Does Light Exposure during Embryonic Development Affect Cognitive Behavior in a Lizard?
2020-04-21XinghanLIChenxuWANGGuoshuaiTANGShuranLILiangMABaojunSUNandYongpuZHANG
Xinghan LI,Chenxu WANG,Guoshuai TANG,Shuran LI,Liang MA,Baojun SUN* and Yongpu ZHANG*
1 Key Laboratory of Animal Ecology and Conservational Biology,Institute of Zoology,Chinese Academy of Sciences,Beijing 100101,China
2 University of Chinese Academy of Sciences,Beijing 100049,China
3 College of Life and Environmental Science,Wenzhou University,Wenzhou 325035,Zhejiang,China
Abstract Light is essential for embryonic development in many oviparous animals including fish,amphibians,and birds.However,light may be harmful for reptile embryos developing underground where they are in complete darkness and beneath thin eggshells.Nonetheless,how embryonic light conditions affect reptile development and offspring remains largely unknown.Here we incubated eggs in dark and light conditions to determine the effects of light exposure on embryonic development and offspring visual ability,spatial cognitive ability and growth in a lacertid lizard,Eremias argus.Our experiments demonstrated that light stimulation shortened incubation duration of eggs,but did not affect hatching success,offspring size,visual ability or survival.More interestingly,light exposure during incubation decreased spatial cognitive ability and post-hatching growth of offspring.On the basis of negative effects on offspring growth rates,our study indicates that in squamate reptiles with thin eggshells,light exposure in early development has negative effects on offspring cognitive ability.
Keywords embryonic development,light,vision,cognitive ability,Eremias argus
1.Introduction
Various environmental parameters during embryonic development play a critical role in shaping the phenotypes in oviparous species (Deeming,2002,2004;Howe,1967;Jezierskaet al.,2009)because embryos develop outside of the mother’s body.Light is one of the most important environmental factors that may govern embryonic development for many animals.Light stimulation may accelerate embryonic development and induce egg hatching in a diversity of oviparous animals ranging from arthropods to birds (Cooperet al.,2011;Horiguchiet al.,2009;Itoh and Sumi,2000;Villamizaret al.,2011).In addition,light exposure during embryonic development is important for the development of vision in fish (e.g.Yamamotoet al.,2004),the modulation of lateralization in fish and birds (e.g.Andrewet al.,2009;Dadda and Bisazza,2012;Rogers,1982;Zappia and Rogers,1983) and,therefore,a number of fitness-related behaviors,such as the analysis of topography and position or the response to prey(Andrewet al.,2000;Bianki and Snarsky,1988).
Unlike many insects,fish,amphibians,and birds that lay eggs exposed to sunlight,most oviparous reptiles bury their eggs underground,resulting in the embryos being sheltered from light.This ecological scenario implies that light is not an important influencing factor for embryonic development in reptiles.As a result,ecologists have largely neglected the effect of light on embryonic development,despite extensive studies on how other environmental factors (e.g.temperature,moisture,and oxygen)affect embryonic development and hatchling phenotypes in reptiles (Deeming,2004;Du and Shine,2015;Nobleet al.,2018).Yet,it would be of great interest to determine how reptile embryos respond to light,because reptiles are a lineage of oviparous animals whose eggs develop in darkness (i.e.underground)under natural conditions.If light exposure during embryonic development imposes negative effects on reptiles,it suggests that reptiles have lost the mechanism to protect themselves from sunlight radiation.
Light exposure during embryonic development has been demonstrated to significantly shorten incubation periods in lizards (one skink,one lacertid and two geckos).In addition,light exposure can impose negative effects on hatchling size and survival in the Chinese skink (Plestiodon chinensis) with very thin eggshells (Zhanget al.,2016).Nonetheless,more research is still needed to conclude the generality of negative effects of light stimulation during embryonic development.Additionally,in contrast to essential effects on vison and cognition of topography and position (e.g.Andrewet al.,2000;Bianki and Snarsky,1988),if reptiles lost the protective mechanisms against light,it is interesting and biologically significant to reveal the effects of light exposure during embryonic development on vision and cognitive behavior.Here,by incubating eggs of a thin eggshell species of lacertid lizard (Eremias argus) under light and dark treatments,we aim to reveal the effect of light exposure during embryonic development on vision and offspring spatial cognitive ability in reptiles to see whether vision in lizards is sensitive to light exposure during embryonic development,and if reptile cognition is also sensitive to light conditions during development,as has been shown for temperature and oxygen (Amiel and Shine,2012;Sunet al.,2014).
