Reversible and irreversible adsorption of bare and hybrid silica nanoparticles onto carbonate surface at reservoir condition
2020-04-25ZinULAbeinArinSrmAlAnssriMuhmmAliShoibMemonMsooAhmeBhttiChristopherLgtMohmmSrmivleh
Zin-UL-Abein Arin,Srm Al-Anssri,b,c,*,Muhmm Ali,c,Shoib Memon,Msoo Ahme Bhtti,Christopher Lgt,Mohmm Srmivleh
a Department of Petroleum Engineering,WA School of Mines: Minerals,Energy and Chemical Engineering,Curtin University,26 Dick Perry Avenue,6151,Kensington,Australia
b Department of Chemical Engineering,College of Engineering,University of Baghdad,Baghdad,Iraq
c School of Engineering,Edith Cowan University,270 Joondalup Drive,Joondalup,WA,6027,Australia
d Premier Oilfield Solutions,Australia
ABSTRACT Realistic implementation of nanofluids in subsurface projects including carbon geosequestration and enhanced oil recovery requires full understanding of nanoparticles (NPs)adsorption behaviour in the porous media.The physicochemical interactions between NPs and between the NP and the porous media grain surface control the adsorption behavior of NPs.This study investigates the reversible and irreversible adsorption of silica NPs onto oil-wet and water-wet carbonate surfaces at reservoir conditions.
Keywords:Nanofluid Silica nanoparticles Wettability Contact angle Surface treatment Adsorption Salinity Carbonate reservoir Carbonate
1.Introduction
Inorganic fillers,particularly silica nanoparticles (NPs),have a wide potential application in many industries including cosmetics,food products,drug delivery,and geological industries including aquifer decontamination,carbon capture and storage,and enhanced oil recovery (EOR)[1].In the oil and gas industry,once primary and secondary oil recovery methods can no longer produce sufficient amount of hydrocarbon,EOR (also called tertiary recovery method)can be utilized for more hydrocarbon production [2].Nanofluid flooding is regarded as a potential EOR technique in oil-wet reservoirs.Nanofluids,dispersion of NPs in a base fluid [3],have been suggested to facilitate oil displacement from the porous media [4,5].Reduction of water contact angle on the oil-wet solid surfaces in addition to the interfacial tension reduction is the main NPs mechanisms in EOR [6,7].In this context,NPs (i.e.silicon dioxide; SiO2)can enhance hydrocarbon recovery via rendering the wettability of oil-wet surfaces to water-wet[1,8]which in turn promotes the spontaneous imbibition of brine into the low-permeability rock [9].Although NPs efficiency as wettability modifier is limited by the adsorption of these fine particles onto the fluid-fluid interface [10]and solid surface [11],there is a dramatic lack of understanding about NPs adsorption onto rock surfaces at reservoirs conditions.According to the available literature,no previous study concerning nanoparticle adsorption was conducted at subsurface pressure,temperature,and salinity.
Recently,there have been several studies on the application of silica NPs to render the oil-wet rock surfaces water-wet for the enhanced oil recovery.Moghaddam et al.[12]have compared the effectiveness of different NPs on wettability alteration of oil-wet calcite surfaces.Their results showed that SiO2nanoparticles are more efficient in terms of contact angle reduction.Zhang et al.[13]proposed that NPs mutually experience reversible and irreversible adsorption on the solid surfaces.In their study,nanofluid was injected into columns packed with a crushed sedimentary rock at ambient condition.The NPs concentration in the effluent stream showed that NPs adsorbed onto the solid surfaces until the adsorption capacity was reached i.e.when the NPs in the effluent equals to that in the injection stream.Subsequent injection of DIwater into the columns led to dramatic desorption of nanoparticle,which was indicated by the significant concentration of NPs in the effluent stream.More recently,Al-Anssari et al.[14]reported the influence of nanofluid concentration and treatment temperature on the reduction of water contact angle on nano-treated calcite samples.Using technologies including scanning electron microscopy (SEM)images,energy dispersive X-ray spectroscopy (EDS),and atomic force microscopy (AFM)measurements,the researchers investigated the adsorption of silica NPs onto calcite samples and the formation of nanotextured surfaces.
Understanding of adsorption behaviour of NPs on rock-solid surfaces is one of the critical issues in the oil and gas industry.Physicochemical interactions between NPs and treated surfaces [15]and between NPs itself [16]are the main controlling factors for the potential irreversibility of NPs adsorption in the porous media [17,18].Typically,physicochemical interactions are mainly controlled by the surfaces charge [3].While the surfaces charge depends on the surface type,and condition [19]and the composition of formation brine[20,21].Theoretically,the Derjaguin - Landau - Verwey - Overbeek(DLVO)theory can demonstrate the effect of physicochemical interactions on NPs behaviour.The theory suggested that the interaction forces between particles and between the particle and the surface control NPs aggregation,deposition,adsorption,adhesion,and desorption [22].In this context,DLOV force is the algebraic summation of the attraction and repulsive forces.The attraction force is the van der Waals force and the repulsive force is the electrostatic force [21].In addition,in case of NPs adsorption,more forces can be considered within DLVO theory including hydrophilic and lipophilic forces [23],repulsive steric forces[24],and magnetic forces [25].
To-date,a limited number of studies have demonstrated the interactions between NPs and solid surfaces.Furthermore,all the previous studies were conducted at ambient condition ignoring the potential impacts of subsurface severe condition on the adsorption scenarios of silica NPs.Moreover,all previous studies have used NPs concentration in the effluent as an indication for NPs adsorption or retention in the porous media.Lecoanet et al.[26],for example,have assessed the transport of NPs in an artificially designed porous media.They reported that NPs display broadly diverse mobility behaviour.Further,surfacemodified NPs (hydrophobic)exhibited maximum mobility.Rodriguez Pin et al.[27]investigated the withholding of silica NPs after injection into sedimentary rocks.They have reported breakthroughs of effluent NP referring to significant mobility in the porous media.
