Effect of An-pressing manipulation on post-stroke muscle spasticity in rats and its mechanism study
2020-04-21ChuXiao初晓LiJiangshan李江山ChenHeng陈恒LiWu李武LiuXiaowei刘小卫
Chu Xiao (初晓), Li Jiang-shan (李江山), Chen Heng (陈恒), Li Wu (李武), Liu Xiao-wei (刘小卫)
1 Changsha Medical University, Changsha 410219, China
2 Hunan University of Chinese Medicine, Changsha 410208, China
Abstract Objective: To explore the mechanism of An-pressing manipulation in improving post-stroke muscle spasticity, by observing the changes of γ-aminobutyric acid (GABA) and glycine (Gly) in plasma and gray matter of L1-L3 spinal cord anterior horn in post-stroke rats with muscle spasticity after An-pressing manipulation intervention.Methods: Ten of 80 adult male Sprague-Dawley (SD) rats were randomly selected as the blank group, and the remaining 70 were used for modeling. The middle cerebral artery occlusion (MCAO) rat model was established by insertion suture occlusion method in the left external carotid artery. Thirty rats with a Longa neurological score of 2-3 points and a modified Ashworth spasticity scale score of 1-, 1+, or 2 were included in the experiment. Using the random number table method, the 30 successfully modeled rats were randomly divided into a model group, an An-pressing tendon group and an An-pressing muscle belly group. Two days after modeling, rats in the An-pressing tendon group and An-pressing muscle belly group received An-pressing manipulation on the tendon and belly of quadriceps femoris muscle respectively, with the pressure of (350±50) g and the frequency of 5 s/time, 15 min per session, once a day for 5 continuous days. After the 5th treatment, the tension of the rat quadriceps femoris muscle was evaluated using the modified Ashworth spasticity scale. The Gly levels in rat plasma and L1-L3 segments of spinal cord were determined by enzyme-linked immunosorbent assay (ELISA). The GABA levels in rat plasma and L1-L3 segments of spinal cord were measured by high performance liquid chromatography (HPLC).Results: The decrease in rat muscle tension scored by the modified Ashworth spasticity scale in the An-pressing tendon group was more significant than that in the An-pressing muscle belly group (P<0.01); the increases in Gly and GABA levels in the rat plasma and L1-L3 segments of spinal cord were more significant in the An-pressing tendon group than those in the An-pressing muscle belly group (all P<0.01).Conclusion: Based on the theory of ‘anti-stretch reflex’ of tendon organs, the use of An-pressing manipulation to induce the ‘anti-stretch reflex’ by stimulating the tendon organs can improve the muscle spasticity of rats, which is better than An-pressing the muscle belly. Increased levels of Gly and GABA in rat plasma and L1-L3 segments of spinal cord may be one mechanism of An-pressing manipulation to improve muscle spasticity by stimulating tendon organs.
Keywords: Tuina; Massage; An-pressing Manipulation; Muscle Spasticity; Reflex, Stretch; Gamma-aminobutyric Acid;Glycine; Rats
Hemiplegic spasticity is a common symptom of motor neuron injury after stroke. About 80% of the stroke patients more or less suffer from limb spasticity,reported by the domestic researches[1], while it is 65%reported abroad[2]. To improve the limb muscle spasticity caused by stroke has also become a hotspot and difficulty in current medical research.
An-pressing manipulation is an important technique in tuina manipulation, and plays an important role in resuscitation and dredging Bi-Impediment syndrome,and inhibiting spasticity and pain. Based on the theory of ‘anti-stretch reflex’ of tendon organs, this work explored the effect and mechanism of An-pressing manipulation in treating post-stroke muscle spasticity in rats, to provide a theoretical basis for An-pressing manipulation in the management of post-stroke muscle spasticity.
1 Materials
1.1 Experimental animal
Eighty clean adult male Sprague-Dawley (SD) rats,weighing 250-300 g, were provided by the Animal Experiment Center of Hunan University of Chinese Medicine. Rats were housed in cages at the Animal Laboratory Center of Hunan University of Chinese Medicine, with the breeding temperature of 24-26 ℃and the humidity of 50%-70%. Ordinary feed was provided by the Animal Experiment Center of Hunan University of Chinese Medicine.