2.Methods
2.1.Animal collection and husbandryThe Mongolian racerunner (E.argus) is a small (up to 70 mm snout-vent length,SVL) oviparous lacertid lizard distributed from northern China to Russia,Mongolia,and Korea.Female adults lay multiple clutches (May to July),with each clutch containing 2 to 5 parchment-shelled eggs (Zhao,1999).We collected gravidE.argusfemales from Xingtai (35°22′ N,117°21′ E) in Hebei Province,China in May.The females were maintained in separate terraria(310×210×180 mm),which were set up in a temperature-controlled room at 24 ± 0.5°C with a 14L:10D (light:dark) photoperiod.Supplemental heating was provided for basking from 08:00 to 16:00.The bottom of the terrarium was lined with moist vermiculite (20 mm thick) where the females laid their eggs.Food (mealworms and crickets dusted with multivitamins and minerals) and water were providedad libitum.
2.2.Egg incubation and hatchling collectionWe checked the terraria for freshly laid eggs three times a day.The collected eggs were weighed (± 0.001 g) immediately and one egg from each clutch was randomly assigned to the full spectrum light (T8,Zilla,USA) or dark incubation treatments to avoid pseudo replication.We allocated 17 and 13 eggs to the full spectrum light and dark incubation conditions,respectively.Eggs were half-buried inside a vermiculite-filled container (-220 kpa) with a plastic membrane cover,and then incubated at 28°C in an incubator (MIR-554,SANYO,Japan).Four data loggers (iButton DS1921G#F50,Dallas,USA) were set beside the eggs in each treatment to record incubation temperatures.We checked the moisture inside the container every week,and added water if necessary.
We followed established methods,with some modifications,to set up the light conditions in the incubator (Andrewet al.,2009;Cooperet al.,2011;Shafey,2004).A Zilla®reptile UV full spectrum light with a wavelength range of 280-780 nm was used for the light incubation condition (Figure S1,available online).In contrast,eggs received no light radiation in the dark incubation conditions (control).The internal space (600×550×300 mm3) of the incubator was divided into two compartments with a double black cloth for the dark and light incubation conditions,respectively.The inner wall of the incubator was covered with black paper to eliminate the reflection from radiation.In the light incubation compartment,the light was located 150 mm above the eggs,with a photoperiod of 10L:14D (from 08:00 to 18:00).During the photoperiod,light availability at the surface of the eggs was measured with a luxmeter (F941,Fluke,USA) at 13:00 every three days.The luminance of light on the egg surface ranged from 690 to 935 lx.
2.3.Post-hatching traitsDuring the end of incubation,we checked for new hatchlings every morning and evening.The hatchlings were measured (SVL to 0.01 mm,body mass to 0.001 g) and were then randomly allocated to terraria (600×450×340 mm3,20 mm sand substrate) in a temperature-controlled room at 26 ± 0.5°C with a photoperiod of 12L:12D (from 07:00 to 19:00).Supplemental heating was provided for basking from 08:00 to 16:00.Food (mealworms and crickets dusted with multivitamins and minerals) and water were providedad libitum.At 30 days after hatching,all hatchlings were reweighed and remeasured(SVL) and the survival of hatchlings was recorded.
2.4.Hatchling visual and cognitive abilityOne week after hatching,visual abilities of hatchlings were tested,using established methods for lizards (Beazleyet al.,2003),with some modifications.Briefly,the hatchlings were fasted for 24 h before the test.During the testing,we used prey items presentation(mealworm larvae) to stimulate the visual attention of one eye of a hatchling.A mealworm was attached to a hand-held stick,which was moved across the monocular field of each eye in a random sequence of left or right eye.Between presentations,the movement of the stick was randomly varied across the nasotemporal or dorso-ventral axes bisecting the main visual axis(Figure S2).If hatchling detected the prey (i.e.turned its head towards the worm,Beazleyet al.,2003) within 60 s,the test was terminated and the hatchling was scored as one for visual ability;if not,the hatchling was scored as zero.Each hatchling was tested separately two times a day over four consecutive days (n=8 tests/hatchling).After the vision test,the hatchlings were returned to their terraria.