Despite studies concerning NPs behaviour on solid surfaces in the lab at ambient condition,challenges in using NPs in real fields are of high potential due to the complex reservoirs conditions such as heterogeneous formations,high pressure,temperature,and salinity [1].This study,thus,investigated the fundamental aggregation,adsorption,and desorption properties of bare and hybrid (silanized)silica NPs onto oil-wet and water-wet carbonate surfaces at different pressures,temperatures,and initial hydrophilicity of NPs.This series of operation conditions help to cover all the potential scenarios in the oil production industry.Dynamic light scattering (DLS),atomic force microscopy(AFM),scanning electron microscope (SEM),energy dispersive X-ray spectroscope (EDS)were used to investigate NP-carbonate surface interactions at the nanoscale.These methods can directly evaluate the interactions of NPs with solid surfaces and their molecular arrangement and order.
2.Materials and methodology
2.1.Materials
Iceland spar (pure calcite,from WARD’S Natural Science)was used as representative for carbonate reservoir.Atomic force microscopy(model DSE 95-200)was used to measure the topography of the calcite samples and the root means square (RMS)surface roughness ranged between 18 and 32 nm,which is very smooth.Toluene (99 mol%,Chem-supply),n-hexane (> 95 mol%,Sigma-Aldrich),acetone and methanol (99.9 mol%,Rowe Scientific)were used to clean calcite samples at different stages.Also,to avoid any contaminant from the air,nitrogen (> 99.99 mol%,BOC)was used as drying agent after each cleaning or nano-treatment step.
Stearic acid (≥98.5%,Sigma Aldrich)was used to shift the wettability of the original calcite surface to oil-wet [28].A stearic acid solution (0.01 M)was initially prepared by dissolving 0.285 g of stearic acid in 100 mL of n-decane (> 99 mol%,from Sigma-Aldrich).Sodium chloride (≥99.5 mol%,Scharlan)and deionized (DI)water (Ultrapure from David Gray; conductivity = 0.02 mS/cm)were used to formulate brine solutions (1-20 wt% NaCl).
Silicon dioxide (Insoluble)NPs (porous spherical,purity = 99.5 wt%,density = 2200-2600 kg/m3,molecular mass = 60.08 g/mole,Sigma Aldrich)with two different initial sizes (5-10 nm and 20-25 nm)were used separately to formulate nanofluids.
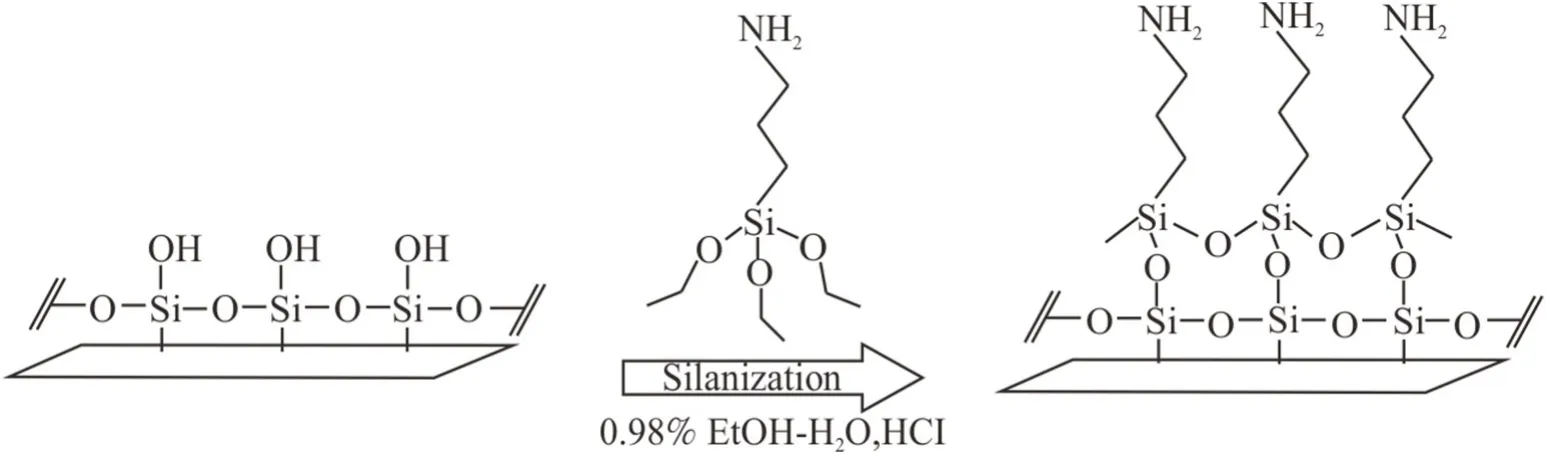
Fig.1.Attachment of (3-aminopropyl)triethoxysilane to silica nanoparticle surfaces [30].
In addition,3-aminopropyl triethoxysilane (H2N(CH2)3Si(OC2H5)3from Sigma Aldrich; Mol wt = 221.37)were used to change the hydrophobicity of hydrophilic silica nanoparticles to a hydrophobic condition (Fig.1)[29,30].
CO2(99.9 mol% from BOC,gas code-082)was used to increase the pressure in the treatment cell to the desired value during surface treatment of calcite with nanofluid.
2.2.Modification of nanoparticles hydrophobicity via silanization
Hydrophobicity of NPs is an essential factor influencing NPs adsorption on a solid surface.Consequently,the adsorption behavior of both hydrophilic and hydrophobic silica NPs was investigated.The hydrophobicity of NPs can be modified by changing the surface coating chemicals.To accomplish this,surface modification of silica NPs was performed by silanization,which is the reaction of solid surface with silane (Fig.1).In general,chemical reaction with silane agent is a practical method to change the hydrophobicity of silica to more hydrophobic condition [29-31].