1.2 Laboratory apparatus
Homemade An-pressing manipulation operation instrument for rat quadriceps femoris muscle; high performance liquid chromatography (HPLC) with a fluorescent detector RF-530 (EM: 430, EX: 338); SCL-6A system controller and LC-2010AHT HPLC (Shimadzu Corporation, Japan); chromatography workstation (Nan Qianpu Software Co., Ltd., China); chromatographic column (N0va-Pak C18 column, 3.9 mm×150 mm, 5 μm,LKB BROMMA company, USA); TGL-16C centrifuge(Hunan Xingke Instrument Factory, China); XW-80A vortex mixer (Shanghai Medical University Instrument Factory, China); 920A acidity meter (Orion, USA).
2 Methods
2.1 Animal grouping
Ten of the 80 rats were randomly selected as the blank group, and the remaining 70 were used for surgical modeling. Three rats died during the modeling process, four rats died after resuscitation, and 63 rats were remained. After the rats revived from anesthesia,the neurological function was evaluated with the five-point scale reported by Longa EZ, et al[3-4], and 48 rats with successful middle cerebral artery occlusion(MCAO) modeling were selected. Modified Ashworth muscle spasticity scale assessment was performed for rats 2 d after resuscitation. Thirty successful muscle spasticity model rats that met the enrollment requirements were subjected to the second round random grouping using a random number table method,and were divided into a model group, an An-pressing tendon group and an An-pressing muscle belly group.There were 4 groups in total including the blank group.When the rat brain was collected at the end of the experiment, it was found that some occlusion sites of the middle cerebral artery were not at the beginning of the middle cerebral artery, therefore, the actual sample size was changed to 6 rats per group.
2.2 MCAO model preparation
The post-stroke muscle spasticity model was established by referring to the method modified by Koizumi J, et al[5], and the rat MCAO model was established using insertion suture occlusion method in the left external carotid artery.
2.3 Criteria for successful model
2.3.1 Criteria for successful MCAO stroke model rat
At the 6th hour after the animal revived from anesthesia, the neurological function was evaluated by the five-point scoring standard reported by Longa EZ,et al[3]. Rats with a score of 2-3 points were included in the experiment, and rats with a score of 0, 1 and 4 points were excluded. When the rat brain was collected at the end of the entire experiment, the location of the suture occlusion at the middle cerebral artery was first checked. Those rats with suture occlusion not at the beginning of the middle cerebral artery were excluded from the statistics.
2.3.2 Criteria for successful muscle spasticity model after stroke
The modified Ashworth spasticity scale was used to evaluate the tension of quadriceps femoris muscle in rats because there was no scoring standard for animal spasticity[6].
Twenty-four hours after the rats were awake, muscle tension was evaluated for those rats with a Longa neurological function score of 2-3 points (n=48).
Rats with grade 1-, 1+or 2 by the modified Ashworth spasticity scale were selected for the experiment (n=30),and rats grade 3 or 4 were excluded.
2.4 Manipulation
Clinical manifestation of post-stroke muscle spasticity is flexor spasticity in the upper limbs and extensor spasticity in the lower limbs. Therefore, the quadriceps femoris muscle was selected as to receive An-pressing manipulation in our current work.
Based on our previous research experience, a homemade An-pressing manipulation operation instrument for rat’s quadriceps femoris muscle was used to simulate the An-pressing manipulation operation (Figure 1 and Figure 2). Two days after modeling, rats in the An-pressing tendon group received An-pressing manipulation at the quadriceps femoris muscle tendon, and rats in the An-pressing muscle belly group received An-pressing manipulation at the quadriceps femoris muscle belly, with the pressure of (350±50) g, frequency of 5 s/time, and An-pressing intensity (the An-pressing distance generated under the pressure by the spring behind the An-pressing head) of 10 cm, 15 min per session, once a day for continuous 5 d[7]. Rats in the blank and the model groups only received bundling at the same time every day.
2.5 Preparation of samples
After the fifth treatment, the rats in each group were scored for muscle tension. Then blood was collected from the abdominal aorta into anticoagulation vacuum blood collection tubes. The blood samples were centrifuged at 3 000 r/min for 15 min. The supernatant was collected and stored at -20 ℃ after the specimen was numbered. Enzyme-linked immunosorbent assay(ELISA) was used to determine the glycine (Gly) change and HPLC was used to determine the γ-aminobutyric acid (GABA) change in rat plasma.
The rats were immediately sterilized, and the gray matter of the L1-L3segment spinal cord anterior horn was separated under a sterile workbench.
ELISA was used for Gly and HPLC was used for GABA detection in the gray matter tissues of the L1-L3segment spinal cord anterior horn.
