Effects of theophylline/chitosan/β-cyclodextrin microspheres for oral drug delivery on an asthmatic rat model
2020-05-07SaisaiWangHaijingZhangXiaomingCuiLipingZhaoMingxiaJiangZhiluXuDongzhenMuWeifenZhang
Saisai Wang, Haijing Zhang, Xiaoming Cui, Liping Zhao, Mingxia Jiang, Zhilu Xu, Dongzhen Mu, Weifen Zhang,4,5 *
ARTICLE
Effects of theophylline/chitosan/β-cyclodextrin microspheres for oral drug delivery on an asthmatic rat model
Saisai Wang1#, Haijing Zhang2#, Xiaoming Cui1, Liping Zhao1, Mingxia Jiang1, Zhilu Xu1, Dongzhen Mu3*, Weifen Zhang1,4,5 *
1School of Pharmacy, Weifang Medical University, Weifang, Shandong 261053, China;2Intensive Care Unit, The First people’s hospital of Hefei, Hefei, Anhui 230061, China;3Department of Immunology, Key lab for Immunology in Shandong Province, School of clinical Medicine, Weifang Medical University, Weifang, Shandong 261053, China;4Institute for Smart Materials and Regenerative Medicine,Weifang Medical University, Weifang, Shandong 261053, China;5Shandong Engineering Research Center for Smart Materials and Regenerative Medicine, Weifang Medical University, Weifang, Shandong 261053, China.
: This study aimed to investigate the effects of TH/CTS/β-CD microspheres by oral administration on ovalbumin allergic asthma of a rat model.
: Spray drying method was applied for preparing the TH/CTS/β-CD microspheres, which were used in treatment of asthmatic rats. At a predetermined time, the levels of eosinophil (Eos), protein, lactate dehydrogenase (LDH), aspartate transaminase (AST), glutamic pyruvic transaminase (GPT), and creatinine (Cr) were ascertained by automatic hematology and biochemical analyzers. Lung tissue histology was performed by hematoxylin-eosin staining.
: No significant differences were found about the contents of Eos in the blood, and the contents of Eos, protein and LDH in the bronchoalveolar lavage fluid between the microsphere-treated and dexamethasone-treated groups, but the levels were lower in both treated groups than in the model group. In the microsphere-treated group, the levels of Cr, AST, and GPT in the serum of rats showed no significant differences compared with the normal group. Based on lung histopathological findings, the microspheres attenuated inflammatory cell infiltration and mucus hypersecretion compared with the model group.
: This study demonstrated that TH/CTS/β-CD microspheres exerted an anti-inflammatory effect and could serve as a novel promising drug delivery system for asthma treatment.
Chitosan, Theophylline, Asthma, Microspheres, Pharmacodynamics

Background
Theprevalence and mortality rates of allergic asthma have increased worldwide in the past two decades. Thus, controlling asthma has become a growing worldwide problem [1]. Various factors may induce allergic asthma, and they include exposure to anaphylactogen, inhalation of isocyanates, and other unknown agents. Although the exact cause of asthma is unknown, it may be related to inflammation or airway stenosis [2]. Gibson discovered that non-specific infiltration of various inflammatory cells, which include eosinophil (Eos), neutrophil (Neu), and macrophage, lead to allergic asthma [3]. Previous studies had reported that the total protein level and lactate dehydrogenase (LDH) activity in bronchoalveolar lavage fluid (BALF) could be considered indexes of lung cell damage [4]. Kim et al. [5] utilized BALF to analyze Eos levels and found increased levels of Eos in the asthmatic group. Pathologically, asthmatic lung tissues were found to exhibit bronchial mucosal thickening by edema, bronchial wall remodeling, broken smooth muscle, and Eos infiltration [6].
Searching for an effective method to control allergic asthma has become a global public health problem. Inhaling corticosteroids and β2-agonists are powerful asthma treatments. Conversely, theophylline (TH), as a phosphodiesterase enzyme inhibitor, is widely used for treating allergic asthma. TH can effectively reduce airway inflammation and bronchial constriction due to its reliable therapeutic effects and low cost [7, 8]. However, the side effects of TH remain a concern at concentrations higher than 20 µg/mL [9]. The short half-life (6 h) of TH presents another problem. Conventional multiple dosing will induce drug residue in the plasma and trigger the risk of side effects [10]. To address these issues, TH-loaded microspheres with a sustained-release feature is a potential novel therapy for asthma treatment in the clinic, they act by prolonging drug action, minimizing side effects, and decreasing administration frequency. The advent of reliable generic preparations of slow-release TH only cost $8.00 per month in the United States [11]. Therefore, TH-loaded microspheres will result in a promising and huge market potential when compared with conventional dosage forms.