Then we tested the spatial cognitive ability of hatchlings from the different treatments in the terrarium where they were fed.One week before the test,hatchlings (21-25 days old) were acclimated in the terraria.Because a familiar environment facilitates spatial cognition,we kept the setting of terraria fixed throughout acclimation and the test (Amiel and Shine,2012;Paulissen,2008).The setting of terrarium and the measurement of cognitive abilities were according to published methods with minor modifications (Sunet al.,2014).In brief,each terrarium contained two hides spaced 350 mm apart.The entrance of one hide was covered with a transparent film to prevent hatchlings’access,but not the other.During the end of acclimation,each hatchling was practiced separately twice a day over two consecutive days before tests conducted with the same methods.In each test,we set a hatchling beneath a container for 30 seconds,then we removed the container and stimulated the hatchlings with a paintbrush.We recorded a successful escape once the hatchling fled into the open hide within 30 seconds,and an error once the hatchling attempted to enter the closed hide.An individual was placed inside the open hide if it did not enter within 120 seconds.We assessed the cognitive ability of hatchling lizards by the probability of successful escape.Tests were conducted for four consecutive days with four tests per day(n=16 tests/lizard).
2.5.Statistical analysisWe analyzed the daily temperatures during incubation with dependentt-test.We analyzed hatching success and hatchling survival (until 30 d of age) between the light and dark incubation conditions with chi-square tests.We compared the initial egg mass and incubation duration with oneway ANOVAs.One-way ANCOVAs were used to compare differences in body size at hatching and 30d between the light and dark conditions,with initial egg mass and hatchling SVL as the covariates,respectively.In analysis,effects of female identify as random factor were redundant and thus were eliminated.Linear mixed model was used to analyze the vision of hatchlings,with test sequence,left or right eye and treatment as factors.We used Generalized Estimating Equations (GEE) to test the effect of light and dark incubation conditions on escape outcome(1=successful escape;0=failed escape) and error rates over 8 consecutive tests,respectively.In the GEE analysis,we considered hatchlings under light incubation conditions as the focal subject for analysis,with the fittest model according to QICC (corrected quasi-likelihood under the independence model criterion).The normality and homogeneity of variance were checked using Kolmogorov-Smirnov and Levene’s tests prior to analysis of variance.All variables met assumptions of normality (allP>0.130) and homogeneity of variance (allP> 0.119).
3.Results
Initial egg mass and incubation temperatures were similar between light and dark incubation conditions.Full spectrum light exposure during embryonic development significantly shortened incubation duration (39.2 ± 0.4) by an average of 2.6 days,compared with dark incubation (41.8 ± 0.3).Embryos developed under full spectrum light produced hatchlings with a significantly lower growth rate.In contrast,light exposure did not have a significant impact on hatching success,hatching SVL,hatchling BM,or survival of one-month-old hatchlings(Table 1).In addition,the vision of hatchlings was not affected by test sequence (F1,33=1.825,P=0.115),to which eye the worm presented (F1,33=1.354,P=0.253),or light treatment (Table 1).
The practice during acclimation made the lizards familiar with the surroundings and the escape retreat.Full spectrum light negatively influenced the spatial cognitive ability (escape probability) of lizards,but the test sequences did not (Figure 1A;Table 2;Table 3,with ‘independent’ working correlation matrix;Table 4).The escape probability of lizards incubated under dark conditions was significantly higher than that of lizards incubated under light conditions (Figure 1A;Table 2).However,light exposure did not affect the number of errors that hatchling lizards made (Figure 1B;Table 2;Table 3,with‘independent’ working correlation matrix;Table 4).
4.Discussion
The effect of light on embryonic development remains poorly understood in reptiles (Sleigh and Birchard,2001;Zhanget al.,2016).Remarkably,our study indicates that even relatively low light intensities (less than 1000 lx for 10 hours per day)have significant effects on embryonic development and hatchling phenotypes.In addition to the negative impact of light stimulation on embryonic survival (Zhanget al.,2016),light exposure during incubation imposed profound effects on cognitive ability (i.e.learning to successfully escape to a safe retreat in this study) and growth rate in lizards.