2.6 Statistical methods
All data were input into a computer and processed with SPSS version 19.0 Windows software. Normal distribution measurement data were presented as mean ± standard deviation (x ±s), and independent sample t-test was used for comparison between the two groups; abnormal distribution measurement data were presented as median (interquartile range), and comparisons between the two groups were performed using the Mann-Whitney rank-sum test. Counting data were presented as frequency and percentage.Comparisons of counting data between groups were performed by Chi-square test or Fisher exact probability method. P<0.05 indicated a statistically significant difference.
3 Results
3.1 Comparison of muscle tension scored by the modified Ashworth muscle spasticity scale
Compared with the blank group, the muscle tension of the model group was significantly increased (P<0.01),suggesting that the post-stroke muscle spasticity model was successfully prepared. There was no significant change in the rat muscle tension score of the model group after intervention (P>0.05), suggesting that no interference was caused by the auto-repair factors of animals within 5 d after the intervention. After intervention, the muscle tension of rats in the An-pressing muscle belly group and An-pressing tendon group decreased significantly compared with that in the model group (both P<0.01), suggesting that An-pressing manipulation can relieve post-stroke muscle spasticity.Rats in the An-pressing tendon group showed significantly lower muscle tension than the An-pressing muscle belly group (P<0.01), suggesting that the improvement of rat muscle spasticity in the An-pressing tendon group was more significant (Table 1).

Figure 1. An-pressing manipulation operation instrument
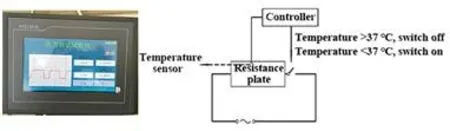
Figure 2. Operation platform and design principles of the An-pressing manipulation operation instrument
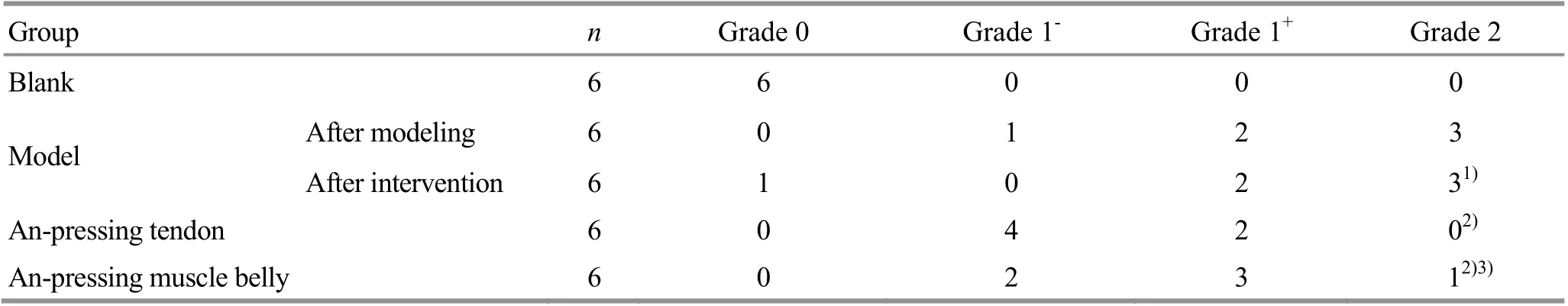
Table 1. Comparison of muscle tension scored by the modified Ashworth spasticity scale among groups (case)
3.2 Comparison of the Gly and GABA levels in rat plasma
Compared with the model group, the Gly and GABA levels in plasma of the An-pressing muscle belly group and the An-pressing tendon group were significantly increased after intervention (all P<0.01), suggesting that An-pressing manipulation increased the level of inhibitory neurotransmitters in plasma of post-stroke spastic rats, and finally improved spasticity. The increases in the Gly and GABA in the An-pressing tendon group were more significant than those in the An-pressing muscle belly group (both P<0.01),suggesting that based on the theory of ‘anti-stretch reflex’ of tendon organs, the effect of An-pressing tendon to increase the plasma Gly and GABA levels of rats with post-stroke spasticity was better than An-pressing muscle belly (Table 2).
