Efficacy of mesenchymal stem cells in the treatment of gastrointestinal malignancies
2020-05-16JianNanLiWeiLiLanQingCaoNingLiuKaiZhang
Jian-Nan Li, Wei Li, Lan-Qing Cao, Ning Liu, Kai Zhang
Jian-Nan Li, Wei Li, Kai Zhang, Department of General Surgery, The Second Hospital of Jilin University, Changchun 130041, Jilin Province, China
Lan-Qing Cao, Department of Pathology, The Second Hospital of Jilin University, Changchun 130041, Jilin Province, China
Ning Liu, Department of Central Laboratory, The Second Hospital of Jilin University,Changchun 130041, Jilin Province, China
Abstract
Key words: Mesenchymal stem cells; Gastrointestinal malignancies; Gastric cancer;Hepatoma; Pancreatic cancer; Colorectal cancer
INTRODUCTION
Gastrointestinal (GI) malignancies, which mainly include stomach, liver, pancreas,small intestine, colon, and rectal cancers, are the major causes of cancer-related death worldwide[1,2]. Based on a survey conducted by the American Cancer Society, there were approximately 328030 new GI malignancy cases and 165640 GI malignancyrelated deaths in the United States in 2019[3]. In China, GI malignancies account for 35% of cancer-related deaths, which is much higher than that in the United States and the United Kingdom[4]. Surgery, radiotherapy, chemotherapy, irreversible electroporation, cryotherapy, molecular targeted therapy, immunity therapy,etc.,have been the main treatment methods for GI malignancies in recent years[5-8]. Despite advances in treatment methods, there are still a large number of patients suffering from GI malignancies every year. Furthermore, many patients with GI malignancies are diagnosed at the late stage of the disease, which limits the treatment efficacy and shortens their survival time. Therefore, it is important and necessary to find other promising treatment methods for the treatment of GI malignancies.
Cancer stem cells (CSCs) are a kind of cancer cell with the characteristics of stem cells that are self-renewing and heterogeneous[9]. In addition, CSCs may not be a single stasis population, but they are diverse and malleable[9,10]. Because of these characteristics, CSCs play a vital role in the development, recurrence, and drug resistance of tumours[11]. However, CSCs possess one or more abnormal signalling pathways that target whether tumour proliferation or invasion can be inhibited[9,12,13].In this review, we aim to summarize the efficacy of mesenchymal stem cells (MSCs) in the treatment of GI malignancies. Therefore, the detailed characteristics of CSCs and their roles in cancer treatment will not be further described in this review.
With the discovery of human body stem cells, researchers have focused on stem cell treatments. Stem cell treatments are being explored widely for their promising potential to treat various tumours. Among many kinds of stem cells, MSCs are mostly used in anticancer studies[14,15]. MSCs have an immune-privileged nature, the ability to migrate to tumour cells, and multi-lineage differentiation[16,17], which make them a kind of biological agent for tumour inhibition. In this review, we introduce the characteristics of MSCs and summarize the effects of MSCs on GI malignancies. Table 1 shows the effect of MSCs in the treatment of GI malignancies.
MSCS
Stem cells were first discovered in 1963 and have been widely implicated in the treatment of various diseases[18]. Stem cells are homogeneous and capable of selfproliferation and renewal. Based on their sources, stem cells can be divided into four categories: embryonic stem cells, capable of self-renewal and differentiating into different tissues[19,20]; foetal stem cells, which exist in foetal tissues and have the potential for multi-differentiation[21]; adult stem cells, which are partially oriented, are present in specific stromal tissues and can be derived from bone marrow, muscle,adipose tissue, synovium, periosteum,etc.[22,23]; and cord blood stem cells, including hematopoietic stem cells and MSCs which are mostly derived from bone marrow[24].
Among these stem cells, the most commonly used for anticancer research is MSCs.MSCs are adult stem cells that were first discovered in the bone marrow. MSCs have the potential for multi-directional differentiation. They can differentiate into bone,cartilage, adipocytes,etc.[25]. In addition, MSCs possess an immune-privileged nature,can migrate to injured tissues and tumour sites, and are easy to culture and storein vitro[25,26].
It has also been reported that MSCs can differentiate into hepatocytes, epithelialcells, and nerve cells, which are endodermal and ectodermal derived[27,28]. In a study conducted by Doanet al[29], MSCs differentiated into endothelial-like cells that produced capillary-like structures and took up low-density lipoprotein. Liuet al[30]found that MSCs could differentiate into neural stem cells and immature neurons. The multi-directional differentiation feature of MSCs makes them important in tissue regeneration.