Experimentally,1 g silica NPs was dispersed in 50 mL ethanol via a sonicator (300 VT Ultrasonic Homogenizer/BIOLOGICS)for 300 s to formulate NPs suspension.Moreover,a pre-hydrolyzed solution also formulated via dissolution of 0.7336 g (3-aminopropyl)triethoxysilane into a solution of 14.82 mL ethanol and 0.18 g H2O [32].The amount of silane,ethanol,and water was controlled by the number of hydroxyl groups moles that situated on 1 g of silica NPs [33,34].In this context,each (3-aminopropyl)triethoxysilane molecule requires three molecules of water for complete hydrolysis [31].Drops of concentrated hydrochloric acid (HCl)were used to maintain the acidity of the modification solution [30]at pH below the isoelectric point of silica nanoparticles which is around 1-2 [35].The silanization formulation was mixed magnetically for 20 min and pipetted to the NPs suspension,and then obtained mixture was magnetically stirred for another 24 h,at ambient condition.Eventually,treated NPs were centrifuged and impregnated with ethanol for 1 day to remove the reversibly absorbed silane compounds and headed 70 °C for 24 h for drying to produce dry hydrophobic (hybrid)NPs.
2.3.Formulation of equilibrated brine and nanofluids
The equilibrium between aqueous phase,carbonate,and CO2is essential to avoid the dramatic dissolution of calcite and the subsequent changes in surface charge and morphology during nano-treatment [14].In addition,the formation brine is naturally at equilibrium with calcite and CO2inside carbonate reservoirs [36].
Different salt concentrations were dissolved in DI water using magnetic starrier (1500 RPM,Across International)to formulate brine at varied salinity.These different brines were used as a base-fluid for the nanofluid.On the other hand,nanosuspensions were prepared via sonication of silica NPs in brine using ultrasonic homogenizer [32].Different weights of dry SiO2hydrophilic (bare)or hydrophobic (hybrid)NPs (0.002,0.004,0.01,0.014,0.02 g)were dispersed in 20 ml of brine at various salinity (0-5 wt% NaCl)to formulate nanodispersions at various NP loads (0.01,0.02,0.05,0.07,0.10 wt% SiO2).Each suspension was sonicated for 120 s to assure a suitable homogeneity.Note that once meet water,NPs tends to rapidly aggregate due to the high surface energy [16,37].Effective mixing is the only way to break down these aggregates and leave the NPs individually suspended.Also,to avoid the potential dissolution of calcite surface during nano-treatment,CO2,calcite,and nanodispersion were equilibrated in a mixing reactor(Fig.2).In this context,the nanosuspension was mixed with carbon dioxide and off-cuts of calcite in the equilibrator at the prescribed pressure and temperature for each experiment for 1 h [14].Characteristically,equilibrium is established when no more CO2dissolves in the nanofluid [38]which was indicated by constant pH.
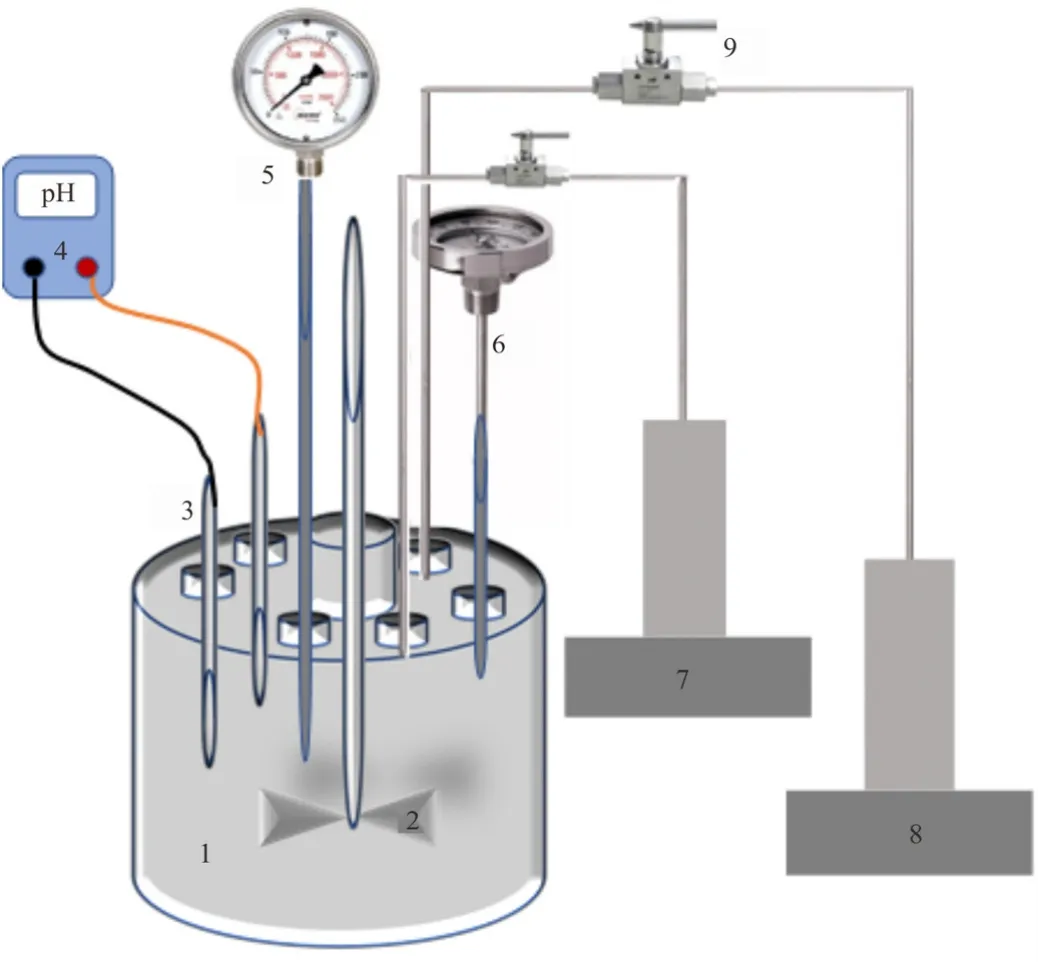
Fig.2.Schematic of the high pressure-high temperature equilibrator: (1)equilibration vessel,(2)the agitator,(3)pH electrode,(4)pH meter,(5)pressure gauge,(6)thermometer,(7)brine or nanofluid pump,(8)CO2 pump,(9)valve.