The microsphere delivery system is a recently developed novel drug delivery system [12]. Drug-loaded microspheres can protect the drug from metabolic activity, increase the life span of active components, control the sustained release of the drug, and reduce the administration frequency and side effects [13]. Chitosan (CTS), the N-deacetylated derivative of chitin, is a natural cationic polysaccharide [14]. CTS and its derivatives are applied to various areas, such as food, cosmetics, biomedicine, and pharmaceutical production, due to its biocompatibility, biodegradability, low toxicity, antibacterial activity, antitumor properties, immunity enhancement, and antioxidant activity [14-16]. CTS microspheres have reportedly been prepared as drug carriers due to their excellent properties [16-18]. Cyclodextrins (CD) are potentially valuable agents for increasing the aqueous solubility of lipophilic compounds [19]. CD can also improve the stability of substances against dehydration, hydrolysis, oxidation, and photodecomposition [19, 20]. Cerchiara et al.[21]used the spray drying method to prepare progesterone-loaded β-CD/CTS microspheres, which exhibited the expected controlled release curve. Kumar et al.reported that pravastatin sodium-loaded β-CD microparticles considerably enhanced therapeutic properties, and CD increased the drug absorption and solubility, decreased the side effects, and controlled drug release from the microspheres [22, 23]. Thus, TH/CTS/β-CD microspheres could improve the bioavailability and diminish the side effects of TH.
This study aimed to investigate the effect of TH/CTS/β-CD microspheres on allergic asthma treatment by establishing an allergic asthma animal model. TH/CTS/β-CD microspheres were prepared by spray drying method. The pharmacodynamics, hematology, histology, and biosafety of the developed microspheres were evaluated.
Materials and methods
Animals
Healthy male Sprague-Dawley rats (250±30 g, 6–7 weeks) were purchased from the Qingdao Drug Inspection Institute. The feeding conditions were as follows: a clear animal house, constant temperature, and humidity under air conditioner, quiet environment, minimum lighting, standard forage, and free drinking water. Room temperature (22±1°C) and humidity (55%±10%) were controlled automatically. All Sprague Dawley rats were provided with food and tap water. Experimental procedures were carried out in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals. Animal handling followed the regulations of the National Animal Welfare Law of China.
Materials
TH (powder, 99% purity) was kindly supplied by Minsheng Pharmaceutical Factory (Hangzhou, China). Ovalbumin (OVA) was purchased from Sigma-A5503, and 10% (w/v) or 1% (w/v) OVA was suspended by 0.9% (w/v) normal saline. The 0.9% (w/v) normal saline was acquired from Shandong Weifang Pharmaceutical Factory Co., Ltd. (Weifang, China). Dexamethasone (DXM) was obtained from Jinan Yongning Pharmaceutical Co., Ltd. (Jinan, China). CTS (molecular weight: 1.3×103kDa, deacetylation degree: ≥85%) was prepared in our laboratory by acetic acid hydrolysis as reported by Chen[24]. Pentobarbital sodium and aminophylline injection were purchased from Guangzhou Chemical Reagent Factory (Guangzhou, China) and Shandong Xinhua Pharmaceutical Co., Ltd. (Zibo, China). Monometallic sodium orthophosphate, disodium hydrogen phosphate, acetone, calm gastrin, heparin, formaldehyde, and other reagents were of analytical grade and manufactured in China.
Preparation of microspheres
Dry TH (3.4 g), CTS, and β-CD (weight ratio: 1:3:1) were fully dissolved in 1% acetic acid solution (200 mL). In the current study, the previously described microspheres were used and this was not another batch of microspheres [25]. After passing through 0.45 μm filtering film, the solution was spray-dried by a spray dryer (Büchi191 Mini Spray Dryer, B-191, Flawil, Switzerland). Using a co-current flow type with a two-fluid nozzle (diameter: 0.7 mm), the liquid formulations were atomized by contacting with compressed air through a nozzle and transformed into a hot-air stream (feed rate: 6 mL·min-1). The operation parameters were as follows: the gas flow was 600 L·h-1, the inlet temperature was 150 ±2 ℃, and the outlet temperature was 81 ±2 ℃. The microspheres were collected and stored in a desiccator containing calcium chloride for 72 h.
Characterization of microspheres
The morphology of microspheres was observed by scanning electron microscope (KYKY2800B, KYKY Technology Development Ltd., Beijing, China). A small amount of sample powder was dusted onto the sample box and sputter-coated with a layer of gold and observed under high pressure. The zeta potential of the microspheres were measured by a laser diffraction particle size analyzer (Malvern Mastersizer 2000, Malvern Instruments Ltd., UK) and a zeta potential/particle sizer (NiComp380ZLS, P.S.S. Particle Size Analyzer Company, USA).
Experimental groups and treatment
The rats were randomly divided into four groups (six rats in each group): the normal, model, DXM, and TH/CTS/β-CD microsphere groups. For all groups except for normal group, the rats were sensitized by intraperitoneal injection with 10% (w/v) OVA and 100 mg aluminum hydroxide in 1 mL normal saline once every other day for a total of 14 days [26]. After 14 days, the rats were placed in an inhalation box and treated with inhalation of 1% (w/v) OVA solution (normal saline as the solvent) powered by 7 L/min compressed air every day until the rats presented asthma symptoms for 5 days. The rats from the normal group were sensitized and challenged with 0.9% (w/v) normal saline using the same method. After 14 days, the normal and model groups were treated with 10 g normal saline (rat body weight per kilogram and every day) by 30 min inhalation before the OVA solution challenged. The DXM group was treated with 2 mg DXM solution (5 mg/mL) (rat body weight per kilogram and every day) by inhaling 30 min before inhaling 1% (w/v) OVA solution. The TH/CTS/β-CD microsphere group was treated with 60 mg TH/CTS/β-CD microspheres (rat body weight per kilogram and every day) in 5 mL normal saline by oral gavage 30 min before inhaling the 1% (w/v) OVA solution.
