Dendrobium huoshanense improves doxorubicin-induced heart failure in C57BL/6 mice
2020-05-07RongHuangYuanyuWangYingquanLiangMaoyunYuYuanliChenJihongHanXiaoxiaoYangYajunDuan
Rong Huang, Yuanyu Wang, Yingquan Liang, Maoyun Yu, Yuanli Chen, Jihong Han,3,Xiaoxiao Yang*, Yajun Duan*
ARTICLE
Dendrobium huoshanense improves doxorubicin-induced heart failure in C57BL/6 mice
Rong Huang1, Yuanyu Wang1, Yingquan Liang1, Maoyun Yu2, Yuanli Chen1, Jihong Han1,3,Xiaoxiao Yang1*, Yajun Duan1*
1Department of Pharmacological Sciences, Key Laboratory of Metabolism and Regulation for Major Diseases of Anhui Higher Education Institutes, College of Food and Biological Engineering, Hefei University of Technology, Hefei, China;2School of Biological and Pharmaceutical Engineering, West Anhui University, Lu’an, China;3Department of Biochemistry and Molecular Biology, College of Life Science, Key Laboratory of Bioactive Materials of Ministry of Education, State Key Laboratory of Medicinal Chemical Biology, Nankai University, Tianjin, China.
: To investigate the therapeutic effect of Shihu (, DH) on doxorubicin (DOX)-induced heart failure in mice and the involved mechanisms.
: Male C57BL/6 mice were randomly divided into 3 groups: Control (Ctrl) group, DOX group and DH group. Chronic heart failure was induced by intraperitoneal injection of doxorubicin solution. Mice in DH group were fed normal chow containing DH powder for 4 weeks. After 4-week treatment, electrocardiograms were measured. At the end of experiment, serum and heart sample were collected for determination of indicators for heart failure indicators. The heart tissues were conducted HE, Masson , Sirius red staining and TUNEL staining to determine cardiac tissue morphology, fibrosis, collagen content and apoptosis, respectively. mRNA and protein expression were determined by qRT-PCR and Western blot, respectively.
:DH reduced the DOX-induced serum biomarkers (creatine kinase, aspartate aminotransferase and lactate dehydrogenase) of heart damage and reduced heart fibrosis. Mechanically, DH inhibited myocardial apoptosis, decreased interleukin 6 and tumor necrosis factoralevels, but increased superoxide dismutase 2 expression.
: DH alleviates DOX-induced chronic heart failure by inhibiting inflammatory pathway and enhancing anti-oxidative enzymes. Our study provides the potential of DH for heart failure treatment.
Heart failure, Dendrobium huoshanense, Fibrosis, Apoptosis, Inflammatory cytokines

Background
Doxorubicin (DOX) is an anthracycline, a typical anticancer medicine, was first isolated from Streptomyces peucetius in the 1960s [1]. It has been used for treatment of epithelial tumors, such as breast, ovarian, and small-cell lung carcinomas or of hematologic malignant tumors, such as Hodgkin's or non-Hodgkin's lymphoma and pediatric leukemia for many years.However, the clinical use of DOX is restricted due to its adverse effects that include reduction of platelets and white blood cells, nausea, alopecia, loss of appetite, cardiotoxicity and liver dysfunctions. Among them, myocardial injury and subsequent heart failure are considered as the major side effects [2]. Heart failure is characterized by heart injury or dysfunction and the final stage of a variety of severe heart diseases [3]. Incidence and prevalence of heart failure have increased year by year, and the 5-year survival rate is similar to that of malignant tumors [4]. Therefore, it is particularly important to develop a specific drug or strategy to prevent DOX-induced heart disease.
Increasing evidence has demonstrated that inflammation and oxidative stress play important roles in the progression of DOX-induced cardiac injury and dysfunction [5-7]. Intense inflammatory response and imbalance of oxidative stress could mediate cardiomyocyte apoptosis and further promote the development of heart failure. Therefore, inhibition of cardiomyocyte apoptosis is considered as an effective strategy to reduce cardiotoxicity during DOX treatment.
Shihu (, DH) is a medicinal plant commonly foundin the Dabie Mountains in Southwestern Anhui. It has been proved that DH can regulate systemic immune functions [8], prevent cataract [9], regulate intestinal microbial population [10], reduce blood glucose [11] and inhibit inflammation [12]. However, whether DH can prevent heart failure remainsunknown. In this study, we used DOX-induced mice as the heart failure model to explore the potential role of DH on DOX-induced heart failure and the involved mechanisms.