Embryonic light stimulation had a negative impact on offspring cognition.Our study provides the first evidence that light exposure during development,in addition to temperature and oxygen,affects the cognitive ability of lizards (Amiel and Shine,2012;Sunet al.,2014).Specifically,after acclimatizing to surroundings and practicing learning the location of safe retreats,lower escaping probabilities demonstrated that light exposure during incubation decreased the spatial cognitive ability of Mongolian racerunner lizard (Figure 1A).The decrease in cognition was not related to visual ability,which may have affected the ability of lizards to locate the hides during the cognitive ability tests,because there was no difference in sight between the light and dark treatments (Table 1).Admittedly,individual boldness could also affect lizard escape behavior(Carazoet al.,2014;Cooper,2009).In this study,we found the attempt to escape into safe retreat of lizards between dark and light treatments were equal,indicated by similar error rates hatchlings made (Figure 1B).
In contrast,embryonic light exposure in birds and fishes can enhance hatchlings’ cognitive abilities.For example,light exposure on chick eggs before hatching increased the hatchlings’ learning ability to find food and at the same time be alert to a predator overhead (Rogerset al.,2004).In addition,absence of light during incubation in zebrafish reduced the spatial cognitive ability of hatchlings to detect potential refuges(Andrewet al.,2009).The decrease in cognition in offspring developed under light exposure ofEremias argusmight be related to brain size and brain structure,which are primary in cognition determination (Lemaireet al.,2000;Solet al.,2008).Alternatively,light exposure during embryonic development could affect brain lateralization ofE.argus.As reported in other lizards and chicken,the lateralization could also determine the cognitive ability,including escaping behavior and predatory responses (Bonatiet al.,2010;Bonati and Csermely,2013;Robinset al.,2005;Rogerset al.,2004).However,we did not find any evidence of lateralization inE.argusas our results were not affected by the eye to which the prey item was presented.Additional experiments are needed to verify the effect of brain lateralization caused by light exposure on offspring cognition.
The effect of light exposure on incubation period inE.argusmay be led by some potential mechanisms.First,light exposure can accelerate embryonic heart rate which is an important proxy of metabolic rate (e.g.Zhanget al.,2016),and thus enhance the developmental rates of embryos.Or alternatively,light exposure can stimulate the behavior of hatching as a visual signal as documented in leopard geckos (Sleigh and Birchard,2001).InE.argus,the effect of light exposure on developmental rate is plausibly led by enhanced metabolic rates.Accordingly,as a cost of higher metabolic rates,post-hatching growth rate might be depressed as indicated in other lizard species (e.g.Sunet al.,2018).
The influence of light on embryonic development depends on light properties.Previous studies have shown that wavelength may have different effects on the development of zygotes and embryos.Ultraviolet (UV) light (300 to 400 nm wavelength) produces free radical oxygen species,such as H2O2,that damage DNA,proteins,and lipids within the cell (Squirrellet al.,1999).Short-wavelength visible light [e.g.,the visible ray of blue wavelength range (445-500 nm)]from ordinary cool white fluorescent lamps is a major component of harmful lighting that affects embryo survival,but is far less damaging to cells than UV (Ohet al.,2007;Seoet al.,2007;Takenakaet al.,2007).In addition,light intensity may also affect embryonic developments.Our previous study (Zhanget al.,2016) revealedthat high-intensity light (1764 to 1970 lx) exposure during embryonic developments decreased the survival of lizard embryos.In contrast,low-intensity light exposure (690 to 935 lx) did not affect hatching success or hatchling survival in this study.Therefore,the severe effects on embryonic developmentand hatchling traits are attributed to the full-spectrum light contained UV light and short-wavelength visible light rather than the light intensity in the current study.