责任护士通过评估患者文化程度和认知能力,选择不同的健康教育方式,可采用讲座、图片、文字资料及通俗语言等方式,讲向患者讲解冠心病相关知识,提升老年人对冠心病的认识度,改善传统生活观念,培养良好的生活习惯。定期组织病友交流会,通过老年患者间相互沟通和交流,能够起到互相鼓励、督促的作用,有利于老年患者身心调节,提高患者主动性参与意识和对治疗的依从性。
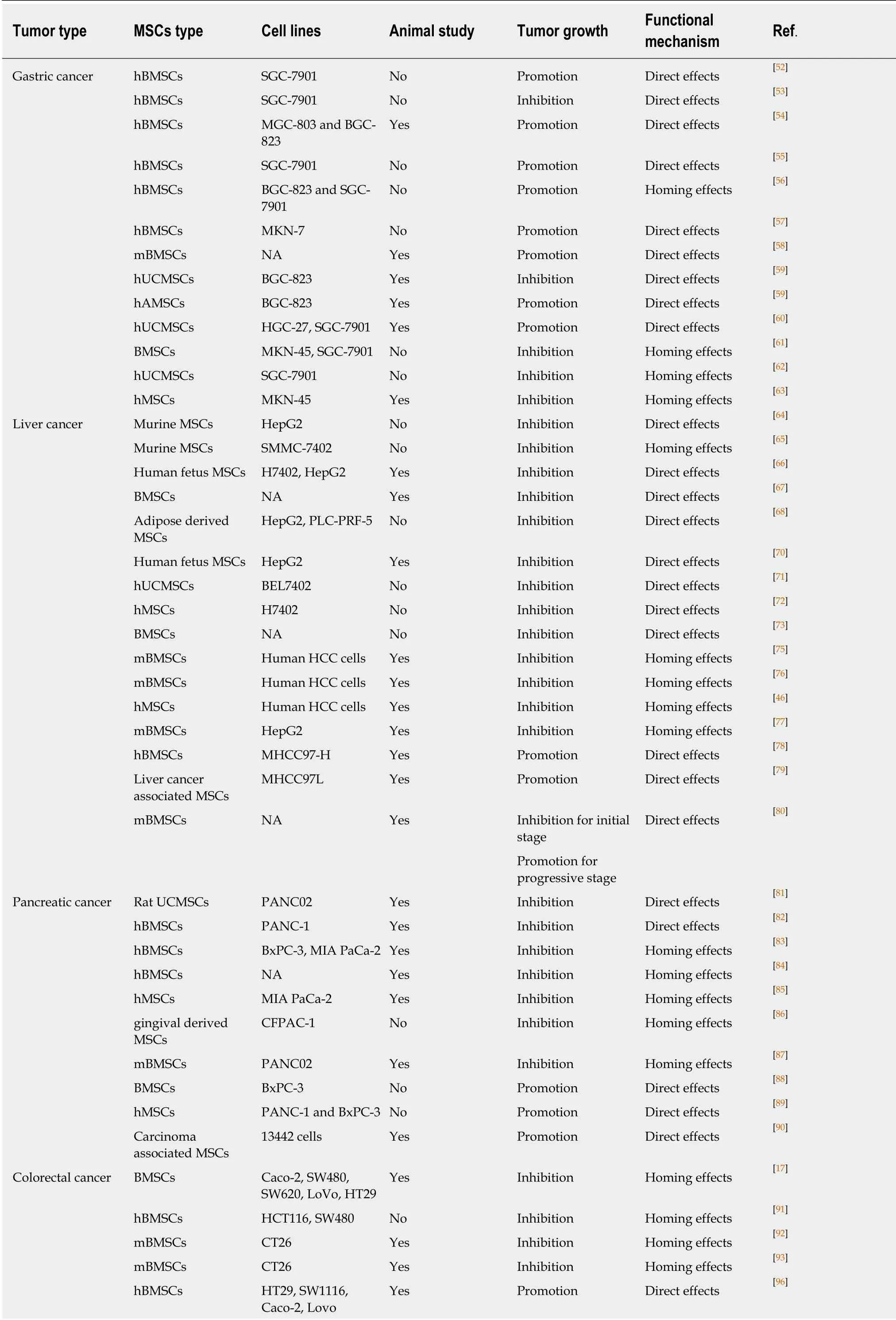
Table 1 The effect of mesenchymal stem cells in the treatment of gastrointestinal malignancies in vitro and in vivo

MSCs: Mesenchymal stem cells; hBMSCs: Human bone marrow-derived mesenchymal stem cells; mBMSCs: Murine one marrow-derived mesenchymal stem cells; hUCMSCs: Human umbilical cord mesenchymal stem cells; hAMSCs: Human amniotic mesenchymal stem cells; hMSCs: Human mesenchymal stem cells; mBMSCs: Murine bone marrow-derived mesenchymal stem cells; NA: Not applicable.
The immune privilege nature is another feature of MSCs. As early as 2000, Liechtyet al[31]transplanted MSCs into sheep and found that MSCs could survive in many tissues of the animals for as long as 13 months. In their opinion, the fact that MSCs could survive in heterogeneous bodies was related to their unique immune regulation function. Some researchers believe that MSCs can inhibit the proliferation of T and NK cells and regulate the activity of B and dendritic cells[32]. As reported in some other studies, the immunosuppressive effect of MSCs is associated with the local immunosuppressive microenvironment formed by bioactive molecules that are secreted after cell contact[33]. Importantly, MSCs express the surface markers of major histocompatibility (MHC) I but hardly express the surface markers MHC II.Meanwhile, MSCs do not express the co-stimulatory molecules CD40, CD80, or CD8[34]. Despite the low expression of MHC I, MSCs cannot activate secondary signals of the immune response, which will result in the absent response status of T cells due to the lack of co-stimulatory factors[34]. Low expression of MHC I molecules can protect MSCs from natural killer cell-mediated cytotoxicity. If MSCs cannot express MHC I molecules, MSCs will be attacked by natural killer cells, leading to the death of MSCs[35]. This innate immune tolerance of MSCs is a necessary condition to achieve allogeneic transplantation.