Materials and methods
Chemicals and antibodies
DH was provided by Tongjisheng Biotechnology Co. Ltd (Lu’an, Anhui, China). DOX was purchased from MedChemExpress (NJ, USA). Rabbit anti-B-cell lymphoma 2 (Bcl-2, 12789-1-AP), Bcl-2 associated X protein (Bax, 50599-2-lg), forkhead box O3 (FOXO3a, 10849-1-AP) antibodies, goat anti-rabbit lgG (H+L)-HRP (LK2001) and goat anti-mouse lgG (H+L)-HRP (LK2003) were purchased from Proteintech Group, Inc (Chicago, Illinois, USA). Rabbit anti-NF-kB (A16271) and SOD2 (AC026) antibodies were purchased from Abclonal (Wuhan, Hubei, China). Cocktail, PMSF and enhanced chemiluminescence (ECL) kits were purchased from Millipore (Darmstadt, Hesse-Darmstadt, Germany). 4’,6-Diamidino-2-phenylindole (DAPI) was purchased from Santa Cruz Biotechnology (Paso Robles, California, USA). Total RNA pure reagent (Trizol, ZP401-2) was purchased from Beijing Zomen Biotechnology Co. Ltd (Beijing, China). The HiScript II Q Select RT SuperMix for qPCR, AceQ SYBR qPCR Master Mix and TUNEL BrightRed Apoptosis Detection Kit were purchased from Vazyme (Piscataway, New Jersey, USA). The other reagents were purchased from Sigma Aldrich (St. Louis, Missouri, USA).
Animals
The animal experiment was conducted with the approval of the Institution Animal Ethics Committee of Hefei University of Technology, and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institute of Health (NIH Publications No. 8023, revised 1978).
Male wild type (C57BL/6) mice (6~8 weeks old, ~22 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. Mice were kept in an air-conditioned atmosphere at 25°C for 12-12 h of light-darkness cycles, allowing free access to food and water. Mice were acclimated to their new surroundings for a week before the experiment. Totally 21 C57BL/6 mice were randomly divided into three groups by random number table method: Control group (Ctrl, n=5), mice were fed normal chow and i.p. injected saline once a week; DOX group (DOX, n=8), mice were fed normal chow and i.p. injected DOX (5mg/kg bodyweight) once a week, 4 times in total; and DH-treated group (DH, n=8), mice were fed normal chow containing DH powder (1.5 g/100 g food) and also i.p. injected DOX (5 mg/kg bodyweight) once a week, 4 times in total. All the experiments were lasted for 4 weeks. Before the end of experiment, all mice were conducted electrocardiography (ECG). At the end of experiment, animals were anesthetized and euthanized in a CO2chamber. Blood and heart samples were collected individually.
ECG
Mice were anaesthetized with 5% hydrated chloral, and conducted ECG. The electrocardiogram was recorded using the BL-420S biological signal acquisition and analysis system (Chengdu Taimer Software Co, Ltd). Briefly, after anaesthesia, mouse was fixed on the foam plate, silver needles were inserted under the skin of their limbs to connect with electrodes, and the two leads were used to collect signals. The red electrode was connected to the upper left limb, the yellow electrode was connected to the right upper limb, the black electrode was connected to the lower left limb and the green electrode was connected to the lower right limb, then the ECG was measured. Analysis of ECG waves was done to calculate heart rate (beats/min), QRS duration (ms), QT interval (Qtc, ms), which was corrected for heart rate using the formula [Qtc=QT/(square root of RR interval)] PR interval (ms) and P wave time (ms) [13].
Detection of the biomarkers of heart damage
The levels of creatine kinase (CK), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) were determined using an automatic biochemical analyzer (Model 7020, Hitachi, Tokyo, Japan) with serum samples (~150 μL/sample).
Hematoxylin and Eosin (HE), Sirius Red and Masson staining
A piece of mouse heart tissue was fixed overnight in 4% formalin solution and embedded in paraffin, and then 5-μm sections were collected [14]. HE, Masson and Sirius Red stainingof sections were conducted according to manufacturer's instructions.
Quantitative real time PCR (qRT-PCR)
To determine mRNA expression, heart tissue (~15 mg) was homogenized in 1 mL Trizol reagent to extract total RNA. cDNA was synthesized using the HiScript II Q Select RT SuperMix (+gDNA wiper) according to manufacturer’s instructions. Quantification of mRNA expression was then performed using the AceQ SYBR qPCR master mix (Piscataway, New Jersey, USA) and the primers listed in Table 1. LightCycler96 Software (Roche, Mannheim, Baden-Württemberg, Germany) was used to calculate the levels of gene expression which were normalized to GAPDH.