Symptoms of asthma exacerbation
The characteristics of allergic asthma include intermittent episodes of wheezing, cyanosis, and coughing [20]. During OVA inhalation, the rats curled up, remained still, and breathed rapidly with abdomen respiration every time. Several rats presented respiratory symptoms of wheezing, cyanosis, persistent coughing, and dyspnea. Inhalation treatment was stopped when the rats developed the symptoms.
Serum collection
After 24 h from the last OVA challenge, all rats were anesthetized by intraperitoneal injection of pentobarbital sodium (40 mg/kg, 0.3%) and fixed on the operating table in a lying posture. The chest fur was shaved and cleaned first with 70% ethanol. The anocelia was opened, and 5 mL blood was collected from the heart into an anticoagulation tube. Leukocyte (Leu) and different types of blood cells were counted using an automatic hematology analyzer. The levels of aspartate transaminase (AST), glutamic pyruvic transaminase (GPT), and creatinine (Cr) in the blood were ascertained by using an automatic biochemical analyzer. Serum samples were centrifuged at 804.96 xg for 10 min at 4 ℃ and then stored in a 2 mL freezing tube at -20 ℃ until further analysis.
Collection of BALF
The detection of BALF, as previously described by Chandler et al. [27], is a crucial method for the basic study of asthma. After collecting blood from the heart, the trachea was exposed and cannulated in the left lung for BALF collection. The BALF was collected immediately by washing the lungs thrice with 3 mL normal saline via trachea. At least 80% of the BALF should be recovered, and no blood mixing should be observed. Otherwise, the BALF should be abandoned. The total protein concentrations in the BALF were determined by Coomassie Brilliant Blue assay (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The BALF was centrifuged, the supernatant was discarded, and the cell pellet was resuspended by a phosphate-buffered solution. Then, the suspension was dropped onto slides and dried naturally. After staining with Giemsa solution, the total cell number in the BALF was counted, and differential cell counts of cytospins were performed under a high-power lens. A minimum of 200 cells were counted per slide. The rest of the BALF was centrifuged at 4 ℃ (357.76 xg) for 10 min, and the supernatants were stored at -70 ℃ until the activity of LDH was determined using a fully automatic biochemical analyzer.
Histopathological evaluation
After blood and BALF collection, right lung tissues were harvested and fixed with 10% formaldehyde. The tissues were embedded in paraffin and cut into 3 μm sections and then stained with hematoxylin-eosin for histological observation. Tissue lesion and inflammatory cell infiltration were then examined using a microscope.
Statistical analysis
Statistical data (mean ±deviation) were analyzed using the statistical software program SPSS 13.0. All statistically significant differences were analyzed by one-way analysis of variance and Student-Newman-Keuls test (S-N-K test). In all cases,0.05 was considered statistically significant. Six samples were used for each test.
Results
Zeta potential of microspheres
In our previous study, TH/CTS/β-CD microspheres were successfully prepared by spray drying with high drug loading, efficient encapsulation, and high yield. TH was sustained-release from TH/CTS/β-CD microspheres when compared with the original drug [25, 28]. The zeta potential of TH/CTS/β-CD microspheres was -37.42 mV (Figure 1).
Leu differential counts in BALF and blood
Inflammatory cells, such as Eos, Neu, and others, significantly increased in the BALF and blood of the model group compared with the normal group (Table 1 and Table 2,< 0.05). TH/CTS/β-CD microspheres significantly decreased the number of Leu and total cells in the BALF and serum in comparison with the model group (Table 1 and Table 2,< 0.05). After DXM administration, the number of Leu in the BALF (1.36 ×109/L) and blood (2.68 ×109/L) decreased and significantly differed in comparison with the model group (the number of Leu in the BALF and blood was 7.14 ×109/L and 7.97 ×109/L, respectively) (< 0.05).

Figure 1 Zeta potential of spray-dried TH/CTS/β-CD microspheres.

Table 1 The number of total cells and differential leukocytes in BALF (mean ± standard deviation, n=6, ×109/L)
Notes: Leu, leukocyte; Eos, eosinophils; Neu, neutrophils; Lym, lymphocytes; Mac, macrophage; DXM, Dexamethasone; TH/CTS/β-CD microspheres: theophylline/chitosan/β-cyclodextrin microspheres.*0.05 indicates statistically significant differences from the normal group.Δ0.05 indicates statistically significant differences from the model group. All statistically significant differences were analyzed by one-way analysis of variance and Student-Newman-Keuls test (S-N-K test).