2.4.Preparation of carbonate surface
Surface cleaning processes are fundamental in surface treatment investigations since any remaining contaminants can impact the surface charges [39]and consequently the adsorption scenario of NPs.Thus,calcite samples were washed with equilibrated water to remove any carbonate dust on the surface.Subsequently,the samples were dried for 60 min at 90 °C and then exposed to air plasma for 10 min (using a Diemer Yocto instrument)to remove any potential organic contaminants [40].
2.5.Modification of original calcite with stearic acid
To simulate all the potential scenarios inside oil reservoirs including oil-wet and water-wet rocks,some calcite samples were treated with stearic acid to achieve oil-wet substrates.This helps in the study of the adsorption phenomena of NPs on both water-wet (hydrophilic)and oilwet (hydrophobic)carbonate formations.Here,the cleaned calcite samples were first submerged for 30 min in 2 wt% equilibrated NaCl brine at pH = 4 to support the later adsorption of acid on calcite surface.Typically,ionization of carboxylic acid groups and the availability of positive sites on the carbonate surface are controlled by the pH and ionic strength of the aqueous phase (equations (1)-(4))[41].Subsequently,the samples were dried with ultra-pure nitrogen to remove the excess brine from the surface and eventually immersed in 0.01 M steric acid at ambient conditions for 7 days.Mechanistically,carboxylate molecules from stearic acid solution are adsorbed on the positive sites of the calcite surface.

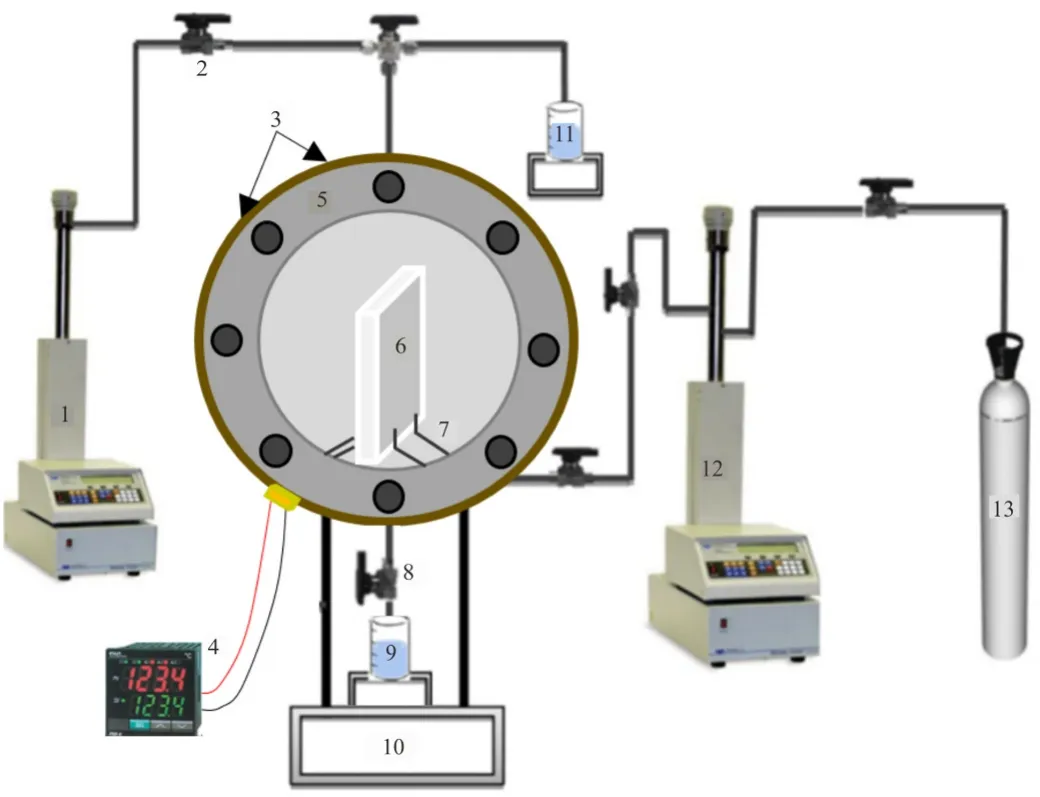
Fig.3.Experimental configuration for high pressure high temperature nanotreatment; (1)syringe pump-liquids,(2)valve,(3)heating tape,(4)thermocouple,(5)high pressure-temperature vessel,(6)calcite substrate,(7)sample holders,(8)pressure relief and drainage valve,(9)collector,(10),stand,(11)nanofluid and flushing liquids feed system,(12)syringe pump-CO2,(13)CO2 source.
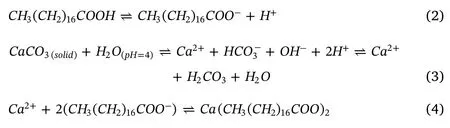
2.6.Nano-treatment of carbonate surfaces
To investigate the adsorption behaviour of silica NPs on different calcite samples at reservoirs conditions,nano-treated samples were prepared by submerging of calcite substrate in a nanofluid at designed exposure time,temperature and pressure.To accomplish this,each clean calcite sample (original or modified with stearic acid)was vertically rested in the nano-treatment vessel (Fig.3).Equilibrated nanofluids were pumped directly from the equilibrator into the treatment vessel which maintained at the same temperature and pressure of the equilibrator using syringe pump (Teledyne D-260,pressure accuracy of 0.1% FS).A constant immersion ratio of 10 g nanofluid for each 1 g of calcite was used to achieve duplicated interaction environment between calcite and nanoparticles.Further,the pressure inside the equilibrator and the nano-treatment vessel was increased using a high precision syringe pump (Teledyne D-500,pressure accuracy of 0.1% FS)to the desired value (0.1,10,20 MPa).Also,a heating tape was used to maintain the temperature at the pre-designed values (296,323,or 343 K).