MSCs possess a migration tendency toward tumour tissues, which has aroused the interest of many researchers. In 1999, Maestroniet al[36]first found that MSCs could migrate to tumour sites. MSCs can interact with tumour cells in many ways to promote or inhibit tumour growth. In a study, researchers found that bone marrowderived MSCs (BMSCs) released a variety of soluble factors that inhibited the growth of lung cancer and melanoma in mice[36]. The migration tendency of MSCs has been confirmed in pancreas, ovarian, lung, colon, rectum, and breast cancers, among others[37-39]. However, the mechanism by which tumours promote MSC migration is unclear and may be related to the biological characteristics of the tumour microenvironment. High concentrations of inflammatory chemotaxis factors and growth factors within the tumour microenvironment are thought to be the main reasons for integrating MSCs into the tumour stroma (Figure 1A). Because of chronic inflammation in tumour tissues, MSCs are promoted to release various soluble factors, such as epidermal growth factor, vascular endothelium growth factor-A,fibroblast growth factor, platelet-derived growth factor, matrix-derived growth factor-1α, interleukin-8, interleukin-6, and transforming growth factor-β (Figure 1B)[27,40,41].These factors also result in the migration tendency of MSCs towards tumour sites.
MSCs can secrete different molecules in distinct microenvironments to interact with tumour cells for the homing, growth, and stemness of tumours (Figure 1B). Whether MSCs promote or inhibit tumour formation depends on multiple factors. Different sources of MSCs have different effects on tumours. One study showed that umbilical cord blood-derived MSCs inhibited the growth of brain tumours, while adipose tissue-derived MSCs promoted brain tumour growth. Furthermore, adipose tissuederived MSCs also promote the growth of gastric cancer, breast cancer and ovarian cancer[42]. Another study demonstrated that allogeneic MSCs were transplanted into the body to promote the transition of melanoma cells into malignant tumours[43]. Some researchers also believe that different types of tumour cells have different responses to MSCs[44].
Currently, an increasing number of researchers have indicated that MSCs with no modification can prevent tumour growthin vitroandin vivoin mice. This may result from the factors released by MSCs that have antitumour effects[36,45]. MSCs have also been modified to overexpress specific genes to secrete therapeutic molecules for cancer treatment[27]. In addition, based on the migration tendency and immune privileged nature, MSCs can also be applied as agent carriers to kill cancer cells[46,47].
MSCS FOR GASTRIC CANCER
The effect of MSCs in the treatment of gastric cancer remains controversial. This section summarizes the studies that applied MSCs for gastric cancer study and analyses their effect on tumour progression.
In some studies, it was reported that BMSCs benefited the angiogenesis of tumours,thus facilitating tumour growth[48]. Previous studies have shown that BMSCs could promote breast, prostate, and liver tumour growth and increase the proliferation of Saos-2 osteosarcomaviaincreasing angiogenesis or other signalling pathways[49-51]. In a study performed by Muet al[52], BMSCs were found to suppress the cell viability of SGC-7901 gastric cancer cells by regulating the expression of apoptosis molecules (e.g.,Bax/Bcl-2, p53) and promoting the release of tumour necrosis factor (TNF)-α. This study indicated the important roles of BMSCs in the development of gastric cancer cells. Zhanget al[53]obtained similar results to some research. They found that human BMSCs (hBMSCs) inhibited the proliferation and migration and activated the apoptosis of SGC-7901 gastric cancer cells by upregulating the TNF-α/JNK pathway[53]. Androgen reversed the BMSC-induced gastric cancer inhibition effect. In another study, researchers found that hBMSC-conditioned medium enhanced the viability of gastric cancer cells (MGC-803 and BGC-823) and promoted tumour development in a xenograft gastric cancer modelviaincreasing the expression of c-Myc[54]. Qiet al[55]found that hBMSC-derived exosomes increased the viability of SGC-7901 gastric cancer cells by activating the Hedgehog signalling pathway. The exosomes of hBMSCs were transfected with miRNA-221 to promote oncogenic activity in gastric cancer in one study[56]. The exosomes of BMSCs acted as a kind of vehicle that might perform tumour homing and immunosuppressive effects during cancer treatment. Nishimuraet al[57]found that hBMSCs could induce an advantageous tumour microenvironment that benefited gastric cancer progression.Other studies also obtained similar results that BMSCs could result in gastric cancer development[58]. It is shown that BMSCs possess different effects on the development of gastric cancer. This may be because of that different gastric cancer cell lines have been applied in above discussed studies. Different cancer cell lines have different features, such as cell malignancy, invasiveness, proliferative capacity, and surface markers. As a result, BMSCs have shown different results towards different gastric cancer cell lines.