Table 2 The number of total cells and differential leukocytes in serum (mean ± standard deviation, n=6, ×109/L)
Notes: Leu, leukocyte; Eos, eosinophils; Neu, neutrophils; Lym, lymphocytes; Mac, macrophage; DXM, Dexamethasone; TH/CTS/β-CD microspheres, theophylline/chitosan/β-cyclodextrin microspheres.*0.05 indicates statistically significant differences from the normal group.Δ0.05 indicates statistically significant differences from the model group. All statistically significant differences were analyzed by one-way analysis of variance and Student-Newman-Keuls test (S-N-K test).
LDH and protein assay of BALF
In this study, protein content and LDH activity in BALF were ascertained as sensitive inflammation biomarkers to measure lung injury. Table 3 presented the results on protein content. Protein content in the BALF significantly increased in the model group (0.97 g/L) compared with the normal group (0.86 g/L) (0.05). Compared with the model group (0.97 g/L), protein content significantly decreased in the DXM (0.85 g/L) and TH/CTS/β-CD microsphere groups (0.88 g/L) (0.05). After administration of TH/CTS/β-CD microspheres, the protein content showed no statistical significance in comparison with the DXM group (0.05). LDH activity in the TH/CTS/β-CD microspheres group (7.33 IU/L) decreased compared with that of the model group (7.67 IU/L) (0.05) (Table 3).
Toxicity of microspheres
Table 4 listed the GPT, AST, and Cr levels in the serum. GPT and Cr levels in TH/CTS/β-CD microsphere group showed no significant difference compared with the normal group (> 0.05). After treatment with TH/CTS/β-CD microspheres, the AST level decreased significantly compared with that of the model group (< 0.05).
Histopathology
In the normal group, the lung tissue structure was complete, the tissue had no Eos infiltration, each group exhibited a regular bronchial lumen, no exudate was observed in the lumen, and a smooth mucosal epithelium was detected (Figure 2A). Histopathological examination of pulmonary bronchus samples in the model group demonstrated a marked infiltration of inflammatory cells into the perivascular and peribronchial areas. Most of infiltrating cells were Eos. Additionally, the membrane of bronchioles and small vessels thickened, the smooth muscles were damaged, hypertrophy and hyperplasia occurred, and the mucous membrane epithelium of the airways exhibited edema and falling (Figure 2B). In the lung section of the DXM group, a small amount of Eos infiltration appeared around the bronchioles, the mucosa was intact, and the lung tissue structure was normal (Figure 2C). TH/CTS/β-CD microspheres inhibited the inflammatory cells from penetrating the perivascular and peribronchial areas, reduced the thickening of airway wall and small vessel (Figure 2D). In summary, airway remodeling was markedly inhibited by TH/CTS/β-CD microsphere treatment.

Table 3 Levels of LDH (IU/L) and content of protein (g/L) in BALF (mean ± standard deviation, n=6)
Notes: LDH: lactate dehydrogenase; BALF: bronchoalveolar lavage fluid; DXM: Dexamethasone; TH/CTS/β-CD microspheres: theophylline/chitosan/β-cyclodextrin microspheres.*0.05 indicates statistically significant differences from the normal group.Δ0.05 indicates statistically significant differences from the model group. All statistically significant differences were analyzed by one-way analysis of variance and Student-Newman-Keuls test (S-N-K test).
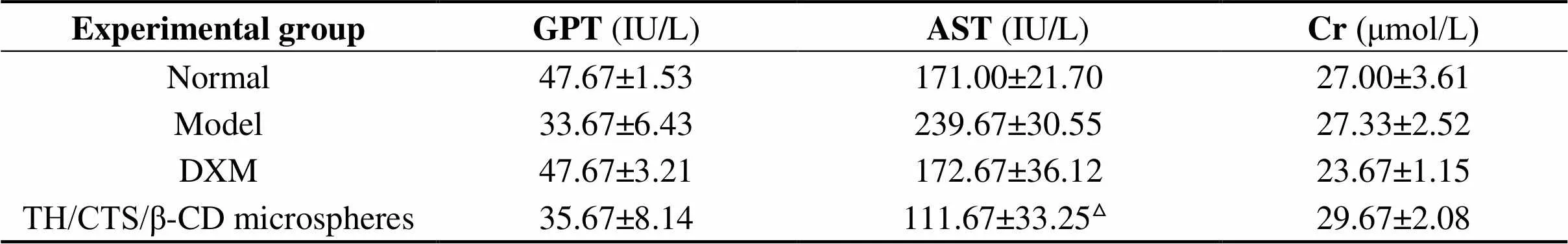
Table 4 Levels of GPT, AST, Cr in the serum of rats (mean ± standard deviation, n=6)
Notes: GPT: glutamate pyruvic transaminase; AST: aspartate transaminase; Cr: creatinine; DXM: Dexamethasone; TH/CTS/β-CD microspheres: theophylline/chitosan/β-cyclodextrin microspheres.Δ0.05 indicates statistically significant differences from the model group. All statistically significant differences were analyzed by one-way analysis of variance and Student-Newman-Keuls test (S-N-K test).