2.7.Characterization of NPs stability and adsorption
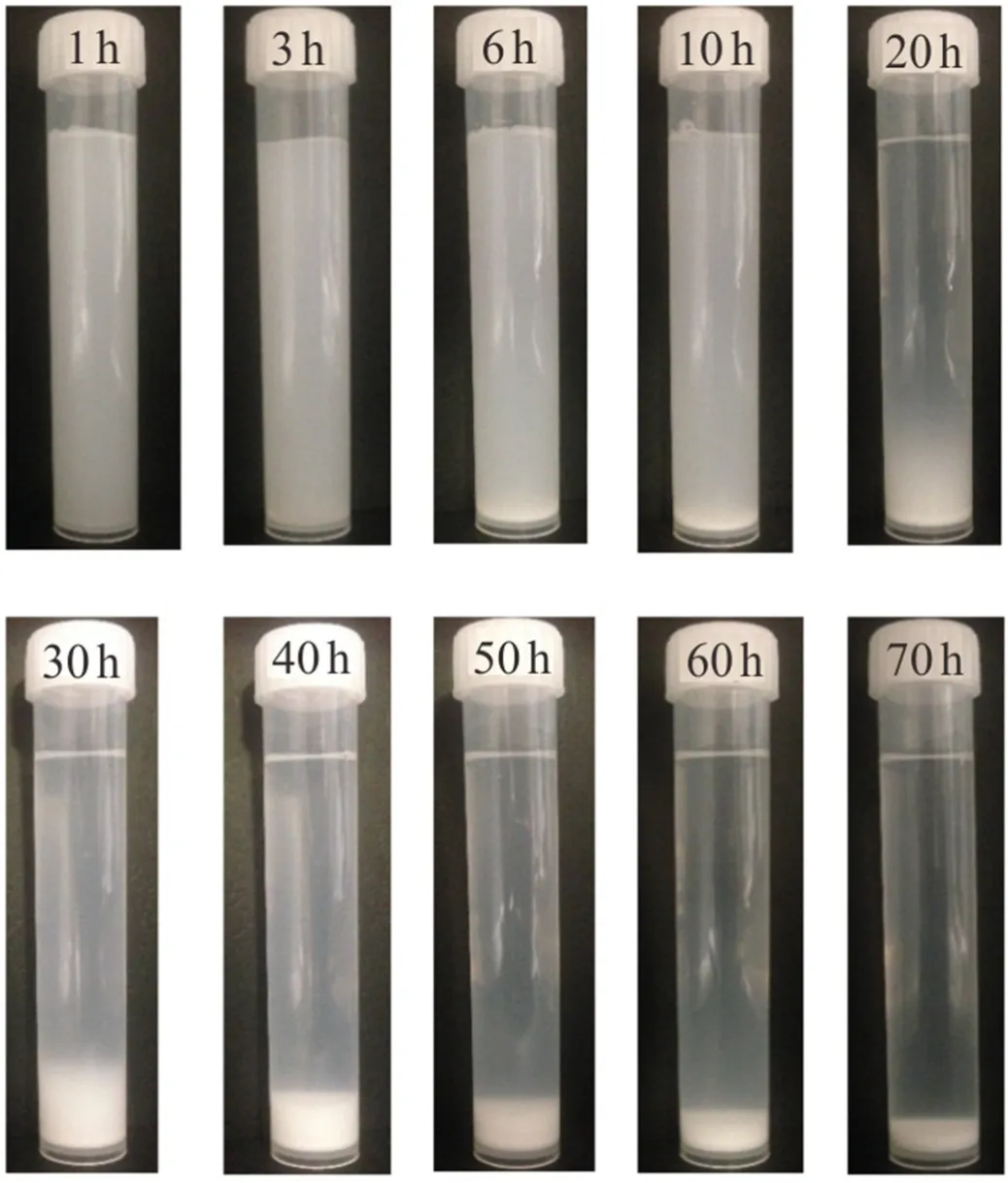
Fig.4.Photograph of the appearance of nano-suspensions (0.05 wt% bare SiO2 dispersed in DI-water at pH = 6.25)at different times (h)after sonication process.
Adsorption of NPs on a solid surface is key to the success of nanofluid in subsurface applications.Thus,adsorption characteristics and particularly the ratio between reversibly and irreversibly adsorbed NPs were investigated using several techniques including atomic force microscopy (AFM,instruments model DME 95-200,Semilab),scanning electron microscope (SEM,Phenom XL,PHENOMWORLD),and energy dispersive X-ray spectroscope (EDS,Phenom XL,PHENOMWORLD).The nano-treated surfaces were exposed to different solvents including DI water and brine in different tests.After each step,the substrate was dried with N2gas,then EDS,AFM,and θ measurements applied to study the irreversibility of NPs adsorption.Before that,stability and aggregation behaviour of NPs in the liquid phase were investigated via particle size distribution (PSD)using dynamic light scattering (DLS,Zetasizer,ZS Malvern).DLS used in this study is a laser-based technique and is very sensitive to the opacity of the fluid.Subsequently,relatively dilute nanofluids (0.05 wt% SiO2)were used to conduct this measurement.
3.Results and discussions
NPs efficiency as EOR agent at reservoirs condition may be different from that evaluated at ambient conditions using pure rock samples[28].In this context,reservoirs conditions including temperature,pressure,and salinity can impact rocks properties [42]and potentially NPs stability [3].Furthermore,rock heterogeneity can limit the mobility and distribution of NPs through the formation [42,43].The presented and discussed data in this section provide the first insight into these potentials.