Human amniotic MSCs (hAMSCs) and human umbilical cord MSCs (hUCMSCs)are two other types of promising stem cells used in clinical applications. The effects of hAMSCs and hUCMSCs on gastric cancer were first analysed by Houet al[59]. The authors found that hUCMSCs not only inhibited the proliferation of BGC-823 gastric cancer cells but also prevented tumour migration. In a gastric cancer xenograft mouse model, hUCMSCs indeed inhibited tumour progression. However, hAMSCs enhanced the proliferation and migration of gastric cancer cells in their study. The authors concluded that, compared with hAMSCs, hUCMSCs were safe for the treatment of gastric cancer[59]. However, in another study, the researchers found that hUCMSCs enhanced the proliferation and migration of HGC-27 and SGC-7901 gastric cancer cells[60]. They fused hUCMSCs and gastric cancer cells and found that the hybrid cells strongly expressed CD44 and CD133. Furthermore, the heterotypic hybrids promoted gastric tumour growth in mice (Figure 2). In comparison of the studies conducted by Houet al[59]and Xueet al[60], we can also find that MSCs may exhert different effects towards different cancer cell lines. BGC-823 and HGC-27 cell lines mixed with hUCMSCs were subcutaneously injected into nude mice in Houet al[59]'s and Xueet al[60]'s studies, respectively. However, hUCMSCs inhibited the tumour formation in Hou's study, while promoted the tumour growth in Xueet al[60]'s study. Zhanget al[53]and Xueet al[60]investigated the effects of hBMSCs and hUCMSCs towards SGC-7901 gastric cancer cells, respectively. However, they obtained opposite results in which BMSCs inhibited the cell viability, but hUCMSCs promoted the cell growth. This demonstrates that different types of MSCs may have different biological features towards GI malignancies.
Because of the tumour homing effect, MSCs have been used as vehicles to load oxygen or other agents to treat gastric cancer. In a study, researchers transfected haemoglobin genes into BMSCs to supply oxygen to the MKN-45 and SGC-7901 gastric cancer cells[61]. The hypoxic microenvironment in gastric cancer was averted,and the chemotherapy efficacy towards the tumour cells increased. They concluded that BMSCs may be an efficient vehicle to improve the hypoxic microenvironment within tumour tissues and increase chemotherapy or radiotherapy treatment efficacy.Zhuet al[62]loaded transgenic LIGHT in hUCMSCs to target tumours. LIGHT is a member of the TNF receptor family and possesses antitumour effects. In this study,the area of tumour necrosis was larger in the MSC-LIGHT group than that in the MSC group. They concluded that hUCMSCs could be an efficient vehicle to carry the LIGHT gene for gastric cancer treatment. Youet al[63]transfected the cytosine deaminase (CD) gene into human MSCs (hMSCs) and found that CD-producing BMSCs could convert 5-fluorocytosine to 5-fluorouracil, which significantly inhibited the development of gastric tumours. In these studies[61-63], the authors applied MSCs as carriers for suicide genes for the treatment of gastric cancer and found that these modified MSCs prevented the development of tumor cells. However, the interaction