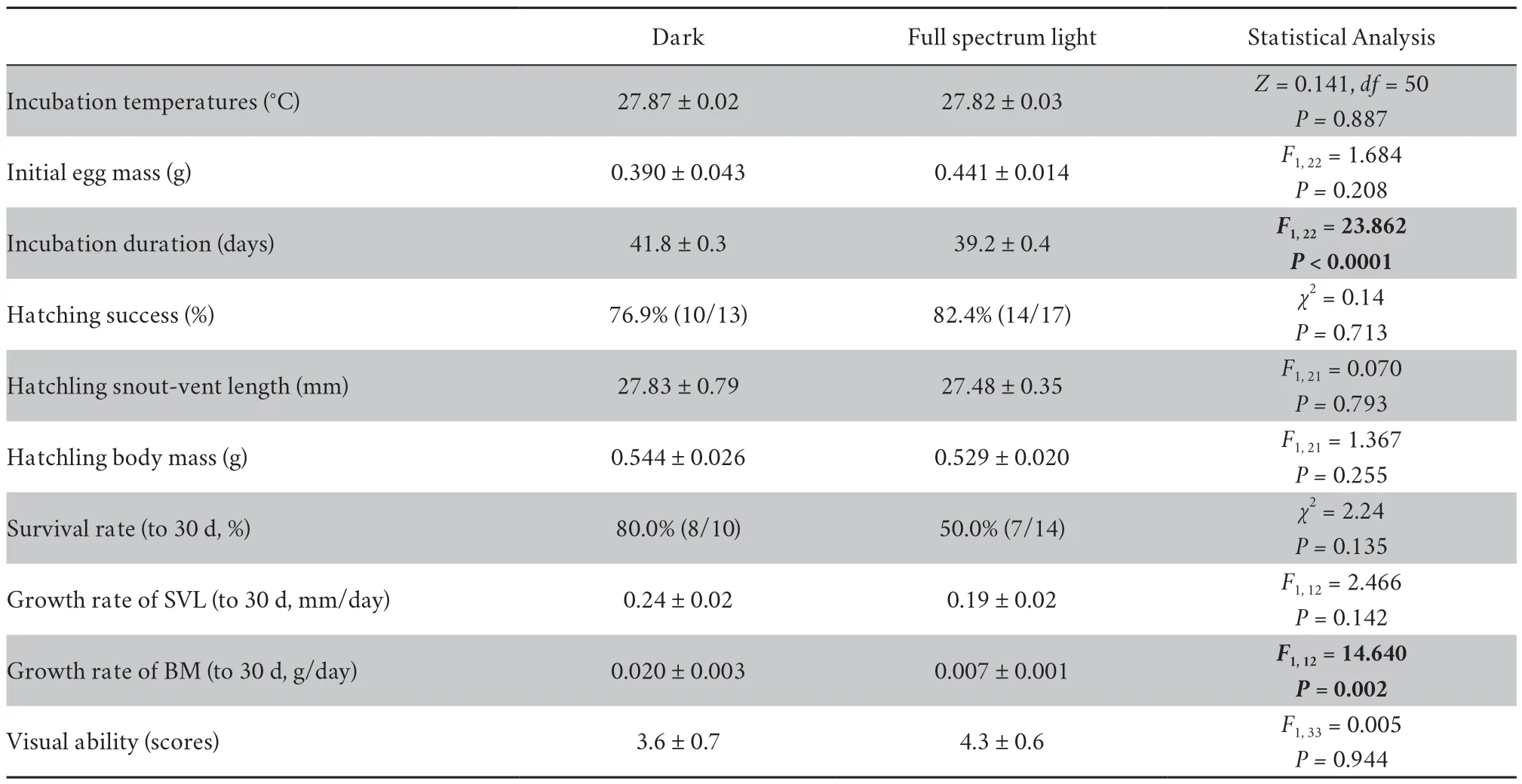
Table 1 Statistical comparisons of Eremias argus hatchlings incubated under dark and light conditions.Significant difference is indicated as boldness.Data are presented as the mean ± s.e.In egg mass and hatchling sizes,the sample sizes are 10 and 14 for dark and full spectrum light conditions,respectively.In hatchling growth and visual ability,the sample sizes are 8 and 7 for dark and full spectrum light conditions,respectively

Figure 1 Spatial cognitive ability of Eremias argus hatchlings incubated under dark and full spectrum light conditions,expressed as (A) the escape probability and (B) the number of errors.Data are presented as the mean ± s.e.,and the sample sizes are 8 and 7 for dark and full spectrum light conditions
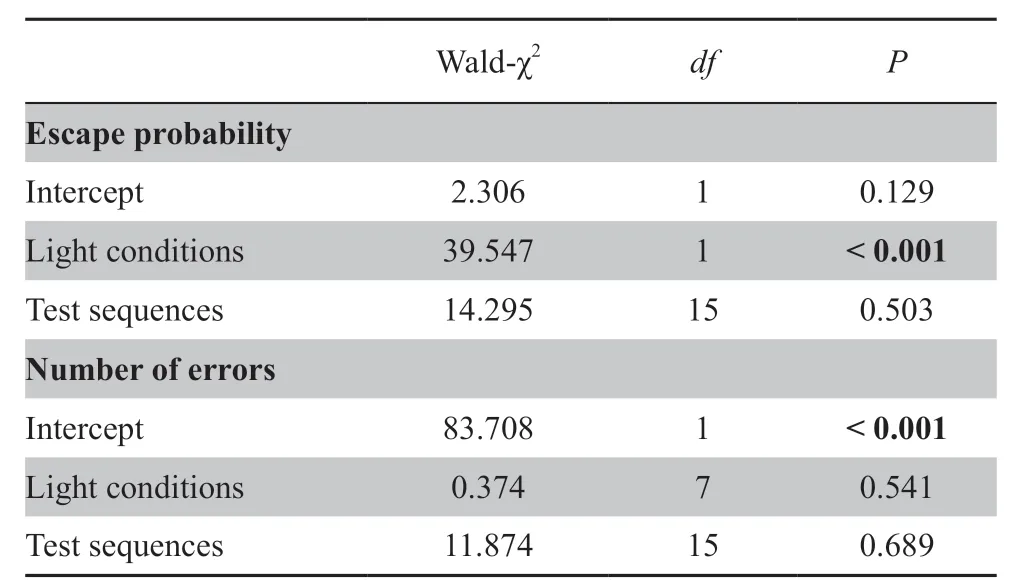
Table 2 Results of Generalized Estimating Equations (GEE) in testing the effects of light incubation conditions and test sequences on escape outcome and the number of errors throughout the testing process.Bold numbers indicate significantly different results.

Table 3 QIC and QICC value of potential working correlation matrixes.The lowest QICC among models are shown in bold.The ‘Independent working correlation matrix’ was selected when running our analysis.QICC of several matrixes were equal for‘number of errors’ test,so we selected the ‘Independent’ for its lowest QIC value.
More generally,the negative effect of light on offspring phenotypes by developmental plasticity has important implications for the culture of reptile embryos.In addition to light exposure damaging embryonic development in mammals(Ohet al.,2007;Seoet al.,2007;Takenakaet al.,2007),our study demonstrates that light stimulation may profoundly and negatively influence hatchling growth and spatial cognitive ability.