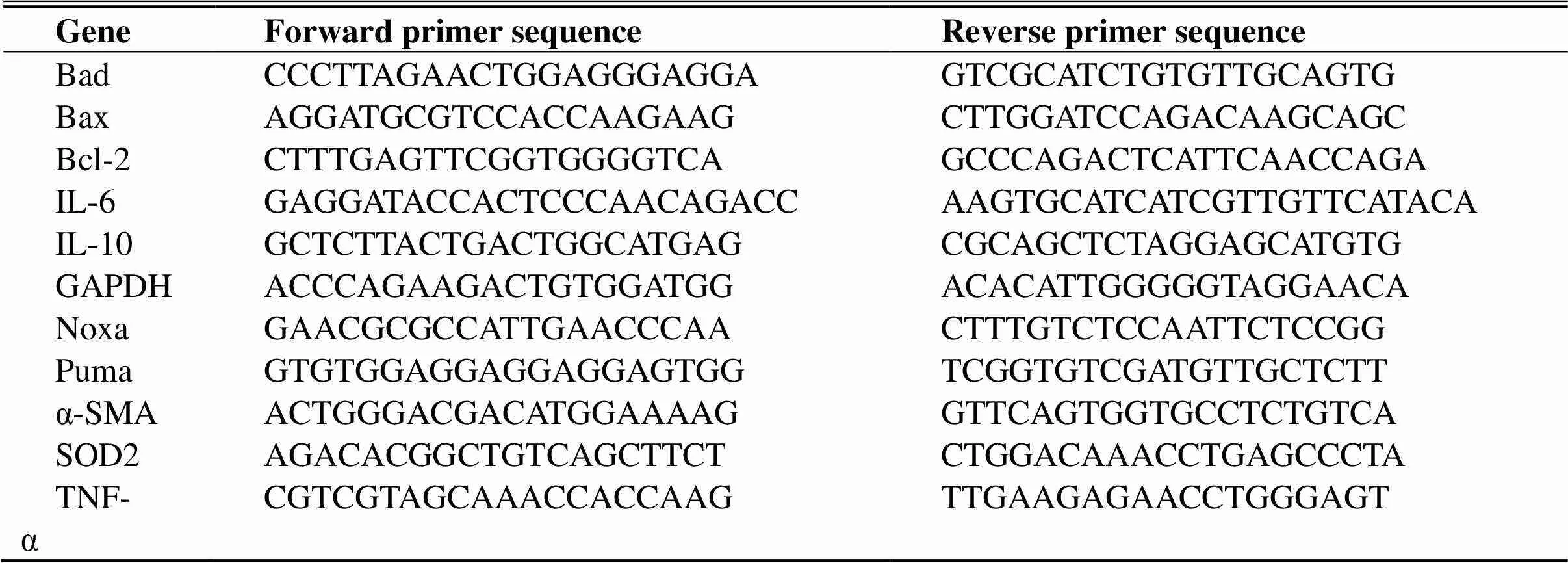
Table 1 qRT-PCR primer sequences
Western Blot
Heart tissues (~15 mg) were homogenized in a lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 1 mM PMSF, 50 mM sodium fluoride, 1 mM sodium orthovanadate, 50 μg/ml aprotinin/leupeptin). The homogenates were centrifuged at 12,000 × g for 10 min, and the supernatant was collected as total protein extract and used to detect Bax, Bcl-2, FOXO3a, GAPDH, NF-kB and SOD2 expressions by Western blot as previously described [15].
TUNEL Staining
Apoptotic cells in heart tissues were determined by TUNEL assay kit (Vazyme, Nanjing, China) according to the manufacturer’s instructions with tissue sections, and the nuclei in sections were stained with DAPI (Paso Robles, California, USA). The images were captured using the fluorescence microscope (Leica, Wetzlar, Hesse-Darmstadt, German). More than 5 microscopic fields (×20) of each heart tissue section were randomly selected to calculate the number of apoptotic cells [16].
Data Analysis
We repeated all experiments at least 3 times and presented the representative results. Data were presented as mean ± S.E.M. Graph Pad Prism 7.0 was used for statistical analysis. One-way ANOVA was used to assess the significance of these groups. For all tests, the significant differences were considered if< 0.05 (n ≥ 3).
Results
DH attenuates cardiac dysfunction in DOX-induced heart failure mice
DOX is an antitumor drug for treatment of several types of cancers. However, DOX also induces cardiotoxicity, characterized by hypotension, arrhythmia and transient depression of left ventricular function. Myocardial toxicity of DOX greatly limits its clinical application [17, 18]. In this study, we used DOX-induced heart failure mice as the model to explore the potential role of DH in heart failure asscheduled in Figure 1A. At the 31stday of treatment, we conducted ECG test. Mice in DOX group demonstrated abnormal ECG, such as increased Qtc interval, QRS interval, PR interval andPwave time. However, DH treatment effectively improved these indicators (Figure 1B, 1D-G).At the end of experiment, we examined mouse heart weight and found that DOX increased the ratio of heart weight to bodyweight but the increase was blocked by DH (Figure 1H). However, the heart rate was not affected by the treatments (Figure 1C).
Serum cardiac isoenzymes CK, AST and LDH are commonly used for clinical diagnosis of myocardial infarction [19]. We found serum CK, AST and LDH levels in DOX group were significantly higher than those in Ctrl group, but were substantially reduced by DH treatment (Figure 1I-K). These results demonstrate that DH can reduce the risk of heart failure in mice.
DH improves fibrosis in heart tissues
We further detected the physiological condition of heart tissue sections to determine histopathological alterations in response to DOX or DOX plus DH treatment. HE staining results show normal morphology of myocardial cells in the Ctrl group with complete and orderly myocardial fibers and normal myocardial interstitial. However, myocardial cells in DOX group showed granular degeneration, widen intermuscular plane as well as broken and disorganized myocardial fibers. DH treatment significantly reversed these symptoms (Figure 2A). Masson and Sirius Red staining also show that DOX induced collagen content and fibrosis in mouse heart tissue, but the induction was substantially attenuated by DH treatment (Figure 2B, C). To determine the molecular role of DH on fibrosis, we conducted qRT-PCR to detect fibrotic markers. We found that DOX induced expression of α-SMA mRNA, which was reduced by DH (Figure 2D).These results suggest that DH can inhibit myocardial fibrosis and collagen accumulation by decreasing α-SMA expression.