Figure 2 Effects of TH/CTS/β-CD microsphere treatment on lung histopathology of asthmatic rats. Lung tissues were collected and fixed with 10% formaldehyde. The histopathology sections (3 μm) from each rat were stained with HE for general morphology. The black arrows represent Eos and the black boxes represent the enlarged area. (A) Normal group: The structures of lung tissue and bronchi were normal, and mucosal epithelial cells were regular with no signs of falling. No inflammatory cells infiltrated the peribronchial area. (B) Model group: Edematous mucosa and broken smooth muscle were observed in the image. Marked inflammatory cells, especially Eos, infiltrated the mucosa. (C) DXM group: A few inflammatory cells infiltrated the peribronchial and perivascular areas, but the structures of lung tissue and mucosa were normal. (D) TH/CTS/β-CD microsphere group: A few inflammatory cells infiltrated the peribronchial and perivascular areas, but the structure of lung tissue was normal.
Discussion
An OVA is a widely available and inexpensive antigen which is often used to prepare the allergic asthma model. The method for preparing the rat asthma model is relatively simple, and the success rate is very high according to Watanabe’s report [26, 29, 30]. OVA-induced asthma is a chronic disease characterized by inflammation, hyperplasia and metaplasia of mucus secreting cells, airway hyper responsiveness (AHR), airway obstruction, and remodeling [31]. Inflammatory mediators promote the maintenance of allergic response by recruiting Eos and Leu to the airways [32]. The notable rising counts of Eos represent a main feature of asthma, and this condition is also positively correlated with airway inflammation and AHR [7]. This allergic inflammation model has been applied to various animal species (rat, guinea pig, and mouse)[29, 30]. In our previous study, we have established both rat and mouse models [8, 13]. In this study, TH/CTS/β-CD microspheres group was treated with TH/CTS/β-CD microspheresby oral gavage. We were more familiar with rats on oral gavage and collection of bronchoalveolar lavage fluid, so rats were chosen as an asthmatic model. In recent years, conventional drugs, such as TH and corticosteroids, were widely used for the management of asthma owing to their low cost and availability [33]. However,the effects of these drugs were not always satisfactory in clinical application due to their side effects. TH can be rapidly absorbed and degraded in a short time, thereby requiring frequent dosage to retain therapeutic concentration and control asthma. However, frequent dosage can cause drug accumulation in the plasma. Compared to β₂agonists and corticosteroids, the oral TH sustained-release preparation can slow release of drugs, reduce the frequency of administration, make plasma-drug concentration steady, is more convenient to use and has a lower price [8]. A previous study revealed that bletilla striata polysaccharide/CTS microspheres could delay the release of oligomeric proanthocyanidins. Furthermore, oral microspheres could decrease the side effects, and the hydrophobicity of CTS could hinder the diffusion of enzymes and water molecules through microspheres [12]. In our previous study, TH was sustained-release from TH/CTS/β-CD microspheres, which provided a release rate of 72.00% within 12 h, indicating that TH prevented transient caffeine-like side effects [28]. Our previous studies on TH/CTS/β-CD microspheres showed that after loading TH in the CTS microspheres, in vitro drug release was evidently delayed, and the pharmacological effect was prolonged [34]. In a previous study, TH/CTS/β-CD microspheres with high performance of drug loading rate and encapsulation efficiency were successfully prepared through spray drying method as a sustained-release drug [34]. The microspheres showed a needle-like structure, which might be caused by the TH whiskers existing on the surface of microspheres [25]. The volume distributions of 50% of the particles (< 4.9 μm) and the particle size distributions (0.5-10 μm) of the microspheres were suitable for oral administration. In our prior research, the initial drug release of TH/CTS/β-CD microspheres reached 39.00%, which was relatively low; however, the release rate totaled 72.00% within 12 h, indicating that that TH/CTS/β-CD could prolong drug release [13]. In this paper, TH was wrapped by CTS and β-CD to form microspheres and reduced toxic side effects. Compared to the previous studies, this study aimed to evaluate the effects of TH/CTS/β-CD microspheres by oral administration for asthma treatment. The clinical symptoms of asthma, such as airway obstruction, coughing, dyspnea, and wheezing, were described in a previous study [35, 36]. After inhaling OVA, the rats exhibited respiratory symptoms of persistent coughing, gasping, and dyspnea. These hallmark manifestations were assumed to underpin asthma symptoms. Thus, the asthmatic model was prepared successfully in our experiment.
