-1a and -1c[J].Mol Cell Biol,2004,24(18):8288-8300.
[63] WANG C,DU X,MOU Z. The Mediator Complex Subunits MED14,MED15,and MED16 Are Involved in Defense Signaling Crosstalk in Arabidopsis[J].Front Plant Sci,2016,7:1947.
[64] LIN X,RINALDO L,FAZLY A F,et al. Depletion of Med10 enhances Wnt and suppresses Nodal signaling during zebrafish embryogenesis[J].Dev Biol,2007,303(2):536-548.
[65] KATO Y,HABAS R,KATSUYAMA Y,et al. A component of the ARC/Mediator complex required for TGF beta/Nodal signalling[J].Nature,2002,418(6898):641-646.
[80] DING N,TOMOMORI-SATO C,SATO S,et al. MED19 and MED26 are synergistic functional targets of the RE1 silencing transcription factor in epigenetic silencing of neuronal gene expression[J].J Biol Chem,2009,284(5):2648-2656.
[81] ZHAO Y,MENG Q,GAO X,et al. Down-regulation of mediator complex subunit 19 (Med19) induces apoptosis in human laryngocarcinoma HEp2 cells in an Apaf-1-dependent pathway[J].Am J Transl Res,2017,9(2):755-761.
[82] ZHANG X,FAN Y,LIU B,et al. Med19 promotes breast cancer cell proliferation by regulating CBFA2T3/HEB expression[J].Breast Cancer,2017,24(3):433-441.
[83] NEVADO J,TENBAUM S P,ARANDA A. hSrb7,an essential human Mediator component,acts as a coactivator for the thyroid hormone receptor[J].Mol Cell Endocrinol,2004,222(1-2):41-51.
[84] HALLBERG M,HU G Z,TRONNERSJO S,et al. Functional and physical interactions within the middle domain of the yeast mediator[J].Mol Genet Genomics,2006,276(2):197-210.
[85] SATO S,TOMOMORI-SATO C,TSAI K L,et al. Role for the MED21-MED7 Hinge in Assembly of the Mediator-RNA Polymerase II Holoenzyme[J].J Biol Chem,2016,291(52):26886-26898.
[86] MO X,KOWENZ-LEUTZ E,XU H,et al. Ras induces mediator complex exchange on C/EBP beta[J].Mol Cell,2004,13(2):241-250.
[87] SHIMOGAWA H,KWON Y,MAO Q,et al. A wrench-shaped synthetic molecule that modulates a transcription factor-coactivator interaction[J].J Am Chem Soc,2004,126(11):3461-3471.
[88] WANG G,BALAMOTIS M A,STEVENS J L,et al. Mediator Requirement for Both Recruitment and Postrecruitment Steps in Transcription Initiation[J].Molecular Cell,2005,17(5):683-694.
[89] GRIFFITHS S J,KOEGL M,BOUTELL C,et al. A systematic analysis of host factors reveals a Med23-interferon-lambda regulatory axis against herpes simplex virus type 1 replication[J].PLoS Pathog,2013,9(8):e1003514.
[90] LIU Z,YAO X,YAN G,et al. Mediator MED23 cooperates with RUNX2 to drive osteoblast differentiation and bone development[J].Nat Commun,2016,7:11149.
[91] CHU Y,GOMEZ R L,HUANG P,et al. Liver Med23 ablation improves glucose and lipid metabolism through modulating FOXO1 activity[J].Cell Res,2014,24(10):1250-1265.
[92] HASEGAWA N,SUMITOMO A,FUJITA A,et al. Mediator subunits MED1 and MED24 cooperatively contribute to pubertal mammary gland development and growth of breast carcinoma cells[J].Mol Cell Biol,2012,32(8):1483-1495.
[93] LEE H K,PARK U H,KIM E J,et al. MED25 is distinct from TRAP220/MED1 in cooperating with CBP for retinoid receptor activation[J].EMBO J,2007,26(15):3545-3557.
[94] RANA R,SURAPUREDDI S,KAM W,et al. Med25 is required for RNA polymerase II recruitment to specific promoters,thus regulating xenobiotic and lipid metabolism in human liver[J].Mol Cell Biol,2011,31(3):466-481.
[95] VERGER A,BAERT J L,VERREMAN K,et al. The Mediator complex subunit MED25 is targeted by the N-terminal transactivation domain of the PEA3 group members[J].Nucleic Acids Res,2013,41(9):4847-4859.
[96] NAKAMURA Y,YAMAMOTO K,HE X,et al. Wwp2 is essential for palatogenesis mediated by the interaction between Sox9 and mediator subunit 25[J].Nat Commun,2011,2:251.
[97] SELA D,CONKRIGHT J J,CHEN L,et al. Role for human mediator subunit MED25 in recruitment of mediator to promoters by endoplasmic reticulum stress-responsive transcription factor ATF6alpha[J].J Biol Chem,2013,288(36):26179-26187.
[98] WIEDERHOLD T,LEE M F,JAMES M,et al. Magicin,a novel cytoskeletal protein associates with the NF2 tumor suppressor merlin and Grb2[J].Oncogene,2004,23(54):8815-8825.