DH inhibits oxidative stress and inflammation in heart tissue
Oxidative stress is mediated by reactive oxygen species (ROS) and can cause contractile failure and structural damage, which play important roles in the pathogenesis of heart failure [20]. SOD2 is a mitochondrial matrix enzyme that protects the mitochondria against deleterious O2·−. FOXO3a plays an important role in maintaining cardiac function and antagonizing oxidative stress responses by regulating SOD2 expression [21]. We found that the protein levels of SOD2 and FOXO3a were reduced by DOX. However, the reduction was substantially restored by DH treatment (Figure 3A, B). Enhanced oxidative stress can further promote inflammation levels. The results of qRT-PCR showed that DH significantly decreased the expression of inflammatory cytokines, IL-6 and TNF-α and moderately increased the levels of anti-inflammatory cytokine, IL-10 (Figure 3C-E). Our results suggest that DH can enhance anti-ROS system and inhibit inflammation pathway to prevent cardiomyocytes toxicity in heart failure mice.
DH attenuates apoptosis of myocardial cells in heart failure mice
Cardiomyocyte apoptosis has important pathophysiological consequences contributing to functional abnormalities in the myocardium [22]. TUNEL staining results show the number of apoptosis cells was increased in DOX group comparedwithCtrlgroup. However, DH significantly reduced the number of apoptosis cells in cardiomyocytes (Figure 4A, B). As one of the tumor suppressor gene, p53 can mediate apoptosis by transcriptionally activating proapoptotic genes, Noxa, Puma and Bax, and repressing expression of antiapoptotic gene, Bcl-2.We found that DOX inhibited Bcl-2 mRNA expression (Figure 4C). At the same time, DOX induced mRNA expression of p53, Bax, Puma and Noxa, which were reduced by DH (Figure 4D-G). In addition, we evaluated the protein expression of apoptosis-related genes, Bax and Bcl-2. As expected, DH treatment significantly reduced expression of pro-apoptotic protein, Bax, but increased expression of anti-apoptotic protein, Bcl-2 (Figure 4H, I). As a nuclear factor with multifunctional transcriptional regulations, NF-kB is widely found in various tissue cells to mediate cell transformation, proliferation, invasion, angiogenesis, apoptosis and metastasis [23]. NF-kB has been found to promote apoptosis by activating proapoptotic proteins, such as p53, Bax, puma, Noxa and death receptor [24, 25].We found that DH treatment reduced DOX-induced NF-kB protein level (Figure 4H, I). These results indicate that DH can reduce DOX-induced apoptosis by regulating NF-kB pathway to inhibit expression of pro-apoptotic genes and induce expression of anti-apoptotic genes

