Complete response to trastuzumab and chemotherapy in recurrent urothelial bladder carcinoma with HER2 gene amplification: A case report
2020-05-14
Qi Jiang, Xiao-Chen Zhang, Department of Medical Oncology, The First Affiliated Hospital,College of Medicine, Zhejiang University, Hangzhou 310003, Zhejiang Province, China
Mi-Xue Xie, Senior Department of Haematology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310003, Zhejiang Province, China
Abstract
BACKGROUND
Targeted treatments may greatly affect the natural history of urothelial carcinoma based on their pharmacokinetics. A phase II trial has explored the combination of cytotoxic chemotherapy with the anti-HER-2 monoclonal antibody trastuzumab in selected patients with metastatic bladder cancer, but it failed.
CASE SUMMARY
Here, we report a case of recurrent urothelial bladder carcinoma (UBC) in a patient who has undergone three operations, and further illuminate its diagnosis and treatment. The diagnosis of UBC was rendered according to the pathological indices. Next-generation sequencing on formalin fixed paraffin-embedded (FFPE)tissue was also performed and suggested HER2 gene amplification in the FFPE tissue. Based on HER2 gene amplification in FFPE, the patient was treated with chemotherapy in combination with trastuzumab after his third surgery.Fortunately, the patient got a clinically complete remission to trastuzumab for 34 mo.
CONCLUSION
There is not enough clinical evidence for incorporating trastuzumab in routine treatment of UBC. This case hinted that recurrent UBC patients with HER2 gene amplification may benefit from targeted trastuzumab. Further studies are needed to further investigate the status of HER2 gene and better determine trastuzumab in the management of UBC.
Key words: Urothelial bladder carcinoma; Trastuzumab; Complete response; Next generation sequencing; HER2; Case report
INTRODUCTION
It has been suggested that a way forward in the treatment of advanced or metastatic urothelial carcinoma may be consistent with the progress made in the targeted therapy of advanced breast cancer, where trastuzumab-based therapy has shown substantial benefit in patients presenting tumors with overexpression and/or amplification of theERBB2gene, which encodes the human epidermal growth factor receptor 2 (HER2). A recent phase II clinical trial (NCT01828736) of advanced or metastatic urothelial carcinoma explored the combination of chemotherapy(gemcitabine and platinum) with trastuzumab. However, the results are similar to those achieved with cytotoxic chemotherapy alone, and the contribution of trastuzumab in this single-arm phase II trial is unclear[1]. Patients were selected for enrollment based onHER2overexpression by immunohistochemistry, gene amplification, and/or elevated serum HER-2. Different tests and “cut-offs” for the putative predictive biomarkers may be the key reasons for the failure of this trial[2].Herein, we present a recurrent urothelial bladder carcinoma (UBC) patient withHER2gene amplification tested by targeted next-generation sequencing (NGS), and the patient has benefited from targeted trastuzumab up to present.
CASE PRESENTATION
Chief complaints
A 43-year-old Chinese man presented to the Medical Oncology Department of our hospital complaining of recurrent UBC for which he has undergone three operations.
History of present illness
In March 2013, the patient presented with pain and intermittent hematuria for 3 mo.On April 12, 2013, he received partial cystectomy for high-grade papillary urothelial carcinoma (WHO grade III). Pathology confirmed that the surgical margin was negative. After four cycles of gemcitabine and carboplatin (GC) as adjuvant chemotherapy, he experienced local recurrence of the bladder, and then received radical cystectomy and ureterocutaneostomy for bladder infiltrating urothelial carcinoma, classified as rpT4aN0M0 on November 22, 2013. From December 2013 to May 2014, he received six cycles of TP (paclitaxel and cisplatin) as first-line chemotherapy. On July 12, 2016, he experienced residual urethra progression and left inguinal lymph node enlargement, and then received the third operation to remove the left inguinal lymph nodes that were pathologically confirmed to have tumor infiltration.
History of past illness
The patient’s main previous medical history was cystolith and pollen allergy. There was a history of pancreatic carcinoma in his patient’s family.
Physical examination
The Eastern Cooperative Oncology Group score of this patient was 0, and the numeric pain intensity scale was 0. An old surgical scar of about 10 cm can be seen in the lower abdomen, and a bladder stoma can be seen in the right lower abdomen with a drainage bag. There was no redness, swelling, or exudation around the stoma, and the urine in the drainage bag was clear.