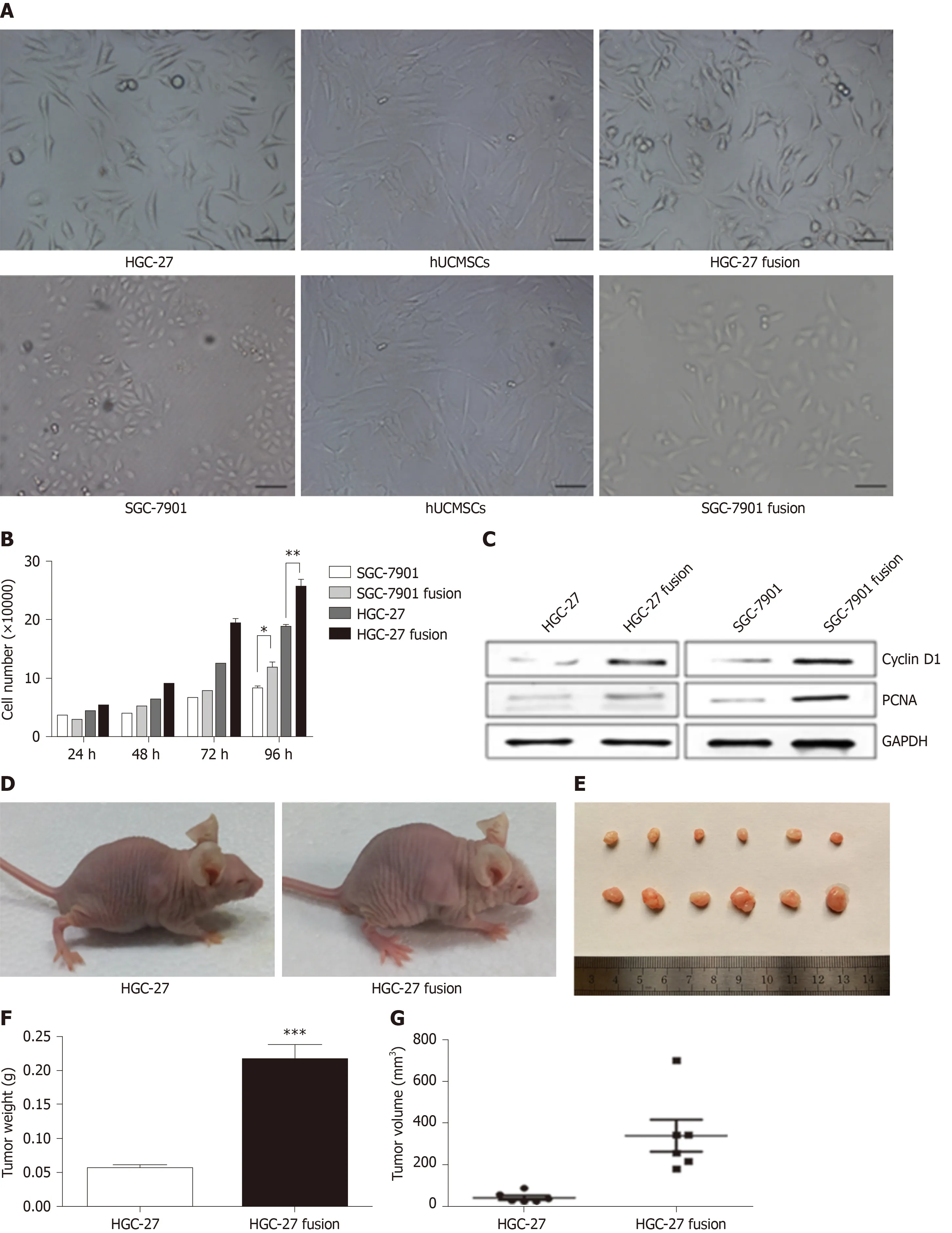
Figure 2 The effects of hybrids (HGC-27 or SGC-7901 fused with mesenchymal stem cells) on gastric cancer[60]. A: The morphology of the two gastric cancer cell lines, hUCMSCs, HGC-27 fusion, and SGC-7901 fusion; B: The growth of the parental and hybrid cells was determined by cell counting assay; C: The expression of PCNA and CyclinD1 proteins in different groups; D: Representative images of the gastric tumor bearing mice; E: The images of the tumor tissues; F: Tumor weight;and G: Tumor volume. hUCMSCs: Human umbilical cord mesenchymal stem cells; PCNA: Proliferating cell nuclear antigen.
MSCS FOR HEPATOMA
MSCs can inhibit or promote hepatoma progression in specific conditions, which is similar to that of gastric cancer.
By TNF-related apoptosis-inducing ligand (TRAIL) transfection, MSCs can activate death receptor (DR) 5 in hepatoma cells (HepG2), thus inducing the apoptosis of cancer cells[64]. The number of apoptotic tumour cells is positively correlated with the expression level of TRAIL on MSCs. However, evidence to confirm whether MSCs can express TRAIL and activate DR on the surface of tumour cellsin vivois lacking. At the same time, thein vivoexpression level and condition of TRAIL are still insufficient.Liuet al[65]established a controllable TRAIL expression adenovirus vector and transfected it into murine MSCs. TRAIL was controllably expressed in the presence of doxycycline, and secreted TRAIL suppressed the viability of human hepatoma cells(SMMC-7402). In this study, MSC-mediated TRAIL expression was artificially controlled. However, the controlled TRAIL expressionin vivoand its safety within the human body need further investigation.
Previous studies have indicated that MSCs could achieve hepatoma cell inhibition by regulating the Wnt signalling pathway. In a study, H7402 and HepG2 hepatoma cells were cocultured with human fetus MSCs[66]. The malignant phenotypes of H7402 and HepG2 cells were inhibited, and the expression of β-catenin, Bcl-2, c-Myc, and PCNA was decreased. The researchers concluded that MSCs could inhibit hepatoma developmentin vivoandin vitroby inhibiting the Wnt pathway. In another study,researchers established a liver cancer model in mice using a chemically induced method[67]. The administration of murine BMSCs (mBMSCs) to mice suppressed tumour progressionviadownregulation of the Wnt signalling pathway. Serhalet al[68]found that human adipose-derived MSCs could inhibit the growth of liver tumours by overexpressing P53 and RB and suppressing the expression of c-Myc and human telomerase reverse transcriptase (hTERT). Other studies have also shown that MSCs prevent hepatoma growthviathe Wnt signalling pathway[67,69].
Other studies also reported that MSCs could induce the apoptosis of hepatoma.Seyhounet al[70]combined sorafenib and human fetus MSCs for the treatment of liver cancer. In another study, hUCMSCs alone inhibited the proliferation of tumour cells by suppressing the expression of Ki-67, prevented tumour angiogenesis by downregulating CD34, and induced the apoptosis of tumour cells[71].