Figure 1 DH attenuates cardiac dysfunctions and decreases the biomarkers of heart damage in heart failure mice. A:modeling and administration of chronic heart failure mice; B-G: ECG on all mice was conducted and the representative graphs were presented (B) with quantitative results of heart rate (C), QRS interval (D), Qtc interval (E), P wave (F) and PR interval (G); H-K: at the end of experiment, mouse heart and blood samples were collected. Heart samples were weighed and used to calculate the ratio of heart weight to bodyweight (H). Blood was used to prepare serum, followed by determination of serum CK, AST and LDH levels (I-K). *P < 0.05, **P < 0.01, ***P < 0.001 vs. Ctrl group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. DOX group (n ≥ 3).
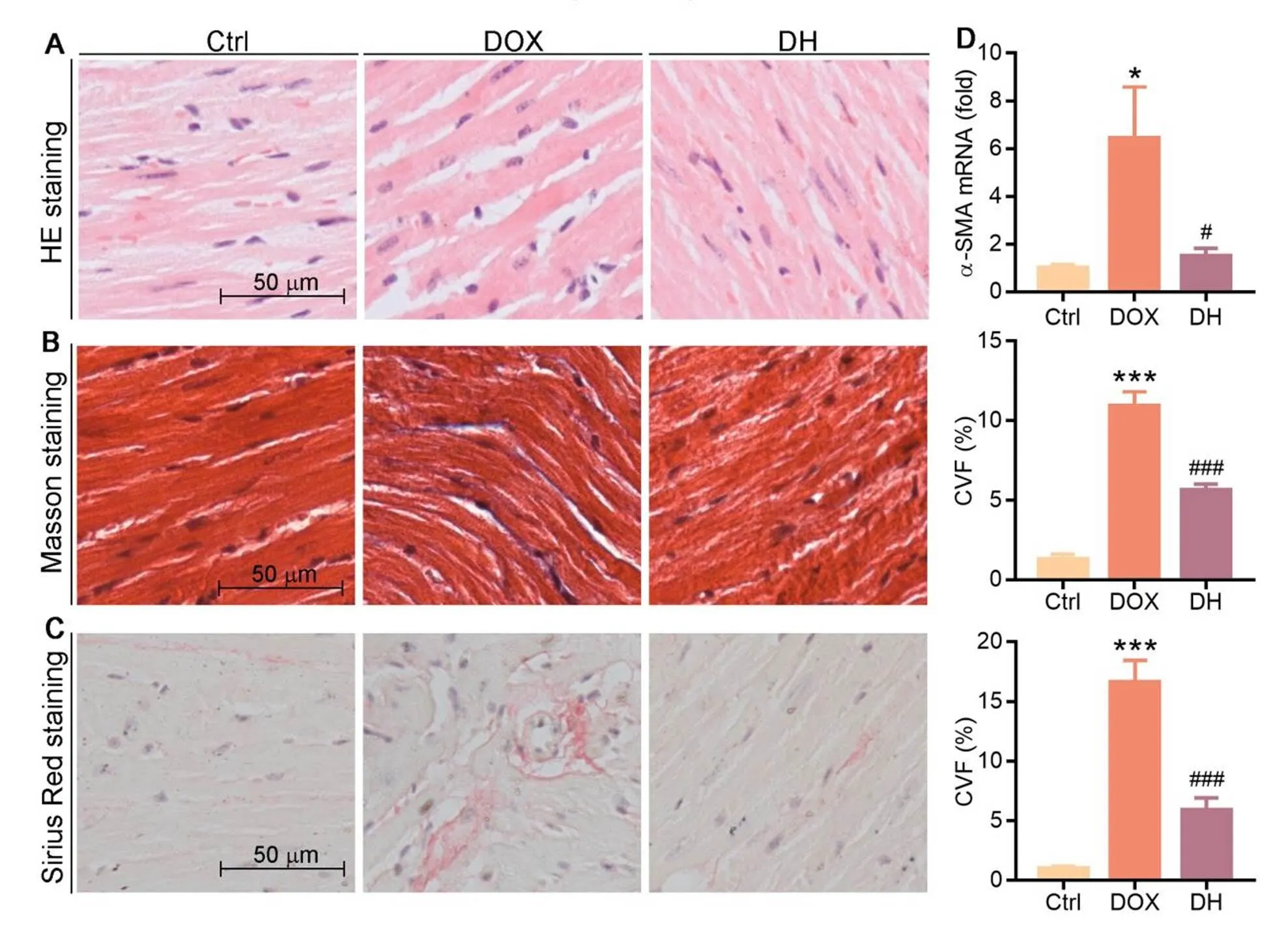
Figure 2 DH improves structural damage and myocardial fibrosis in myocardial cells in DOX-treated mice. A-C: heart tissue sections were prepared and used to conduct HE staining (A), Masson staining (B) and Sirius Red staining (C) with quantitation of collagen content; D: mRNA levels of α-SMA in heart tissues were determined by qRT-PCR. * P < 0.05, *** P < 0.001 vs. Ctrl group; # P < 0.05, ### P < 0.001 vs. DOX group (n ≥ 3).
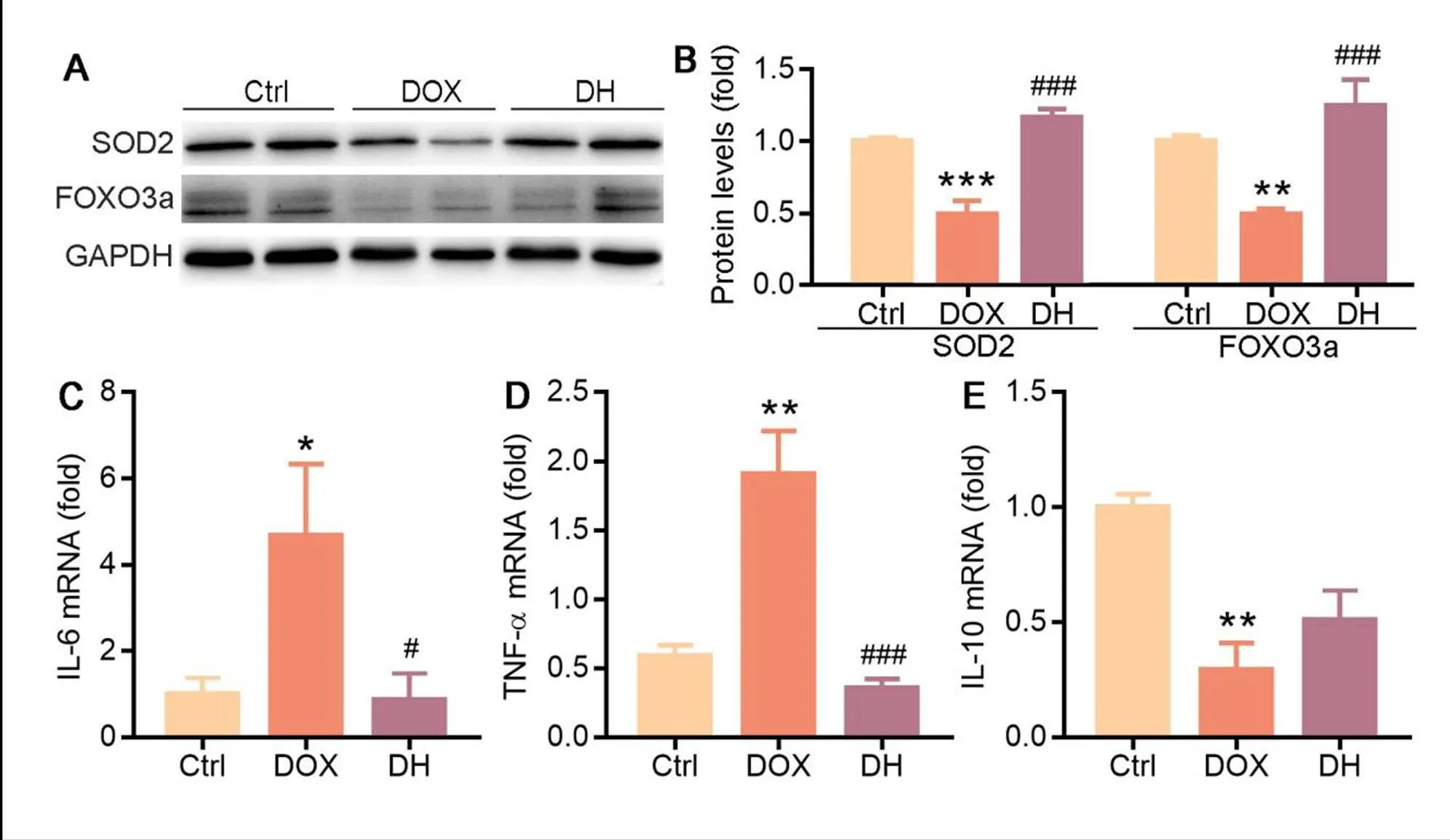
Figure 3 DH inhibits oxidative stress and inflammation in heart failure mice. A-C: expression of IL-6 (A), TNF-α (B) and IL-10 (C) mRNA in mouse heart tissue were determined by qRT-PCR; D, E: expression of FOXO3a and SOD2 protein in mouse heart tissue were determined by Western blot (D) with quantitative analysis of band density (E); *P < 0.05, **P < 0.01, ***P < 0.001 vs. Ctrl group; #P < 0.05, ###P < 0.001 vs. DOX group (n ≥ 3).

Figure 4 DH attenuates apoptosis of myocardial cell in heart failure mice. A-B: mouse heart sections were conducted TUNEL staining (A) and the numbers of apoptotic cells were obtained by quantitating 5 different fields in each section (B); C-G: mRNA levels of Bax (C), Bcl-2 (D), p53 (E), Puma (F) and Noxa (G) in heart tissues were determined by qRT-PCR; H, I: expression of NF-kB, Bax and Bcl-2 protein in mouse heart tissue were determined by Western blot (H) with quantitative analysis of band density (I); **P < 0.01, ***P < 0.001 vs. Ctrl group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. DOX group (n ≥ 3).
Discussion
In this study, we show that DH can protect mice from DOX-induced chronic heart failure with unveiled potential mechanisms. Our results show that DH can reduce the abnormal ECG in mice. Mechanically, DH decreases cardiac fibrosis and myocardial cell apoptosis by inhibiting inflammation pathway and expression of apoptosis-related molecules while increasing expression of antioxidant enzymes.