3.1.Stability-aggregation characterization of NPs
Characteristics of the formulated nanosuspensions including NPs zeta potential,suspensions acidity,and salinity can impact the stability and aggregation rate of NPs [18,37,44].Thus,different nanofluid composed of 0.1 wt% bare or hybrid NPs dispersed in base fluids under constant acidity (pH = 6.25)was formulated.The stability was monitored visually (Fig.4)and by particle size distribution (PSD)measurement (Figs.5 and 6).
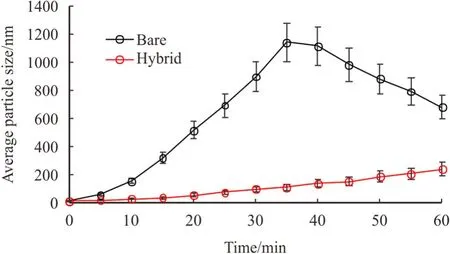
Fig.5.Average particle size (APS)measurements for both bare and hybrid silica nanoparticles dispersed in DI-water as a function of time.
The surface of bare silica NPs is appreciably negatively charged owing to the dissociation of the surface silanol (SiOH)groups [31].Although,the pH of the suspension was 6.25 ± 0.25 which is way above the isoelectric point of silica suspension (IEP of SiO2occurs at pH = 2-3)[3],visual evaluation of bare silica nanofluid showed a dramatic precipitation and sedimentation after only 6 h with total phase separation after less than 3 days even when the NPs were dispersed in DI-water (Fig.4).Here,the instability of nanofluid was characterized by the clarity of the liquid phase (base fluid)rather than the sediment height considering the impact of water content in the precipitant on sediment height.Characteristically,the relatively high surface energy of NPs owing to the highest surface area to particle size ratio [10]is the main reason for the observed aggregation activities of NPs.
Average particle size (APS)measurements,based on particle size distribution (PSD)measurements were conducted on nanofluid samples.To assure an identical condition and thus measurements consistency,all samples were taken from the same point of nanofluid container,just below the top of the container.Samples were taken after different intervals from the end of sonication process.At these times,samples were taken from a point just below the upper surface of the nanofluid.PSD measurement on silica nanofluid reveals the formation of nano-aggregates directly after preparation of nanodispersion (Fig.5).
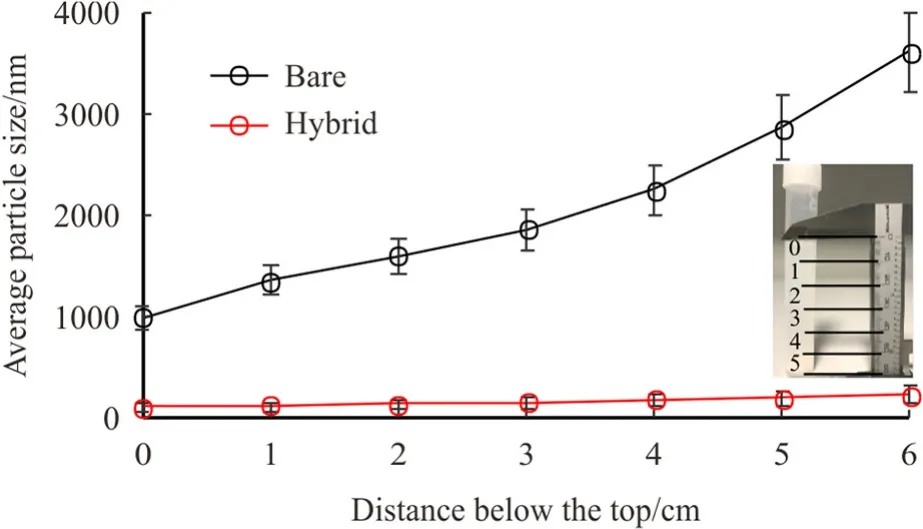
Fig.6.Average particle size (APS)measurements,for both bare and hybrid silica NPs,dispersed in DI-water as a function of distance from the top interface.Nanofluids were left for 45 min then samples were taken.
Results showed that the PSD of bare NPs dramatically increased with time reaching a plateau (i.e.after 35-40 min from the end of sonication)then unexpectedly decreased with time.Different PSD trend,however,was observed for the hybrid silica nanofluid.Significantly smaller aggregates were formulated with time and continued to grow over the test period (60 min).Typically,the aggregation of NPs after sonication in the liquid phase was consistent with the reported data in the literature [3,16].Nevertheless,the later decreased in APS was questionable.Further investigation was carried out to study this phenomenon.The APS was measured for each nanofluid using samples taken from six different points at a different depth of the nanofluid (Fig.6).To achieve this,6 duplicated tubes were filled with the exact same nanofluid.Then a syringe with a long nick needle was used to take samples from each tube from the desired height of the fluid.A similar approach has been conducted in our earlier work [3].This was to help measure aggregates size distribution in the nanofluid at different heights.
Fig.6 indicates that aggregates sizes of bare NPs were significantly bigger in the lower parts of the samples.The average size of these aggregates can reach very large sizes on the bottom side of the nanofluid(i.e.≈ 3.7 μm in the bottom),which appears to be out of the nanoscale.The observations are consistent with those in the literature[15,18].Considering the efficient sonication processes which most likely produces homogeneous nano-suspension,the variation of nanoaggregated size at different heights of the nanofluids is possibly related to the precipitation of larger aggregates from the top to the bottom side of the sample.Typically,the heavier weight of large aggregated promotes the precipitation of these aggregates.This explains the sudden reduction of aggregate size after 35-45 min (Fig.5)due to the downward mobilization of heavier clusters to the bottom by the effect of gravity leaving the sprightly and swift (smaller)once on the top.
3.2.Reversible and irreversible adsorption of NPs
Adsorption and the potential desorption of NPs on a solid surface is a complicated phenomenon.Nano-treatment conditions including pressure and temperature have a direct impact on NPs settlement onto the solid surface and consequently the quality of surface treatment.Thus,it is crucial to investigate the effect of treatment conditions on the adsorption-desorption behavior of NPs on the carbonate surface.To accomplish this,calcite samples were treated with the same nanofluid at different temperatures and pressures and the adsorption behavior was investigated by AFM,EDS measurements,and SEM images.