The regulation of metabolic levels may be another way for MSCs to inhibit hepatoma growth. Qiaoet al[72]found that hMSCs depressed H7402 proliferation by downregulating the mRNA level of NF-κB. However, the further concrete mechanism of the hepatoma inhibition effect of MSCs at the metabolic level is still unclear. In another study, the researchers applied portal vein embolization and hBMSC infusion for the treatment of postsurgical patients with liver cancer[73]. Compared with the control group, the treatment efficacy of embolization and hBMSC infusion was higher.This may be because stem cells can be affected by growth-promoting factors in the liver microenvironment, such as hepatocyte growth factor and epidermal growth factor, resulting in liver regeneration[74]. Unfortunately, we cannot conclude that MSCs directly participate in the inhibition of liver cancer cells or reduce the recurrence rate.
Because MSCs have the ability to migrate to tumour tissues, many researchers have applied MSCs as a tool for loading antitumour components, including various cytokines, suicide genes, radioactive substances,etc., to achieve tumour treatment. In a study, mBMSCs were adenovirally engineered to secrete interleukin-12 (IL-12) and injected subcutaneously to prevent the formation of hepatoma[75]. They found that mBMSCs could express IL-12 and inhibit tumour formation efficiently. However, the specific mechanism is unknown, and there is no study on whether MSCs migrate to other parts of the body. Niesset al[76]transfected the thymidine kinase suicide gene into mBMSCs and injected the modified MSCs into a liver cancer mouse model. It was found that such MSCs can be enriched in the tumour site, and the quality of the tumour was reduced compared with the control group after the suicide gene was activated by the promoter. Unfortunately, this study did not perform safety analysisin vivo, and it is not known whether suicide gene-modified MSCs have a negative impact on other parts of the body. Yoonet al[46]infected MSCs with hepatoma carcinoma-targeted oncolytic adenovirus. The modified MSCs homed to the hepatoma site and resulted in the accumulation of a large number of virions that lysed the tumour cells and led to the inhibition of tumour growth. In another study,mBMSCs were engineered with vectors expressing IFN-gamma and IL-10[77]. The combination of these two kinds of cytokines in MSCs significantly suppressed the viability of hepatoma cells. However, these studies only focused on the tumour homing effects of MSCs, the safety evaluation of MSCs application should be further studied.
Some researchers also hold the opinion that MSCs promote the progression of hepatoma, and the possible reasons include that MSCs participate in the formation of tumour stroma, promote the expression of transforming growth factor β, and enhance tumour angiogenesis. In a study, researchers co-cultured hBMSCs with MHCC97-H hepatoma cells and found that the proliferation of hepatoma cells increased and the invasion ability decreased[78]. Consistent with thein vitroresults, thein vivoexperiments in nude mice showed that the tumour volume increased rapidly with the administration of hBMSCs, but the lung metastasis rate decreased. The authors pointed out that this may be related to the fact that MSCs participated in the formation of the local tumour matrix, which was conducive to tumour colonization.However, Yanet al[79]showed that liver cancer associated MSCs can not only promote the growth of liver cancer cells but also enhance distant metastasis. They first discovered the presence of MSCs in liver cancer tissues. The enhancement effect of MSCs on hepatoma cells may be related to the secretion of S100A4, which can increase the expression of miR-155, thus downregulating the cell signal transduction inhibitor SOCS1 and activating STAT3 signalling. In comparison of the studies performed by Qianet al[66]and Liet al[78], we can find that different types of MSCs may play different roles in tumor growth. In addition, the animal modeling methods, animal species, and administration routes of MSCs may affect the roles of MSCs in the development of GI malignancies. In Qian's[66]study, SCID mice were subcutaneously injected with human liver cancer cells (H7402 cell line) and human fetus MSCs. In Li's[78]study,MHCC97-H cells were subcutaneously injected into nude mice and hBMSCs were intravenously injected three times a week.
Interestingly, Zonget al[80]found that mBMSCs could play different roles in different stages of hepatoma. In the initial stage of liver cancer, MSCs can suppress tumour growth by reducing DNA damage and the levels of reactive oxygen species(ROS) and exhibit anti-fibrosis and anti-inflammatory effects. However, for the progressive stage of liver cancer, MSCs could promote the proliferation of cancer cells by enhancing the epithelial-mesenchymal transition of the tumour cells (Figure 3).
MSCS FOR PANCREATIC CANCER
Because of the anti-inflammatory and tumour homing effects, MSCs have also been applied for the study of pancreatic cancer (PC)[14]. However, the treatment efficacy is still controversial, and the specific mechanism is poorly understood.
Many studies have reported the prevention effect of naïve MSCs in PC. Doiet al[81]co-cultured UCMSCs and PAN02 PC cell lines and found that the PC cells were arrested at the G0/G1 phase; thus, the proliferation of the tumour cells was inhibited.Thein vivostudies showed that UCMSC treatment significantly reduced the peritoneal tumour burden and increased the survival time of mice. Kiddet al[82]injected hBMSCs or hBMSCs expressing interferon (IFN)-β intraperitoneally into mice with PC. The authors found that MSCs alone could exert antitumour effects on PANC-1 cells and that MSCs could also act as vehicles for IFN-β transportation for further tumour inhibition.