The mechanism of DOX-induced cardiotoxicity has intrigued both basic and clinical investigators for many years and is thought to be mediated by several different cellular processes [26]. DOX causes severe cardiotoxicity with enhanced oxidative stress in cardiomyocytes, resulting in elevated inflammatory cytokines. Our results demonstrate that DOX can induce myocardial fibrosis and cardiomyocyte apoptosis (Figure 2B, C and 4A). Therefore, it is urgent to find a drug to alleviate the toxicity of DOX. Traditional Chinese medicine (TCM) has the advantage of safety and multi-targets in prevention and treatment of heart failure [27]. Thus, treating with TCM provides a new way for amelioration of chronic heart failure. TCM, such as Ginseng [28], Astragalus [29] and Aconite [30], can enhance the heart contraction function. Salvia miltiorrhiza and safflower [31] can dilate blood vessels, improve heart blood supply, and reduce peripheral vascular resistance.In addition, ginsenosides in Ginseng can prevent ventricular remodeling, and astragaloside IV in Astragalus can inhibit myocardial cell apoptosis [32]. The main components contained in DH are polysaccharides and alkaloids. DH has been proved to have a variety of biological properties, such as immune regulation, antioxidant, neuroprotection, lowering hyperglycemia, anti-hypertension, anti-liver injury and anti-tumor activity. In this study, we first revealed the therapeutic effect of DH on heart failure.
Expression of normal p53 was found to cause rapid loss of cell viability with morphological characteristics of apoptosis [33]. p53 is a tumor suppressor that plays an important role in regulating cell growth, DNA repair and apoptosis [34]. Numerous studies have shown that DOX-induced cardiomyocyte apoptosis is associated with increased p53 expression [17]. p53-regulated cell death pathway has been shown to mediate acute DOX cardiotoxicity. Our results demonstrate that DH plays an anti-apoptotic role by increasing Bcl-2 expression and inhibiting p53 expression (Figure 4C, E). Besides, Bcl-2 family, such as Puma and Noxa, can also contribute to p53-mediated apoptosis via indirect induction of mitochondrial outer membrane permeabilization [13]. Studies have shown that DOX cardiotoxicity was attenuated by cardiac-specific overexpression of anti-apoptotic protein Bcl-2 [35]. Our results also show that DH can increase Bcl-2 expression and decrease the expression of Bcl-2 family related genes Puma and Noxa (Figure 4F, G).