3.2.1.AFM measurement
Although the used calcite was smooth (18-54 nm),adsorption of nanoparticles may change the surface roughness and influence the morphology of the sample [1].Fig.7 shows the dramatic effect of nanotreatment conditions on NPs adsorption and thus surface roughness of carbonate surfacers.
Immersing pure calcite sample in nanofluid at 70 °C and 0.1 MPa will raise the surface roughness to around 2490 nm (Fig.7,A).On the other hand,nano-treatment of the similar sample with identical nanofluid but at 25 °C and 15 MPa can only raise the surface roughness up to 396 nm.These results revealed a vital impact of nano-treatment operation condition on NPs adsorption and thus on the change in surface roughness (Fig.7,B).Also,hybrid NPs adsorption on calcite was examined (Fig.7C).Thus,the effect of nano-treatment on surface morphology and roughness were addressed after each treatment step(Table 1)to understand the nature of NPs adsorption on carbonate surfaces.

Fig.7.Atomic Force Microscope of nano-treated calcite samples using bare NPs: A)at 70 °C and 0.1 MPa,B)25 °C and 15 MPa,and C)using hybrid NPs at 25 °C and 15 MPa.
Table 1 reports the statistics for the effect of NPs adsorption on calcite surface roughness.Although adsorption of bare NPs at ambient pressure can significantly increase carbonate surface roughness [8],treating the samples with nanofluid at high pressure showed insignificant influence on surface morphology.Further,nano-treatment with hybrid NPs has the minor influence of surface roughness indicating a uniform nano-coating of the carbonate surface.Mechanistically,the rapid aggregation of bare NPs and the subsequent accelerated precipitation of the formed aggregates leads to dramatic sedimentation of these nano or possibly micro-aggregates on solid surfaces [3,8].Mostly,these aggregates reversibly attached to the surface after precipitation by gravity effect [7].On the other hand,the limited aggregation of hybrid NPs keeps these nano-structure suspended in the liquid phase.Subsequently,hybrid NPs can only reach the solid surface via a Brownian motion to adsorb irreversibly into the surface.This limited adsorption of hybrid NPs forms a uniform mono or double nano-layers[15,18].This phenomenon was confirmed by the ultimately limited change in surface roughness after treatment with hybrid silica nanofluid.
The last three rows of Table .1 provide data about the sole effect of temperature on nano-treatment at reservoirs pressure (15 MPa),and low NPs concentration (0.05 wt%).Keeping all other variables constant(Pure calcite,bare,15 MPa,0.05 wt% NPs),the increase in temperature from 23 to 70 results in surface roughness increase from 116 to 236 nm.This change in surface roughness is very limited referring to slight adsorption of NPs.On the other hand,at ambient pressure (0.1 MPa),and relatively high NPs concentration (0.1 wt%)the effect of temperature increase was more significant and surface roughness increased from 1270 to 2500 nm.This more significant changes in surface roughness at ambient pressure is mainly related to the dissolution of the carbonate surface [19,21]rather than the adsorption of NPs.
3.2.2.EDS measurements
EDS results (Table 2)provides data about adsorption of silica (Si)NPs after immersing with the same nanofluid (0.05 wt% bare or hybrid SiO2)but at different pressure,temperature and initial surface wetness of calcite.Consistent with the outcomes of AFM measurements,regarding treatment with hybrid silica nanofluid,traces amount of Si was detected on all the tested points on carbonate sample.Note that,five different points were selected for EDS measurements at the tested surface of each sample and the average was calculated.Characteristically,the low measured Si ratio proves the absence of large aggregates and indicate the formation of thin NPs-layer.Experimentally,significantly higher Si ratios were detected after treatment with bare silica nanofluid with relatively high variation between different points.These high ratios result from the accumulation of large silica-aggregates [13].In this case,most of the NPs are reversibly attached to each other and not adsorbed directly on carbonate surface [1].
EDS results also confirm the effect of pressure and temperature on NP adsorption.In this context,results show that the increase in temperature until 50 °C has no significant effect on the adsorption of bare NPs.In this context,Hamouda and Gomari [19]revealed that below 50 °C,the carbonate surface entirely positively charges.However,with further increase in temperature (≤50 °C),the adsorption of such bare NPs decreases with temperature and reached a minimum value at 70 °C(the highest tested temperature).Mechanistically,the change in surface charge of carbonate from positive to zero and then negative as temperature increased [19]is the main reason for this reduction in NPs adsorption with a potential desorption process of already attached NPs at this higher temperature range.In contrast,the adsorption of hybrid NPs increases with temperature on both oil-wet and water-wet carbonate surfaces.Basically,hybrid NP carries no surface charge [27]and the reduction of carbonate surface charge with temperature support the deposition of hybrid NPs.
3.2.3.SEM images
NPs adsorption was evaluated with a scanning electron microscopy(SEM)and images showed the presence of silica clusters on both oil-wet and pure calcite surfaces (Fig.7)when treated with bare silicananodispersion at ambient and high pressure.

Table 1 The surface roughness of pure and nano-treated carbonate surface with different nanoparticles at different treatment conditions.
SEM images indicated a partial agglomeration of bare silica NPs into larger clusters confirming the APS results in Figs.5 and 6.Moreover,the size of clusters decreases with pressure (Fig.8 A and B).Typically,the effect of CO2-pressure on the pH of the nanofluid explains the formation of these larger clusters.Characteristically,the original pH of silica nanofluid was 6.25 which is far enough from the isoelectric point(IEP,pH = 2-3)of silica nanofluid [14]; however,the increase in CO2-pressure brings the pH to a value close to the IEP leading to an accelerated agglomeration process between neighboring NPs.Mechanistically,the low or zero repulsive force between particles at this low pH(e.g.at or near the isoelectric point IEP)increases the aggregation rate after each collision between particles [3,20].Consequently,the more and larger clusters will be formed and electrochemically (irreversibly)adsorbed or gravitationally precipitated (reversibly)on carbonate surface.