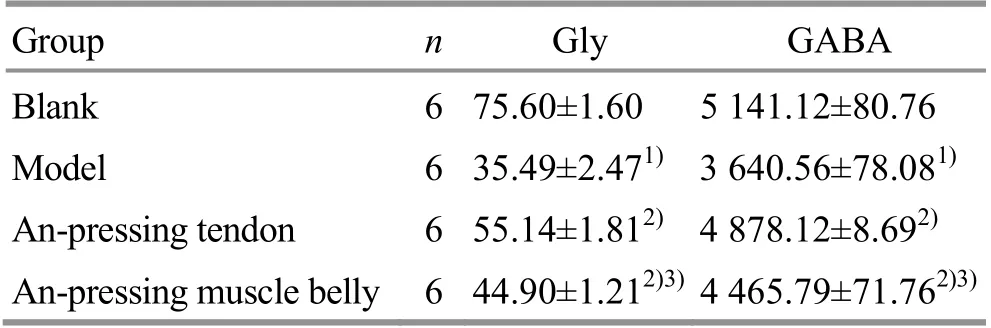
Table 2. Comparison of the Gly and GABA levels in plasma of rats among groups (x ±s, μmol/L)
3.3 Comparison of the Gly and GABA levels in rat spinal cord
Compared with the blank group, the Gly and GABA levels in the gray matter tissues of the L1-L3segment spinal cord anterior horn of the model group were significantly reduced (both P<0.01). After intervention,compared with the model group, the Gly and GABA levels in rat spinal cord of the An-pressing muscle belly group and the An-pressing tendon group were significantly increased (all P<0.01). Among them, the increases of the Gly and GABA levels in the An-pressing tendon group were more significant than those in the An-pressing muscle belly group (both P<0.01),suggesting that based on the ‘anti-stretch reflex’ theory of tendon organs, the effect in increasing the Gly and GABA levels in spinal cord of post-stroke spastic rats was better by An-pressing tendon than by An-pressing muscle belly (Table 3).
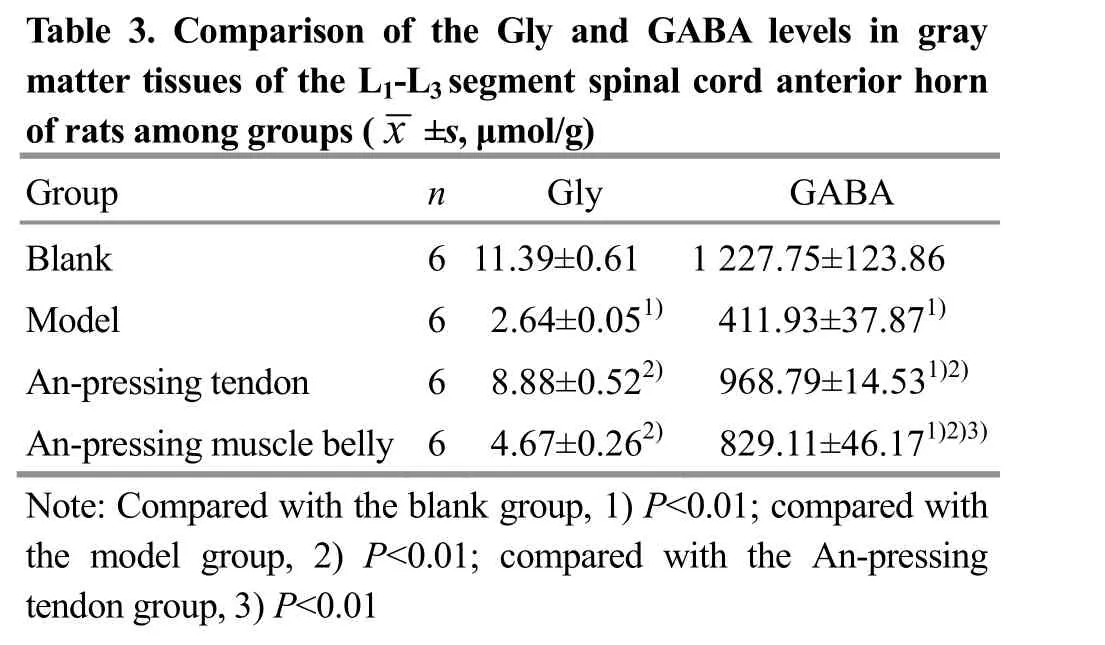
Table 3. Comparison of the Gly and GABA levels in gray m of artat e ts r atmi ss o unegs gorfo tuhpes L(1x- L±3 ss,e μg mme o n l/tg )s pinal cord anterior horn Group n Gly GABA Blank 6 11.39±0.61 1 227.75±123.86 Model 6 2.64±0.051) 411.93±37.871)An-pressing tendon 6 8.88±0.522) 968.79±14.531)2)An-pressing muscle belly 6 4.67±0.262) 829.11±46.171)2)3)Note: Compared with the blank group, 1) P<0.01; compared with the model group, 2) P<0.01; compared with the An-pressing tendon group, 3) P<0.01
4 Discussion
Increased muscle tension is a hyperactive stretch reflex and manifested in the form of spasticity. The restraint to the lower-level neurons such as the spinal cord is weakened after stroke due to the damaged high-level central motor neurons, which causes the hyperactive ‘stretch reflex’ of the spinal cord segment,especially the increased excitability of the ‘α-γ loop’[8].The normal voluntary movement is replaced by the abnormal movement pattern characterized by spasticity[9], which causes the spasticity of the limb muscles after stroke[10]. ‘Anti-stretch reflex’ is aimed at the increased excitatory α motor neurons. It stimulates tendon organs to induce nerve impulses, stimulates intermediate neurons in the spinal cord to release inhibitory neurotransmitters such as Gly and GABA, and inhibits the excessively excited α motor neurons, and suppresses stretch reflexes[11], thereby alleviating the limb spasticity. Based on the relationship between the two nerves, we proposed to verify the mechanism of‘anti-stretch reflex’ of tendon organs by testing the Gly and GABA levels in rat plasma after stimulating the tendon organs with An-pressing.