Additionally, one of the consequences of DOX-induced cardiac injury is exhaustion of the intracellular antioxidant enzymes thereby enhancing the cardiomyocytes vulnerability to oxidative damage [36]. FOXO3a plays a crucial role in controlling mitochondrial metabolism and redox balance [37], and is involved in formation of the first line to defense against oxidative stress. Moreover, FOXO3a is a ROS-sensitive transcription factor that regulates expression of SOD2, catalase and glutathione peroxidase 1. Our study demonstrates that DH may reduce ROS levels by increasing expression of FOXO3a and SOD2 (Figure 3A, B), another mechanism contributing to the protecting effects of DH.
Conclusion
In this study, we demonstrate that DH can reduce the biomarkers of heart damage, improve heart function, inhibit heart fibrosis and myocardial apoptosis, mainly by reducing expression of inflammatory factors and enhancing anti-oxidative enzymes. Collectively, these results suggest that DH might be a promising herbal medicine for treatment of heart failure in clinic.
1. Yu J, Wang C, Kong Qet al. Recent progress in doxorubicin-induced cardiotoxicity and protective potential of natural products. Phytomedicine 2018, 40: 125-139.
2. Singal PK and Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med 1998, 339: 900-905.
3. Shuai Y, Guo JB, Peng SQet al. Metallothionein protects against doxorubicin-induced cardiomyopathy through inhibition of superoxide generation and related nitrosative impairment. Toxicol Lett 2007, 170: 66-74.
4. Zhang X, Ji R, Liao Xet al. MicroRNA-195 regulates metabolism in failing myocardium via alterations in sirtuin 3 expression and mitochondrial protein acetylation. Circulation 2018, 137: 2052-2067.
5. Octavia Y, Tocchetti CG, Gabrielson KLet al. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol 2012, 52: 1213-1225.
6. Yuan YP, Ma ZG, Zhang Xet al. CTRP3 protected against doxorubicin-induced cardiac dysfunction, inflammation and cell death via activation of Sirt1. J Mol Cell Cardiol 2018, 114: 38-47.
7. Akolkar G, da Silva Dias D, Ayyappa P net al. Vitamin C mitigates oxidative/nitrosative stress and inflammation in doxorubicin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol 2017, 313: H795-H809.
8. Ye Q, Qin G and Zhao W. Immunomodulatory sesquiterpene glycosides from Dendrobium nobile. Phytochemistry 2002, 61: 885-890.
9. Zha XQ, Deng YY, Li XLet al. The core structure of a Dendrobium huoshanense polysaccharide required for the inhibition of human lens epithelial cell apoptosis. Carbohydr Polym 2017, 155: 252-260.
10. Xie SZ, Liu B, Ye HYet al. Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barrier function and intestinal microbiota in mice. Carbohydr Polym 2019, 206: 149-162.
11. Wang HY, Li QM, Yu NJet al. Dendrobium huoshanense polysaccharide regulates hepatic glucose homeostasis and pancreatic beta-cell function in type 2 diabetic mice. Carbohydr Polym 2019, 211: 39-48.
12. Lam Y, Ng TB, Yao RMet al. Evaluation of chemical constituents and important mechanism of pharmacological biology in dendrobium plants. Evid Based Complement Alternat Med 2015, 2015: 841752.
13. Mantawy EM, Esmat A, El-Bakly WMet al. Mechanistic clues to the protective effect of chrysin against doxorubicin-induced cardiomyopathy: Plausible roles of p53, MAPK and AKT pathways. Sci Rep 2017, 7: 4795.
14. Yang X, Li Y, Sun Let al. NaoXinTong enhances atorvastatin-induced plaque stability while ameliorating atorvastatin-induced hepatic inflammation. J Cardiovasc Pharmacol 2017, 69: 55-64.
15. Sun L, Yang X, Li Qet al. Activation of adiponectin receptor regulates proprotein convertase subtilisin/kexin type 9 expression and inhibits lesions in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 2017, 37: 1290-1300.
16. Hou S, Xian L, Shi Pet al. The Magea gene cluster regulates male germ cell apoptosis without affecting the fertility in mice. Sci Rep 2016, 6: 26735.
17. Zhu W, Soonpaa MH, Chen Het al. Acute doxorubicin cardiotoxicity is associated with p53-induced inhibition of the mammalian target of rapamycin pathway. Circulation 2009, 119: 99-106.
18. Abd El-Aziz TA, Mohamed RH, Pasha HFet al. Catechin protects against oxidative stress and inflammatory-mediated cardiotoxicity in adriamycin-treated rats. Clin Exp Med 2012, 12: 233-240.
19. Ghormade PS, Kumar NB, Tingne CVet al. Distribution & diagnostic efficacy of cardiac markers CK-MB & LDH in pericardial fluid for postmortem diagnosis of ischemic heart disease. J Forensic Leg Med 2014, 28: 42-46.
20. Gomez-Samano MA, Grajales-Gomez M, Zuarth-Vazquez JMet al. Fibroblast growth factor 21 and its novel association with oxidative stress. Redox Biol 2017, 11: 335-341.
21. Guo Y, Li Z, Shi Cet al. Trichostatin A attenuates oxidative stress-mediated myocardial injury through the FoxO3a signaling pathway. Int J Mol Med 2017, 40: 999-1008.