The exact mechanisms of asthma remain unclear, but airway inflammation is specific to bronchial asthma [37]. Airway inflammation in bronchial asthma consists of cellular infiltration, submucosal edema and increased airway secretions [35]. Increase in eosinophilic inflammation and enhancement of AHR are regarded as cardinal features of asthma for afflicted individuals. Numerous inflammatory cells, including Eos, Neu, lymphocytes, and basophils, are also necessary mediators of airway inflammatory response [37, 38]. A rat model of allergic asthma was developed in our study, and the serum and BALF were analyzed to assess the effect of TH/CTS/β-CD microspheres on airway inflammation in asthma. Our results showed that the total cell, Eos, Neu, and lymphocytes in the BALF and serum of the model group significantly increased and significantly differed from those of the normal group (0.05). Thus, the asthmatic model had been prepared successfully in our experiment according to previous studies. DXM is an anti-inflammatory drug that belongs to a glucocorticoid class. Glucocorticoids can inhibit several inflammatory mediators implicated in bronchial asthma. DXM can markedly suppress Eos activation and reduce pulmonary vascular permeability and the leakage of anti-inflammatory plasma proteins [39]. Compared with the DXM, TH/CTS/β-CD microspheres had the following advantages: low cost; could reduce the stimulation of the respiratory tract; had the characteristics of slow release, could significantly extend the local blood concentration time limit, and improve bioavailability, reduce the dosage, minimize systemic side effects to patients, and reduce the number of dosing, increase compliance, thereby reducing patient suffering. The total number of Leu, especially the percentage of Eos in BALF and serum, showed a substantial reduction in the TH/CTS/β-CD microsphere and DXM groups compared with the model group (0.05). Thus, TH/CTS/β-CD microspheres and DXM markedly suppressed the increase in inflammatory cells. In our previous study, TH/CTS/β-CD microsphere administered by inhalation also significantly inhibited the infiltration of inflammatory cells [8]. There was no direct comparison between the results of inhaled and oral administration, which was the limitation of current research.
In addition to the presence of inflammatory cells, airway remodeling, smooth muscle hyperplasia and hypertrophy are also important features of asthma [40]. A previous study has suggested that Eos could contribute to airway inflammation and tissue damage [37]. Lung lesions, inflammatory cell infiltration (including thick bronchiole and vessel membranes), and damaged smooth muscles were defined as histopathological characteristics of asthma [41]. In our studies, the infiltration extent of inflammatory cells, especially Eos, and airway wall thickness in the model group significantly increased compared with the normal group. In addition, after administration with TH/CTS/β-CD microspheres and DXM, the damage histopathology improved by various degrees compared with the model group. Therefore, an asthmatic airway remodeling model was successfully established, and the TH/CTS/β-CD microspheres exhibited a curative effect on asthma.
Evidence from previous studies showed that a number of inflammatory corpuscle and cellular elements, such as LDH and proteins, played prominent roles in the pathogenesis of allergic asthma [42]. As an intracellular enzyme, LDH would leak into the BALF during cell damage, this event could be an indicator of inflammatory and cytotoxicity response. Total protein and LDH levels have been routinely used as toxicity indexes that indicate tissue damage. Protein content in BALF of the model group significantly increased compared with that of the normal group (Table 3). These findings were consistent with the presented proof on successful development of an asthmatic airway remodeling model. Additionally, the results indicated that total protein levels and LDH activity decreased after TH/CTS/β-CD microsphere administration compared with the model group. Overall, these observations revealed that as a safe drug, TH/CTS/β-CD microspheres could provide protection against cell damage associated with allergen-induced inflammation.
Elevated serum level of Cr is a conventional indicator of renal damage. Hepatic function is evaluated by means of AST and GPT serum levels. In this study, the Cr, AST, and GPT levels were evaluated to determine renal toxicityandhepatotoxicity. After treatment with TH/CTS/β-CD microspheres, the GPT and Cr levels exhibited no significant difference with other groups. The AST level decreased in the groups treated by TH/CTS/β-CD microspheres compared with the model group (0.05). In the present research, changes in Cr, AST, and GPT levels suggested that the microspheres induced no toxicity to the liver and kidneys. Therefore, TH/CTS/β-CD microsphere administration by oral route caused no harmful effects on kidney and hepatic functions.
Conclusion
This study confirmed the anti-asthmatic effects of TH/CTS/β-CD microspheres in a rat OVA-induced asthma model in the aspects of pathophysiology and histopathology. A toxicology test indicated TH/CTS/β-CD microspheres as safe for drug administration by oral route, showing no harmful effects on kidney and hepatic function. Given the similar mechanisms of human and rat bronchial asthma, TH/CTS/β-CD microspheres might be a new potential therapy for the management of asthma in humans while avoiding TH toxicity to maintain a stable blood drug level. Further studies should be conducted to clarify the details on the mechanism of action.
Our study proved the effects of TH/CTS/β-CD microspheres on asthma, hepatotoxicity, and renal toxicity. However, the trend of dose response was excluded, thereby requiring further experiments.
Reference
1. Choi MS, Park S, Choi T. Bee venom ameliorates ovalbumin induced allergic asthma via modulating CD4+ CD25+ regulatory T cells in mice. Cytokine 2013, 61:256-265.
2. Simpson JL, Daly J, Baines KJ. Airway dysbiosis: Haemophilus influenzae and Tropheryma in poorly controlled asthma. Eur Respir J 2016, 47:792-800.
3. Huang XP, Tao EX, Feng ZQ. Inhibitory effect of sihuangxiechai decoction on ovalbumin-induced airway inflammation in guinea pigs. Evid Based Complement Alternat Med 2014, 2014:1-8.
4. Sharkuu T, Doerfler DL, Copeland C. Effect of maternal exposure to ozone on reproductive outcome and immune, inflammatory, and allergic responses in the offspring. J Immunotoxicol 2011, 8:183-194.
5. Kim CK, Kim SW, Park CS. Bronchoalveolar lavage cytokine profiles in acute asthma and acute bronchiolitis. J Allergy Clin Immunol 2003, 112:64-71.