4.1 Peripheral nerve mechanism
Stroke hemiplegia is mainly manifested as muscle spasticity in the lower limbs. The quadriceps femoris muscle, as the largest extensor of the lower limbs, also presents with the most severe spasticity. An-pressing manipulation has the effects of soothing tendon and setting bone, strengthening tendon and nourishing muscle. An-pressing manipulation stimulates local muscles to make them contract and relax rhythmically,promotes local blood circulation, and reduces local edema of the quadriceps femoris muscle, thus to improve spasticity.
The basic feature of the release of many neurotransmitters is membrane depolarization and Ca2+entry[12]. The release of GABA in nerve endings is dependent on membrane depolarization and Ca2+entry[13]. Therefore, we believe that An-pressing manipulation promotes local blood circulation and reduces local edema, which is beneficial to the improvement of the substance level at the nerve-muscle junction. Enhancing substance transport may increase the inhibitory neurotransmitter level at the nerve-muscle junction, as a result, the concentrations of excitatory neurotransmitters and inhibitory neurotransmitters in the body tend to balance, thereby improving the muscle spasticity.
4.2 Central nerve mechanism
The impulse of the tendon organs is increased by pressing the tendon organs and transmitted into the spinal cord through class Ib fibers to excite the inhibitory intermediate neurons in the spinal cord for releasing inhibitory neurotransmitters (Gly and GABA),thus to inhibit α motor neurons and reduce the excitability of α motor neurons. As a result, the external spindle muscle contraction and the stretch reflex are weakened, so as to achieve the purpose of relieving muscle spasticity.
Gly has a protective effect on cells through direct or indirect pathways, though the exact mechanism of its protective effect is unknown. Many experiments have found that Gly plays an important role in antiinflammatory, immunomodulatory and cytoprotective effects in various disease states[14-17]. Increased GABA may have protective effects on ischemic brain injury[18-19]. GABA allows chloride ions to enter the cells to hyperpolarize the membrane, and antagonize the depolarization caused by excitatory amino acid (EAA) to protect cells[3,20]. Therefore, the increased inhibitory neurotransmitters, such as Gly and GABA in plasma, are possible to improve the inhibitory neurotransmitter levels in the high-level central nervous system through humoral circulation, speed up the reorganization of central nervous function and improve the high-level central function, thus to relieve limb muscle spasticity.
The An-pressing manipulation used in this study effectively improved the post-stroke muscle spasticity of rats, especially the An-pressing tendon manipulation based on the ‘anti-stretch reflex’ theory of tendon organs. Our result is helpful for further scientific research of An-pressing manipulation.
Conflict of Interest
The authors declare that there is no potential conflict of interest in this article.
Acknowledgments
This work was sponsored by Fund Project of Hunan Province Education Office (湖南省教育厅科学研究项目,No. 18C1174).
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria.
Received: 3 July 2019/Accepted: 30 August 2019
猜你喜欢
杂志排行
Journal of Acupuncture and Tuina Science的其它文章
- Clinical efficacy of acupuncture in treatment of chronic urticaria and its effects on the content of IgE and the imbalance of Th1/Th2 cell function
- Therapeutic observation of ‘warming-unblocking needling technique’ for knee osteoarthritis due to deficiency of liver and kidney
- Therapeutic observation of arthrolysis under brachial plexus anesthesia for adhesive capsulitis of the shoulder
- Clinical study of thumb-tack needle therapy for cervical radiculopathy based on meridian differentiation
- Clinical observation of acupuncture plus repetitive transcranial magnetic stimulation in the treatment of post-stroke insomnia
- Clinical observation on prevention of chemotherapy infection in gastric cancer by moxa-stick moxibustion plus rhG-CSF and its effect on immune function