[99] GARRETT-ENGELE C M,SIEGAL M L,MANOLI D S,et al. intersex,a gene required for female sexual development in Drosophila,is expressed in both sexes and functions together with doublesex to regulate terminal differentiation[J].Development,2002,129(20):4661-4675.
[100] SATO S,TOMOMORI-SATO C,BANKS C A,et al. A mammalian homolog of Drosophila melanogaster transcriptional coactivator intersex is a subunit of the mammalian Mediator complex[J].J Biol Chem,2003,278(50):49671-49674.
[101] KUUSELO R,SAVINAINEN K,SANDSTROM S,et al. MED29,a component of the mediator complex,possesses both oncogenic and tumor suppressive characteristics in pancreatic cancer[J].Int J Cancer,2011,129(11):2553-2565.
[102] BEADLE E P,STRAUB J A,BUNNELL B A,et al. MED31 involved in regulating self-renewal and adipogenesis of human mesenchymal stem cells[J].Mol Biol Rep,2018.45(5):1545-1550.
[103] ZHANG X,ZHOU W,CHEN Q,et al. Mediator subunit MED31 is required for radial patterning of Arabidopsis roots[J].Proc Natl Acad Sci USA,2018,115(24):E5624-E5633.
[104] EBERHARDY S R,FARNHAM P J. Myc recruits P-TEFb to mediate the final step in the transcriptional activation of the cad promoter[J].J Biol Chem,2002,277(42):40156-40162.
[105] PARK J M,WERNER J,KIM J M,et al. Mediator, not holoenzyme,is directly recruited to the heat shock promoter by HSF upon heat shock[J].Mol Cell,2001,8(1):9-19.
[106] YERGIYEV O,GARIB G,SCHOEDEL K,et al. CDK8 Expression in Extrauterine Leiomyosarcoma Correlates With Tumor Stage and Progression[J].Appl Immunohistochem Mol Morphol,2018,26(3):161-164.
[107] KOLESKE A J,BURATOWSKI S,NONET M, et al. A novel transcription factor reveals a functional link between the RNA polymerase II CTD and TFIID[J].Cell,1992,69(5):883-894.
[108] RANISH J A,YUDKOVSKY N,HAHN S. Intermediates in formation and activity of the RNA polymerase II preinitiation complex:holoenzyme recruitment and a postrecruitment role for the TATA box and TFIIB[J].Genes Dev,1999,13(1):49-63.
[109] EYCHENNE T,NOVIKOVA E,BARRAULT M B,et al. Functional interplay between Mediator and TFIIB in preinitiation complex assembly in relation to promoter architecture[J].Genes Dev,2016,30(18):2119-2132.
[110] AKOULITCHEV S,CHUIKOV S,REINBERG D. TFIIH is negatively regulated by cdk8-containing mediator complexes[J].Nature,2000,407(6800):102-106.
[111] JANG M K,MOCHIZUKI K,ZHOU M,et al. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription[J].Molecular cell,2005,19(4):523-534.
[112] YANG Z,YIK J H N,CHEN R,et al. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4[J].Molecular cell,2005,19(4):535-545.
[113] DONNER A J,EBMEIER C C,TAATJES D J,et al. CDK8 is a positive regulator of transcriptional elongation within the serum response network[J].Nat Struct Mol Biol,2010,17(2):194-201.
[114] GUGLIELMI B,SOUTOURINA J,ESNAULT C,et al. TFIIS elongation factor and Mediator act in conjunction during transcription initiation in vivo[J].Proc Natl Acad Sci USA,2007, 104(41):16062-16067.
[115] BENTLEY D L.Coupling mRNA processing with transcription in time and space[J].Nat Rev Genet,2014,15(3):163-175.
[116] BEN-YISHAY R,ASHKENAZY A J,SHAV-TAL Y. Dynamic encounters of genes and transcripts with the nuclear pore[J].Trends genet,2016,32(7):419-431.
[117] SCHNEIDER M,HELLERSCHMIED D,SCHUBERT T,et al. The nuclear pore-associated TREX-2 complex employs mediator to regulate gene expression[J].Cell,2015,162(5):1016-1028.
[118] SOUTOURINA J. Transcription regulation by the Mediator complex[J].Nature reviews molecular cell biology,2017,19(4):262-274.
[119] 汪 煒,尹景雯,刘艾洁,等.中介体复合物——真核转录调控中的中央控制器[J].中国细胞生物学学报,2011(6):597-607.
[120] KLUMPER N,SYRING I,OFFERMANN A,et al. Differential expression of Mediator complex subunit MED15 in testicular germ cell tumors[J].Diagn Pathol,2015,10:165.
[121] siRNA-mediated silencing of CDK8 inhibits proliferation and growth in breast cancer cells[Retraction][J].Int J Clin Exp Pathol,2018,11(3):1836.
收稿日期:2019-01-06
基金项目:国家自然科学基金项目(31470731)
作者简介:王 雯(1993-),女,山西长子人,在读硕士研究生,研究方向为生物化学与分子生物学,(电话)18202278662(电子信箱)18202278662@163.com。
