New Semiconducting Polymer Nanoparticles for Antibacterial Agent by the Synergetic Effect of Positive Charge and Photothermal Conversion
2020-04-13PANGuoyongLIYawenMALijunMAYufanAIWentingWANGZhenguoHOUXinhuiGrigoryZyryanovWANGZhuo
PAN Guoyong,LI Yawen,MA Lijun,MA Yufan,AI Wenting,WANG Zhenguo, HOU Xinhui,Grigory V·Zyryanov,WANG Zhuo*
(1.State Key Laboratory of Chemical Resource Engineering,Beijing Advanced Innovation Center for Soft Matter Science and Engineering,College of Chemistry,Beijing University of Chemical Technology,Beijing 100029,China; 2.Ural Federal University Yekaterinburg 620002,Russian Federation; 3.Postovsky Institute of Organic Synthesis,Ural Branch of the Russian Academy of Sciences,Yekaterinburg 620108,Russian Federation)
Abstract Because of the abuse of antibiotics and the emergence of bacterial resistance,the new antibacterial agents are required urgently.Herein,we prepared semiconducting polymer nanoparticles(SP-PPh3 NPs) with synergistic antibacterial activity due to photothermal properties and positive charge.SP-PPh3 NPs have broad-spectrum antibacterial properties against Gram-negative Escherichia coli(E.coli) and Gram-positive Staphylococcus aureus(S.aureus).The photothermal conversion efficiency of SP-PPh3 NPs is 43.8%.Moreover,the positive charge of SP-PPh3 NPs can adhere to bacteria,which is helpful to transmit heat to bacteria effectively.Under the synergistic effect of heat and positive charge,the antibacterial rates of E.coli and S.aureustreated with SP-PPh3 NPs are 99.9% and 98.6% in vitro,respectively.In addition,SP-PPh3 NPs have good biocompatibility and have almost no side effects on the major organs of mice.The bacteria-infected skin wounds on mice can completely heal after 12 d treated with SP-PPh3 NPs.
Keywords Semiconducting polymer; Positively charged nanoparticles; Photothermal conversion; Antibacterial
Bacterial resistance,caused by excessive abuse of antibiotics,is probably becoming serious than cancer[1].Over the past few decades,antibiotics are the main choice for the treatment of bacteria-induced diseases[2—6].However,with the extensive use of antibiotics and the emergence of multi-drug resistant bacte-ria,the therapeutic effect of antibiotics has greatly reduced[7—10].Considering the slow development of new drugs and the evolution of resistant bacterial pathogens,the new antibacterial solutions with high efficiency are urgently needed.Photothermal therapy(PTT),a new alternative solution,has received widespread attention due to its non-invasiveness and fewer side effects[11—13].Unlike conventional antibiotic therapy mechanisms,PTT uses photothermal agents to generate local heat to destroy bacteria,inducing less multi-drug resistant bacteria and also can eliminate the biofilm[14,15].
In the past few years,many photothermal agents have been extensively studied,such as metal nanoparticles,two-dimension materials,organic molecules and so on[16—19].Among them,organic molecules show advantages on the basis of the clear chemical structure and metabolic pathway.The conjugated semiconducting polymer possesses unique optical properties such as high photothermal conversion efficiency and good biocompatibility compared with inorganic materials,which has been widely used in optical imaging and treatment[20,21].Diketopyrrolopyrrole(DPP) with eucalyptus coplanar structural unit and the electron-deficient unit,has good photostability and high molar absorption coefficients,so it is one of the good photothermal materials for PTT[22,23].By regulating donor and acceptor structural units of semiconducting polymers,the energy gap between the highest occupied molecular orbital(HOMO) and lowest unoccupied molecular orbital(LUMO) can be reduced,therefore shifting the absorption maxima to the NIR range with good photothermal conversion efficiency[24—26].The materials with positive charge have the ability to destroy bacterial cell membranes by electrostatic attraction and insertion into lipid membranes.This physical damage to membranes makes it difficult for bacteria to self-repair,and inhibit the development of bacterial resistance[27,28].The combination of positive charge groups with photothermal polymers can get new antibacterial agents.