Based on the tumour homing characteristics, MSCs can also be applied as carriers to load cytokines and genes for PC treatment. Shanget al[83]found that a low expression level of miR-1231 in blood was correlated with the pathological stage of PC, which indicated the inhibitory role of miR-1231 in PC growth. As a result, they transfected miR-1231 into the exosomes of hBMSCs and found that the modified exosomes restricted the development of PC (Figure 4). In another study, hBMSC exosomal miR-126-3p was developed and tested to inhibit the proliferation, invasion,and metastasis of PC cells[84]. Hanet al[85]designed a kind of TRAIL-secreting hMSC in which photochemical internalization was applied to enhance the entrapping and transfection efficacy into MSCs. Photochemical internalization increased TRAIL expression in MSCs, and the tumour homing effect enhanced tumour targeting, which resulted in a significant tumour apoptosis effect. Briniet al[86]obtained MSCs from human gingival interdental papilla and applied gingival-derived MSCs as vehicles to transport paclitaxel to CFPAC-1 cells. Zischeket al[87]transfected mBMSCs with the suicide gene thymidine kinase (TK), which is driven by chemotactic cytokines. These mBMSCs resulted in 50% PC growth and reduced the tumour metastasis rate. Even though, MSCs can be used as vehicles in PC treatment, the primitive effects of MSCs towards PC were not clearly demonstrated[83-87].
Contrary to the reports that support the anticancer effect of MSCs, some studies hold the opinion that MSCs can promote pancreatic cancer progression. In one study,researchers found that the coexistence of pancreatic ductal adenocarcinoma (PDAC)and MSCs upregulated the expression of amphiregulin (AREG) and MMP-3 in cancer cells and MSCs, respectively[88]. This phenomenon resulted in enhanced tumour invasion. Blockage of AREG expression prevented the MSC-induced PDAC progression effect. Because of the inflammatory nature of PC, increasing the expression of inflammatory cytokines within the tumour microenvironment may lead to the development of cancer. In one study, the researchers found that MSCs could promote the secretion of TNF-α and IFN-γ, thus leading to the invasion of PC cells[89].Mathewet al[90]found that MSCs within tumour tissues could promote tumour growth by alternating macrophage polarization.
MSCS FOR COLORECTAL CANCER
Colorectal cancer (CRC) ranks as the third most common cancer leading to cancerrelated modalities worldwide. Due to their anti-inflammatory and tumour homing properties, MSCs have also been studied in CRC studies. However, the controversial treatment efficacy of MSCs on CRC induces concerns for further research.
MSCs are widely used as vehicles to carry agent or specific genes for the treatment of CRC. Xuet al[17]transfected miR-16-5p into the exosomes of BMSCs and found that exosomes inhibited the proliferation, invasion, and migration and increased the apoptosis of CRC cells by decreasing the expression of integrin alpha 2. Thein vivostudy demonstrated that BMSC-derived exosomes transfected with miR-16-5p significantly suppressed CRC growth. Chenet al[91]found that the expression of miR-4461 was lower in CRC tissues than in normal tissues and that the expression of COPB2 was negatively correlated with miR-4461 because miR-4461 targeted COPB2.The authors transfected miR-4461 genes into hBMSC-derived exosomes and found that exosomes could downregulate the expression of COPB2, thus inhibiting the proliferation of CRC cells. Many other studies also transfected MSCs with suicide genes or loaded MSCs with tumour killing agents for CRC treatment[92-95].
Similar to many other kinds of cancer, MSCs can also interact with cancer cells extensively and promote CRC development. Chenet al[96]investigated TNF-αpreactivated hBMSCs in the growth of CRC cells. The authors found that TNF-αpreactivated MSCs could secrete high levels of CCL5, which binds with its receptor in CRC cells, thus enhancing the proliferation, invasion, migration, and epithelialmesenchymal transition of cancer cells. The tumour-promoting effect of TNF-αpreactivated MSCs is associated with the upregulation of the beta-catenin signalling pathway. Ohet al[97]co-cultured hBMSCs and CRC cells and found that the proliferation and invasion of CRCs were increased with the upregulation of TGF-β1 and downregulation of p53. In one study, the authors showed that mBMSCs could increase C26 CRC growthin vivo[98]. The tumour-promoting effect of MSCs could be further enhanced by inflammatory cytokines (TNF-α and IFN-γ), which are common in the tumour microenvironment. Inflammation-prestimulated MSCs could increase the tumour angiogenesis effect. Wuet al[99]found that high expression levels of C-C chemokine receptor type 5 (CCR5) were associated with the poor prognosis of CRC patients. Strong CCR5 expression has been confirmed in many CRC cell lines and within the cytoplasm of BMSCs. The authors co-cultured HCT116 cells with BMSCs and found that the proliferation of CRC cells was significantly promoted, especially in HCT116 cells with high CCR5 expression (Figure 5). Furtherin vivostudies also indicated that BMSCs promoted CRC progressionviaCCR5. In a study performed by Wuet al[100], nude mice were intraperitoneally injected with HCT116 cells suspended in hBMSCs concentrated medium. The authors found that MSCs performed a tumorpromoting effect. Wanget al[101]established xenograft tumour model by injecting the flanks of nude mice with CRC cells (SW480, LS174T, HT29) mixed with hBMSCs. The results indicated that hBMSCs could promote the growth of CRC.