Laboratory examinations
The routine blood examination, blood biochemistry, and urine analysis were normal.Electrocardiogram, chest X-ray, and arterial blood gas were also normal. Serum tumor markers including alpha-fetoprotein, carcinoembryonic antigen, cancer antigen 125,cancer antigen 19-9, and ferritin were routinely monitored, and only ferritin was higher than the upper limit of reference range and trended to be associated with tumor burden. Detailed monitoring values are shown in Figure 1. Left inguinal lymph nodes were resected during the third operation, and the pathology suggested urothelial carcinoma metastasis, Immunohistochemistry showed hepatocyte (-), GPC-3 (-), PSA (-), TTF-1 (-), CK7 (+), CK20 (+), P63 (+), GATA-3 (+), CK5/6 (+), P504S(part +), and CD44 (+).
Imaging examinations
Pelvic magnetic resonance indicated postoperative changes of bladder cancer (after the third operation).
Further diagnostic work-up
A customized NGS panel targeting 416 genes was further carried out on formalin fixed paraffin-embedded sample, with white blood cells used as a negative control.The sequencing results suggestedERBB2(HER2) amplification in formalin fixed paraffin-embedded sample (Figure 2).
FINAL DIAGNOSIS
Recurrent urothelial bladder carcinoma (TNM stage: rpT4aN0M1a).
TREATMENT
After a multidisciplinary consultation, a second-line regimen was decided with trastuzumab 6 mg/kg every three weeks after a loading dose of 8 mg/kg and cisplatin 75 mg/m2every three weeks, from September 2016. After five cycles, this treatment was disrupted because of the patient’s economic condition.
OUTCOME AND FOLLOW-UP
Serum tumor markers including alpha-fetoprotein, carcinoembryonic antigen, cancer antigen 125, cancer antigen 19-9, and ferritin were routinely monitored, and only ferritin was higher than the upper limit of reference range and trended to lower(Figure 1). Fortunately, there was no recurrence until now. The patient got a clinically complete remission to trastuzumab for 34 mo.
DISCUSSION
At diagnosis, approximately 25% of patients with UBC presentde novowith metastatic disease[3]. Unfortunately, approximately 11% of patients with UBC have regional or distant metastases at initial presentation, with 5-year survival rates of about 35% and 5%, respectively[4]. Compared with other solid tumors, UBC is a chemosensitive malignancy characterized by relatively high response rates to combination chemotherapy. Cisplatin-based combination chemotherapy has been shown to improve survival benefit, not only in adjuvant and neoadjuvant therapy for patients with locally advanced carcinoma but also in the patients with metastatic disease.Methotrexate, vinblastine, doxorubicin, cisplatin, and GC are currently the standard first-line regimens (Table 1) for locally advanced or metastatic disease[2]. Available therapies for management of platinium-refractory metastatic UBC are not satisfactory with a response rate that is low in second-line therapy with some agents (Table 2) and with no validated third-line therapy[2].

Figure 1 Changes of serum ferritin levels in this patient.
HER2gene plays a critical role in the pathogenesis of UBC. Several studies have reported that the overexpression rate of HER2 protein is between 5% and 80% in UBC[3]. Laéet al[5]analyzed the HER2 status of tissue specimens from 1005 patients with muscle-invasive bladder cancer and found that overexpression of HER2 protein andHER2gene amplification accounted for 11.4% and 5.1%, respectively[5]. Several clinical trials have explored inhibitors of the HER2 pathway in selected patients with metastatic UBC (Table 3)[1,6,7]. A phase II trial of patients with metastatic bladder cancer explored the combination of cytotoxic chemotherapy (GC and paclitaxel) with the anti-HER-2 monoclonal antibody trastuzumab. The patients were selected for enrollment based HER-2 overexpression by immunohistochemistry, gene amplification, and/or elevated serum HER-2. Thirty-one of forty-four (70%) patients achieved objective responses (5 complete and 26 partial). However, these results are similar to the results achieved with cytotoxic chemotherapy alone, and the contribution of trastuzumab in this single-arm phase II trial is unclear. Another phase II clinical trial explored lapatinib [the dual HER-2/ epidermal growth factor receptor(EGFR) pathway inhibitor] as second-line therapy in metastatic BC patients. The patients were eligible provided that they had 1+, 2+, or 3+ expression of either EFGR or HER-2 by immunohistochemistry (from either primary or metastatic tumor samples). An objective response to treatment was observed in 1.7% (95% confidence interval: 0.0%–9.1%) of patients; however, 18 (31%; 95% confidence interval:19%–44%) patients had a stable disease. Further analysis revealed that clinical benefit was associated with EGFR overexpression, and, to some extent, HER-2 overexpression. The same pathway inhibitors were used in these trials, but the patients were selected for enrollment based on HER-2 overexpression by immunohistochemistry, gene amplification, and/or elevated serum HER-2. Different tests and ‘‘cut-offs’’ for the putative predictive biomarkers may be the critical factors in the era of targeted therapeutics. The rate ofHER2gene mutation is about 2% in breast cancer, but it was not reported in urinary epithelial carcinoma[8]. Studies have shown thatHER2gene mutation is one of the mechanisms of anti-HER2 therapy (e.g.,herceptin and lapatinib) for drug resistance[9,10]. In this case, the patient harboredHER2gene amplification but noHER2mutation tested by NGS achieved more than two years of disease-free progression after his third surgery, which might be the important factor in the effectiveness of trastuzumab.