6. Girodet PO, Ozier A, Bara I. Airway remodeling in asthma: new mechanisms and potential for pharamacological intervention. Pharmacol Ther 2011, 130:325-337.
7. Llmarinen P, Kankaanranta H. Eosinophil apoptosis as a therapeutic target in allergic asthma. Basic Clin Pharmacol Toxicol 2014, 114:109-117.
8. Feng ZQ, Sun CG, Zheng ZJ. Optimization of spray-drying conditions and pharmacodynamics study of theophylline/chitosan/β-cyclodextrin microspheres. Dry technol 2015, 33: 55-65.
9. Margay SM, Farhat S, Kaur S. To study the efficacy and safety of doxophylline and theophylline in bronchial asthma. J Clin Diagn Res 2015, 9: FC05-08.
10. Mellstrand T, Svedmyr N, Fagerström PO. Absorption of theophylline from conventional and sustained-release tablets. Eur J Respir Dis Suppl 1980, 109: 54-60.
11. Jenne JW. What role for theophylline. Thorax 1994, 49: 97-100.
12. Liu K, Feng ZQ, Shan L. Preparation, characterization, and antioxidative activity of Bletillastriata polysaccharide/chitosan microspheres for oligomeric proanthocyanidins. Dry Technol 2017, 35: 1629-1643.
13. Zhang WF, Zhou HY, Chen XG. Biocompatibility study of theophylline/chitosan/β-cyclodextrin microspheres as pulmonary delivery carriers. J Mater Sci Mater Med 2009, 20: 1321-1330.
14. Zhou X, Kong M, Cheng XJ. Investigation of acetylated chitosan microspheres as potential chemoembolic agents. Colloids Surf B Biointerfaces 2014, 123: 387-394.
15. Shan L, Tao EX, Meng QH. Formulation, optimization, and pharmacodynamic evaluation of chitosan/phospholipid/β-cyclodextrin microspheres. Drug Des Devel Ther 2016, 10: 417-429.
16. Zhou X, Cheng XJ, Liu WF. Optimization and characteristics of preparing chitosan microshperes using response surface methodology. J Appl Polym Sci 2013, 127: 4433-4439.
17. Borchard G. Chitosans for gene delivery. Adv Drug Deliver Rev 2001, 52: 145-150.
18. Zhou X, Kong M, Cheng XJ. In vitro and in vivo evaluation of chitosan microspheres with different deacetylation degree as potential embolic agent. Carbohydr Polym 2014, 113: 304-313.
19. Gustafsson H, Akesson J, Lau CL. A comparison of two formulations of intradermal capsaicin as models of neuropathic pain in healthy volunteers. Br J Clin Pharmacol 2009, 68: 511-517.
20. Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci 1996, 85: 1017-1025.
21. Cerchiara T, Luppi B, Bigucci F. Effect of chitosan on progesterone release from hydroxypropyl-β-cyclodextrin complexes. Int J Pharm 2003, 258: 209-215.
22. Kumar Y, Philip B, Pathak K. High-efficiency loading and controlled release of highly water-soluble drug, pravastatin sodium by use of cross-linked β-cyclodextrin. Int J Pharm Investig 2011, 1: 10-16.
23. Fernandes CM, Veiga FJ. Effect of the hydrophobic nature of triacetyl-beta-cyclodextrin on the complexation with nicardipine hydrochloride: physicochemical and dissolution properties of the kneaded and spray-dried complexes. Chem Pharm Bull 2002, 50: 1597-1602.
24. Chen XG, Zheng L, Wang Z. Molecular affinity and permeability of different molecular weight chitosan membranes. J Agric Food Chem 2002, 50: 5915-5918.
25. Zhang WF, Chen XG, Li PW. Preparation and characterization of theophylline loaded chitosan/β-cyclodextrin microspheres. J Mater Sci Mater Med 2008, 19: 305-310.
26. Waserman S, Xu Lj, Renzi PM. Association between late allergic bronchoconstriction in the rat and allergen-stimulated lymphocyte proliferation in vitro. Am J Respir Crit Care Med 1995, 151: 470-474.
27. Chandler MJ, Zeiss CR, Leach CL. Levels and specificity of antibody in bronchoalveolar lavage (BAL) and serum in an animal model of trimellitic anhydride-induced lung injury. J Allergy Clin Immunol 1987, 80: 223-229.
28. Zhang WF, Chen XG, Li PW. Chitosan and β-cyclodextrin microspheres as pulmonary sustained delivery system. J Wuhan Univ Technol 2008, 4: 541-546.
29. Blesa S, Cortijo J, Martinez-losa M. Effectiveness of oral N-acetylcysteine in a rat experimental model of asthma. Pharmacol Res 2002, 45: 135-140.
30. Suchankova J, voprsalova M, kottova M. Effects of oral alpha-tocopherol on lung response in rat model of allergic asthma. Respirology 2006, 11: 414-421.
31. Hoffman S, Nolin J, McMillan D. Thiol redox chemistry: Role of protein cysteine oxidation and altered redox homeostasis in allergic inflammation and asthma. J Cell Biochem 2015, 116: 884-892.