Interestingly, some researchers pointed out that the effects of MSCs on CRC might depend on the malignancy of cancer cells and the expression levels of polypyrimidine tract-binding protein 1 (PTBP1). Fuet al[102]found that hBMSCs increased the progression of the low malignancy CRC cell line HT29 but showed no obvious effect on the high malignancy CRC cell line HCT 116. The expression level of PTBP1 was decreased in HT29 cells co-cultured with MSCs. Upregulation of PTBP1 decreased the MSC-induced invasion of HT29 cells. In this study, the researchers indicated that MSCs can play different roles for cancer cells with different malignancy. In addition,MSCs are dominated by cancer cells with high malignancy and cannot perform significant changes on the cancer cells. Some researchers also thought that adipose tissue-derived MSCs might play different roles in different kinds of CRC cell lines.Ipliket al[103]co-cultured adipose derived MSCs with HT29, HCT116, and RKO. After 48 hours of incubation, the expression level of caspase 3 decreased in RKO and HT29 cells but increased in HCT116 cells. Their results indicated that adipose tissue-derived MSCs significantly induced apoptotic cell death in HCT116 cells compared with the other cell lines. In another study, Kanget al[104]reviewed studies concerning the effects of MSCs on inflammatory bowel disease (IBD). Based on the interaction effects and paracrine actions, MSCs have shown therapeutic properties towards IBD. The authors also anticipate that the treatment of CRC treatment by MSCs holds promise due to the tumour homing and inflammation inhibition effects. Prakashet al[105]also summarized MSC treatment for IBD in both animal and clinical studies. The authors further focused on the therapeutic effects of MSCs on IBD-induced CRC.
CONCLUSION
Recent studies have shown that there are still controversies regarding the effects of MSCs on the proliferation and invasion of GI malignancies. The reasons why MSCs may play different roles in GI malignancies are summarized here: (1) The types of MSCs used in previous studies are different (e.g., hBMSCs, hAMSCs, hUCMSCs,foetal epithelium MSCs, porcine MSCs, murine MSCs). Different MSCs possess different biological features. (2) Different types of GI cancer cells have been applied,such as SGC-7901, MGC-803, BGC-823, HGC-27, MKN-45, MKN-7,etc., for gastric cancer studies. Different cancer cell lines affect the experimental results in terms of cell malignancy, invasiveness, proliferative capacity, and surface markers. (3) The administration routes of MSCs for GI malignancies are different. These routes mainly include intravenous administration ahead of the animal model, intravenous administration post the animal model, and direct injection into the tumour body. And(4) The animal models, modelling methods, and animal species are different. Nude mice are commonly used in most studies, but the occurrence and development of tumours are closely related to the immune status of the body. We cannot observe the effects of MSCs on the immune system due to immunodeficiency in nude mice.
Concerning the current research, an increasing number of researchers have turned their attention to modified MSCs, which have been applied as carriers for agent and gene therapy. The main methods for modifying MSCs include the transfection of various suicide genes and the transportation of many therapeutic agents.Nevertheless, it should be noted that most of the inherent properties and biological characteristics of MSCs are not changed. The effects of primitive MSCs and tumours are not uniform, and the interaction between primitive MSCs and tumours and the detailed mechanisms need to be further studied, especially regarding the safety of MSCsin vivo.
Great progress has been made in anticancer studies that applied modified MSCs as gene or agent carriers. However, most researchers mainly focus on the tumour homing effects of MSCs but do not consider the fact that a small amount of MSCs can be present in other parts of the body. When foreign genes are transfected, the expression products may have an effect on normal tissues. As a result, studies that can enhance the tumour homing effects of MSCs while decreasing their accumulation in other parts are necessary. In addition, recent studies investigating the effects of MSCs on GI malignancies were mainly performed in cancer cells and/or animal models.Clinical trials are important and are needed to clearly demonstrate the safety and concrete treatment efficacy of MSCs on GI malignancies.
猜你喜欢
杂志排行
World Journal of Gastrointestinal Oncology的其它文章
- Potential microRNA panel for the diagnosis and prediction of overall survival of hepatocellular carcinoma with hepatitis B virus infection
- LlNC00511 promotes gastric cancer cell growth by acting as a ceRNA
- Primary tumor location and survival in colorectal cancer: A retrospective cohort study
- Robotic- vs laparoscopic-assisted proctectomy for locally advanced rectal cancer based on propensity score matching: Short-term outcomes at a colorectal center in China
- Diagnostic ability of multi-detector spiral computed tomography for pathological lymph node metastasis of advanced gastric cancer
- Nomogram using F-18 fluorodeoxyglucose positron emission tomography/computed tomography for preoperative prediction of lymph node metastasis in gastric cancer