22. Reeve JL, Szegezdi E, Logue SEet al. Distinct mechanisms of cardiomyocyte apoptosis induced by doxorubicin and hypoxia converge on mitochondria and are inhibited by Bcl-xL. J Cell Mol Med 2007, 11: 509-520.
23. Dang Y, Li Z, Wei Qet al. Protective effect of apigenin on acrylonitrile-induced inflammation and apoptosis in testicular cells via the NF-kappaB pathway in rats. Inflammation 2018, 41: 1448-1459.
24. Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene 2006, 25: 6680-6684.
25. Li J, Yuan S, Qi Let al. Functional conservation and innovation of amphioxus RIP1-mediated signaling in cell fate determination. J Immunol 2011, 187: 3962-3971.
26. Carvalho FS, Burgeiro A, Garcia Ret al. Doxorubicin-induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Med Res Rev 2014, 34: 106-135.
27. Wen J, Zhang L, Liu Het al. Salsolinol attenuates doxorubicin-induced chronic heart failure in Rats and improves mitochondrial function in H9c2 cardiomyocytes. Front Pharmacol 2019, 10: 1135.
28. Zhu H, Clinical observation of ginseng treatment for chronic heart failure. Guangming Journal of Chinese Medicine 2019,34:1208-1210.
29. Sang ZC, Liu ZJ, Effect and mechanism of astragalus on improving heart failure. Systems Medical 2016, 1:163-165.
30. Wang SL, Dong YR, Effect of aconite decoction on hemodynamics in rats with heart failure after myocardial infarction. Shaanxi Journal of Traditional Chinese Medicine 2007, 06:745-748.
31. Zhou W, Effects of traditional Chinese medicine on the treatment of elderly patients with chronic heart failure. Medical Equipment 2019,32:80-81.
32. Zhou YD, Zou DH, Chen S, et al. Study on the medication regularity of TCM decoction in the treatment of chronic heart failure based on data mining. China's Naturopathy 2019,27:45-48.
33. Yu J and Zhang L. The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun 2005, 331: 851-858.
34. Li J, Wang PY, Long NAet al. p53 prevents doxorubicin cardiotoxicity independently of its prototypical tumor suppressor activities. Proc Natl Acad Sci U S A 2019, 116: 19626-19634.
35. Letai A, Bassik MC, Walensky LDet al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2002, 2: 183-192.
36. Doroshow JH, Locker GY and Myers CE. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Invest 1980, 65: 128-135.
37. Tothova Z, Kollipara R, Huntly BJet al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 2007, 128: 325-339.
Shihu (, DH) can alleviate doxorubicin-induced chronic heart failure, which is associated with inhibition of cardiomyocyte apoptosis, myocardial fibrosis, and inflammatory factor expression.This study provides a promising approach for the treatment of heart failure in clinics.
DH, also known as Mihu, is a medicinal plant commonly foundin Huoshan county, Anhui province, Dabie Mountains. Both the dry and fresh DH stems can be used as herbal medicine. Itcan effectively improve immune functions, prevent cataracts, delay aging, and reduce tumorigenesis.
Abbreviations: TH, theophylline; CTS, chitosan; β-CD, β-cyclodextrin; Eos, eosinophil; LDH, lactate dehydrogenase; AST, aspartate transaminase; GPT, glutamic pyruvic transaminase; Cr, creatinine; Neu, neutrophil;BALF, bronchoalveolar lavage fluid; CD, cyclodextrins; OVA, ovalbumin; DXM, dexamethasone; AHR, airwayhyper responsiveness.
Funding: This work was supported by the foundation for visiting scholar abroad in Weifang Medical University,National Natural Science Foundation of China (No. 81973671, 81774125) and Weifang Science and TechnologyDevelopment Plan Project (2018YX060).
Competing interests: The authors declare that there is no conflict of interests regarding the publication of this paper.
Online: 26 March 2020.
: Wang SS, Zhang HJ, Cui XM, et al. Effects of theophylline/chitosan/β-cyclodextrin microspheres for oral drug delivery on an asthmatic rat model. TMR Modern Herbal Medicine 2020, 3(2): 66-76.
Executive Editor: Chaoyong Wu
Submitted: 23 February 2020,
3 March 2020,
*Correspondence to: Yajun Duan, PhD, College of Food and Biological Engineering, Hefei University of Technology, Feicui Road 420, Hefei 230601, China. Email: yduan@hfut.edu.cn;
Xiaoxiao Yang, PhD, College of Food and Biological Engineering, Hefei University of Technology, Feicui Road 420, Hefei 230601, China. Email: yangxiaoxiao@hfut.edu.cn
杂志排行
TMR Modern Herbal Medicine的其它文章
- Effects of theophylline/chitosan/β-cyclodextrin microspheres for oral drug delivery on an asthmatic rat model
- The research idea of Xuebijing injection in influencing severe pneumonia-pulmonary fibrosis with blood stasis syndrome evolution by inhibiting inflammation, endotoxin and dispersing blood stasis
- Research progress on chemical composition, pharmacological effects of Forsythia suspensa (Thunb.) Vahl and predictive analysis on Q-marker
- The functional components and mechanism of Linderae Radix in treating diabetic nephropathy based on the network pharmacology
- The roles of traditional Chinese herbal medications in regulating mitochondrial activity to reverse cancer