AcknowledgementsWe thank Tingting WANG,Yige CHEN and Peng CAO for assistance.All procedures were performed under the approval (IOZ14001) from the Animal Ethics Committee at the Institute of Zoology,Chinese Academy of Sciences.Funding was provided by National Natural Science Foundation of China (31500324 for Baojun SUN and 31801977 for Shuran LI).
Appendix
Figure S1 Spectral energy distribution of full spectrum lights.Data are from the website of Zilla®3 (http://www.zilla-rules.com/assets/00a6/20616.pdf).

Figure S2 Sketch map that how the worm was presented to the lizard during vision test.The figure was imitated from published method by Beazley et al.,2003.N-T and D-V in the figure indicate naso-temporal and dorso-ventral axes,along which the worm was presented,respectively.
Reference
Beazley L.D.,Rodger J.,Chen P.,Tee L.B.G.,Stirling R.V.2003.Training on a visual task improves the outcome of optic nerve regeneration.J Neurotrauma,20:1263-1270
杂志排行
Asian Herpetological Research的其它文章
- A New Species of the Asia n Toad Genus Megophrys sensu lato(Anura:Megophryidae) from Guizhou Province,China
- Genetic Differentiation of the Forest Crested Lizards,Calotes emma alticristatus Schmidt,1925 and C.emma emma Gray,1845 (Squamata:Agamidae) Relating to Climate Zones in Thailand
- A Revised Checklist of Amphibians and Reptiles in Camiguin Sur,Misamis Oriental,Mindaanao,Philippines
- Species Diversity and Elevational Distribution of Amphibians in the Xianxialing and Wuyishan Mountain Ranges,Southeastern China
- Changes on Anuran Tadpole Functional Diversity along an Environmental Gradient at the Southernmost Atlantic Rainforest Remnant