CONCLUSION
This case hinted that recurrent UBC patients withHER2gene amplification may benefit from targeted trastuzumab. Further studies and cases are needed to further investigate the status ofHER2gene and better determine trastuzumab in the management of UBC, particularly after failure of routine therapy.
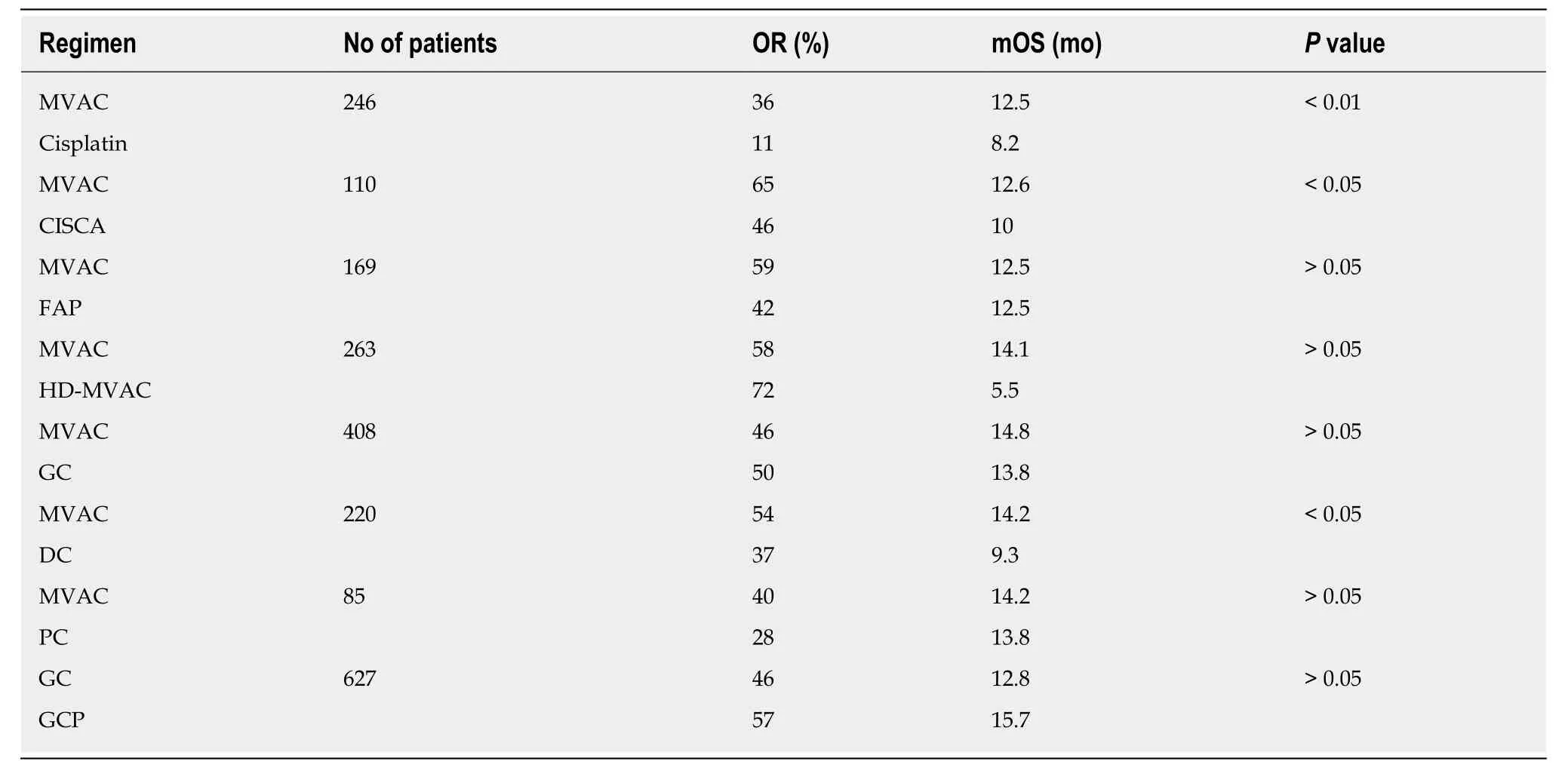
Table 1 Randomized trials in metastatic bladder cancer
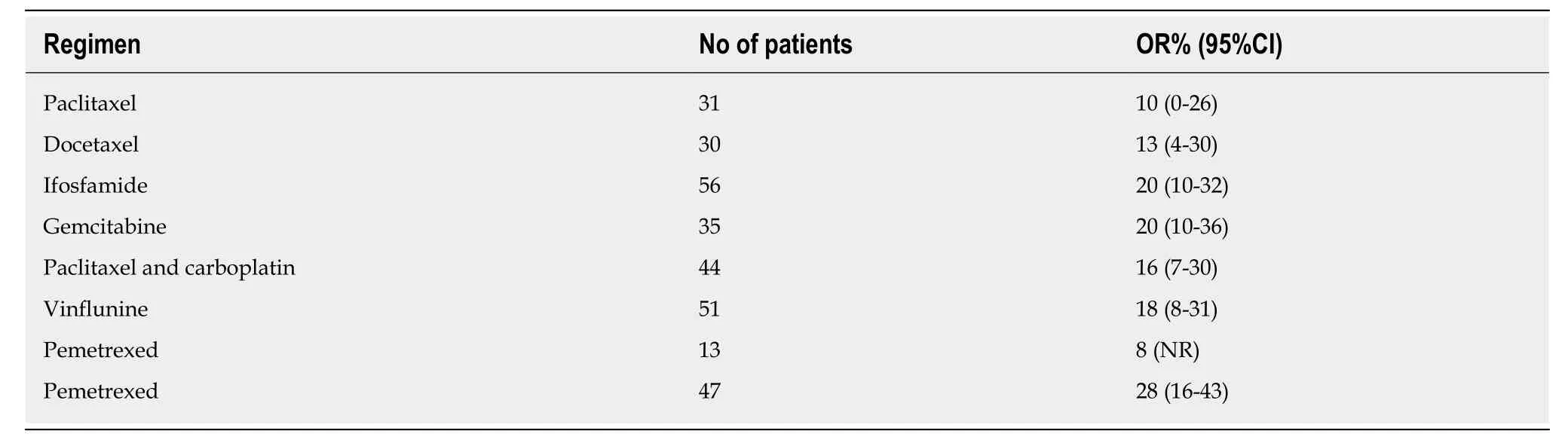
Table 2 Second-line chemotherapy in metastatic bladder cancer
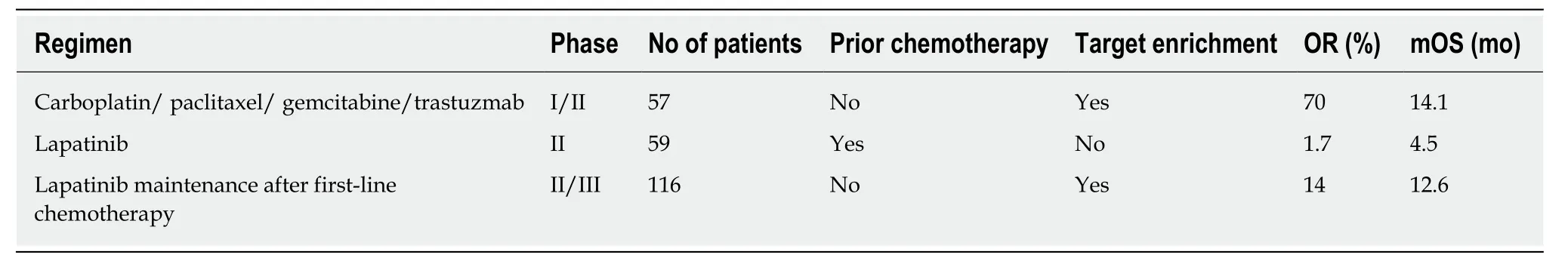
Table 3 Selected trials of anti-HER2 agents in advanced bladder cancer
杂志排行
World Journal of Clinical Cases的其它文章
- Comprehensive review into the challenges of gastrointestinal tumors in the Gulf and Levant countries
- Can cyclin-dependent kinase 4/6 inhibitors convert inoperable breast cancer relapse to operability? A case report
- Ruptured splenic peliosis in a patient with no comorbidity: A case report
- Successful kidney transplantation from an expanded criteria donor with long-term extracorporeal membrane oxygenation treatment: A case report
- Boarding issue in a commercial flight for patients with cavitary pulmonary tuberculosis: A case report
- Cytomegalovirus ileo-pancolitis presenting as toxic megacolon in an immunocompetent patient: A case report