Furthermore,immersing these nano-treated surfaces in DI-water at the same pressure led to smaller nano-silica cluster,which confirmed the degradation and detachment of nanoparticles from silica agglomerates and re-dispersing in the water phase due to the break of equilibrium condition.
4.Conclusions
Nanofluid is a dispersion of nanoparticles (NPs)in a base fluid.Nanofluid flooding is a potential approch for enhanced oil recovery(EOR).To give the first insight on NPs adsorption onto carbonate surface at the subsurface condition,this study has conducted a series of dynamic light scattering (DLS),scanning electron microscope (SEM),energy dispersive X-ray spectroscope (EDS),and atomic force microscopy (AFM)measurements.It was found that only a limited amount of hybrid (silanized)silica NPs could be adsorbed by oil-wet and waterwet calcite surface at ambient [15,18]and reservoirs conditions.Also,the average particle size (APS)measurement which based on particle size distribution measurement,showed a limited aggregation of hybrid NPs once dispersed in the liquid phase [3,20].This stable behaviour of hybrid NPs supports the formation of a monolayer NP cluster on the treated surfaces.In contrast,the dramatic agglomeration of bare silica NPs when dispersed in the base fluid result in large silica aggregates[16].Additionally,immersing the carbonate (calcite)sample in baresilica nanofluids result in precipitation of large silica clusters on the solid surface.This was proved by the significant increase in surface roughness when measured using AFM.Most of bare NPs clusters,however,are reversibly adsorbed on calcite surface and easy to getdetached when flushed with solvent (i.e.DI-water).It was also found that at high pressure,small nano-clusters were distributed more uniformly along the nano-treated substrate.Further,EDS measurements proved the presence of silica in all the tested points with narrow and adequate ratios.Nevertheless,at ambient pressure,significantly large silica clusters were separately distributed on the nano-treated surfaces with very wide differences in silica ratios (1.2-5.2% Si).Moreover,although temperature values below 50 °C showed no significant effect on nanoparticle adsorption,increasing the temperature above 55 °C decreases silica adsorption and thus the effect of nanofluid on surface wettability.Further,flushing the nano-treated surfaces with DI-water can lead to dramatic desorption of adsorbed bare NPs from the surface.We conclude that the attachment of NPs,after nanofluid injection into carbonate surface,are mostly reversible adsorption process.
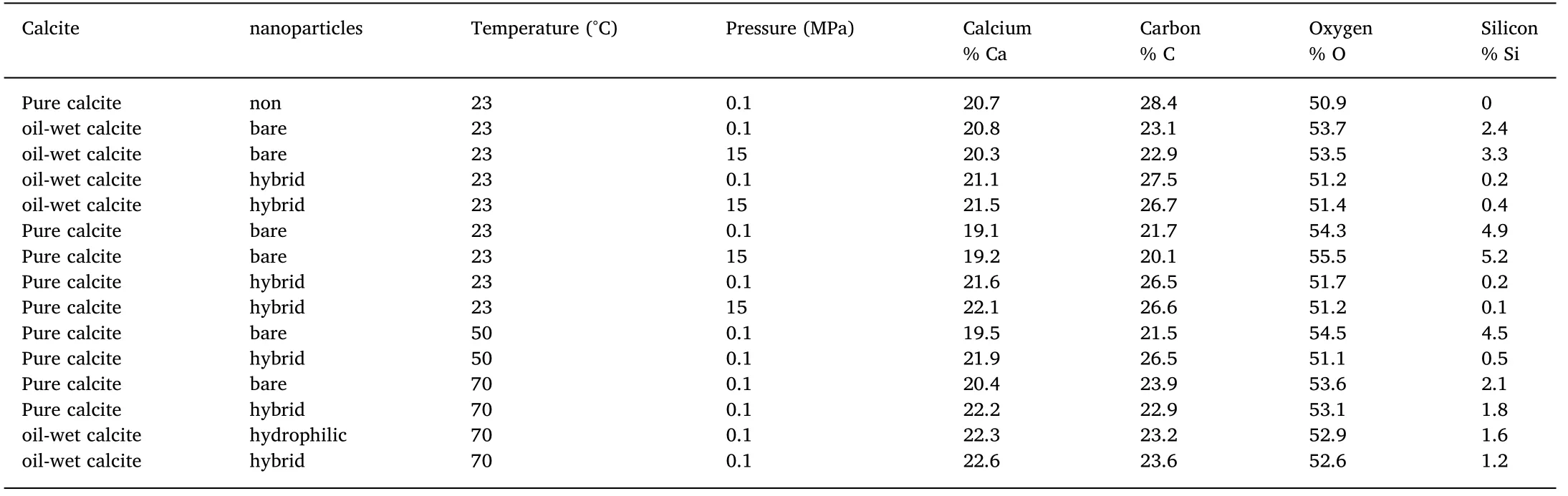
Table 2 Chemical characterization of carbonate surfaces that treated with different nanofluids base on the average of five different points on each surface.

Fig.8.SEM images of nano-treated oil-wet carbonate surface: (A)hydrophilic NP at 0.1 MPa,(B)hydrophilic NP at 20 MPa,(C)nano-treated sample after immersing DI-water.The amorphous looking material is the nano-clusters and the small pyramid looking structures are the dissolved carbonate surface.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.petlm.2019.09.001.
杂志排行
Petroleum的其它文章
- Contribution to the ageing control of onshore oil and gas fields
- Reservoir rock properties estimation based on conventional and NMR log data using ANN-Cuckoo: A case study in one of super fields in Iran southwest
- Effects of synthesized nanoparticles and Henna-Tragacanth solutions on oil/water interfacial tension: Nanofluids stability considerations
- Comparative study of well soaking timing (pre vs.post flowback)for water blockage removal from matrix-fracture interface
- β-cyclodextrin assists salt-resistance polymer flooding: Injectivity improvement
- Polymeric microsphere injection in large pore-size porous media