32. Holgate ST. Innate and adaptive immune responses in asthma. Nat Med 2012, 4: 673-683.
33. Dubuis E, Wortley MA, Grace MS. Theophylline inhibits the cough reflex through a novel mechanism of action. J Allergy Clin Immunol 2014, 133: 1588-1598.
34. Zhang WF, Chen XG, Li PW. Chitosan and chitosan/β-cyclodextrin microspheres as sustained-release drug carriers. J Appl Polym Sci 2007, 103: 1183-1190.
35. Fanta CH. Asthma. N Engl J Med 2009, 360: 1002-1014.
36. Lommatzsch M, Virchow CJ. Severe asthma: Definition, diagnosis and treatment. Dtsch Arztebl Int 2014, 111: 847-855.
37. Abdureyim S, Amat N, Umar A. Anti-Inflammatory, immunomodulatory, and heme oxygenase-1inhibitory activities of ravan napas, a formulation of Uighur traditional medicine, in a rat model of allergic asthma. Evid Based Complement Alternat Med 2011, 2011: 40706-40713.
38. Wei M, Xie X, Chu X. Dihydroartemis in suppresses ovalbumin-induced airway inflammation in a mouse allergic asthma model. Immunopharmacol Immunotoxicol 2013, 35: 382-389.
39. Barnes PJ. Mechanisms of action of glucocorticoids in asthma. Am J Respir Crit Care Med 1996, 154: 26-27.
40. Chen G, Tang JH, Ni ZH. Antiasthmatic effects of resveratrol in ovalbumin-Induced asthma model mice involved in the upregulation of PTEN. Biol Pharm Bull 2015, 38: 507-613.
41. Bonsignore MR, Profita M, Gagliardo R. Advances in asthma pathophysiology: stepping forward from the Maurizio Vignola experience. Eur Respir Rev 2015, 24: 30-39.
42. Patel B, Gupta N, Ahsan F. Aerosolized montelukast polymeric particles-an alternative to oral montelukast-alleviate symptoms of asthma in a rodent model. Pharm Res 2014, 31: 3095-3105.
Theophylline/chitosan/β-cyclodextrin (TH/CTS/β-CD) microspheres attenuated inflammatory cell infiltration and mucus hypersecretion compared with the model group. This study demonstrated that TH/CTS/β-CD microspheres exerted an anti-inflammatory effect and could serve as a novel promising drug delivery system for asthma treatment.
Theophylline is a transient metabolite which from the caffeine catabolism of tea tree and it is a natural methylxanthine. It is not only relaxing the smooth muscles of the bronchial airways and pulmonary blood vessels, but also reduces the airway responsiveness to histamine, adenosine and methacholine. In 1922, it was introduced for the clinical treatment of asthma. It was one of the most widely drugs for the treatment of asthma and chronic obstructive pulmonary disease worldwide.
Abbreviations: TH, theophylline; CTS, chitosan; β-CD, β-cyclodextrin; Eos, eosinophil; LDH, lactate dehydrogenase; AST, aspartate transaminase; GPT, glutamic pyruvic transaminase; Cr, creatinine; Neu, neutrophil; BALF, bronchoalveolar lavage fluid; CD, cyclodextrins; OVA, ovalbumin; DXM, dexamethasone; AHR, airway hyper responsiveness.
Funding: This work was supported by the foundation for visiting scholar abroad in Weifang Medical University, National Natural Science Foundation of China (No. 81973671, 81774125) and Weifang Science and Technology Development Plan Project (2018YX060).
Competing interests: The authors declare that there is no conflict of interests regarding the publication of this paper.
Online: 26 March 2020.
:Wang SS, Zhang HJ, Cui XM, et al. Effects of theophylline/chitosan/β-cyclodextrin microspheres for oral drug delivery on an asthmatic rat model. TMR Modern Herbal Medicine 2020, 3(2): 66-76.
Executive Editor: Chaoyong Wu
Submitted: 23 February 2020,
3March 2020,
#These authors contributed equally to this work.
*Corresponding to: Weifen Zhang, Department of pharmaceutics, College of Pharmacy, Weifang Medical University, No.7166 West Baotong Street, Weifang, Shandong 261053, China. Email: zhangwf@wfmc.edu.cn;
Dongzhen Mu, Department of Immunology, Weifang Medical University, No.7166 West Baotong Street, Weifang, Shandong 261053, China. Email: mdzyxx@163.com
杂志排行
TMR Modern Herbal Medicine的其它文章
- The research idea of Xuebijing injection in influencing severe pneumonia-pulmonary fibrosis with blood stasis syndrome evolution by inhibiting inflammation, endotoxin and dispersing blood stasis
- Research progress on chemical composition, pharmacological effects of Forsythia suspensa (Thunb.) Vahl and predictive analysis on Q-marker
- The functional components and mechanism of Linderae Radix in treating diabetic nephropathy based on the network pharmacology
- Dendrobium huoshanense improves doxorubicin-induced heart failure in C57BL/6 mice
- The roles of traditional Chinese herbal medications in regulating mitochondrial activity to reverse cancer