Herein,we link triphenylphosphine with the DPP structure to synthesize a positive charged polymer SP-PPh3.SP-PPh3can form nanoparticles,which have a good dispersed status in water.SP-PPh3NPs(SP-PPh3nanoparticles) show high photothermal conversion efficiency(43.8%) and low cytotoxicity.SP-PPh3NPs can killE.coliandS.aureusby the synergistic of positive charge and photothermal effect.Invivo,SP-PPh3NPs can promote the healing ofS.aureusinfected wound.
1 Experimental
1.1 Reagents and Instruments
2,5-Bis(6-bromohexyl)-3,6-bis(5-bromothiophen-2-yl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione(TDPP-Br) and {4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b′]dithiophene-2,6-diyl}bis(tributylstannane)(CPDT-Sn) were purchased from Derthon Optoelectronic Materials Science Technology Co.Ltd..Tris(dibenzylidene-acetone)dipalladium(0)[Pd2(dba)3] and tri-o-tolylphosphine[P(o-tolyl)3] were purchased from Acros.3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide(MTT) was purchased from KeyGEN BioTECH.Escherichiacoli(E.coli,Gram-negative bacteria) andStaphylococcusaureus(S.aureus,Gram-positive bacteria) were obtained from Beijing Tiantan Hospital.
The synthesized polymers were characterization by nuclear magnetic resonance(NMR) spectra with a Bruker Avance Ⅲ(400 MHz) NMR spectrometer,Fourier transform infrared spectroscopy(FTIR) with FTIR spectrometer(Nicolet 6700,Thermo Fisher Scientific Incorporated,USA).Gel permeation chromatography(GPC) tests were performed on Agilent 1260 GPC(Agilent,USA); PS was used as a calibration standard; the mobile phase was DMF+NaNO3(0.06 mol/L) and the flow rate was 1 mL/min.The UV-Vis spectrum was recorded by a spectrophotometer(U-3900H,HITACHI,Japan).The absorbance was measured with a PerkinElmer Multimode plate reader in the MTT and IC50assay.The dynamic light scattering(DLS),zetapotentials and the morphology of the nanoparticles were confirmed by Zetasizer Nano(Malvern,Mastersize 2000,UK) and transmission electron microscope(TEM,Hitachi HT7700,Japan).Morphological observation of bacteria was verifiedviaa scanning electron microscope(SEM,JEOL JSM-7401F,Japan).An 808 nm laser was obtained by a laser device(STL808T1-4.0W,Stone-laser LTD,Beijing,China).The temperature change was recorded by an IR-thermal camera(E8,FLIR,USA).
1.2 Syntheses of SP-Br and SP-PPh3
TDPP-Br(0.5 mmol,392.1 mg),CPDT-Sn(0.5 mmol,490.4 mg),Pd2(dba)3(0.01 mmol,9.2 mg),and P(o-tolyl)3(0.04 mmol,12.2 mg) were added into a Schlenk tube.Anhydrous toluene(10.0 mL) was added to the tube under nitrogen.Nitrogen was extracted/charged under the freeze.The reaction solution was vigorously stirred at 110 ℃ for 2 h.And the resulting mixture was poured into MeOH(200 mL) and filtered.The polymer was purified by Soxhlet extraction using methanol,acetone,andn-hexane,respectively.The residue was washed with chloroform and dried in vacuo to get a black-green solid SP-Br(240.1 mg,yield 23.1%).1H NMR(400 MHz,CDCl3),δ: 8.90(s,2H),7.06(d,J=97.9 Hz,4H),3.94(d,J=160.1 Hz,4H),3.40(s,4H),1.90(d,J=48.2 Hz,12H),1.37—1.11(m,26H),0.87(s,12H).
SP-Br(51.4 mg,0.05 mmol),triphenylphosphine(1.0 g) and ethanol(10 mL) were added to a flask.The reaction solution was stirred at room temperature for one day in a dark environment and then heated to 90 ℃ for one day.The polymer was purified several times by methanol and dichloromethane.The residue was dried under vacuum to give a dark purple solid SP-PPh3(27.0 mg,34.8%).GPC analysis:Mn=137520,Mw=280462.1H NMR(400 MHz,DMSO-d6),δ: 8.81(s,2H),7.75(s,30H),7.32—6.81(m,4H),4.02—3.64(m,8H),1.55—0.73(m,50H).
1.3 Preparation of SP-PPh3 Nanoparticles
SP-PPh3(1.0 mg) was sonicated by bath in a solution of F127(20.0 mg) in methanol(1.0 mL).It was filtered through a polyvinylidene fluoride(PVDF) syringe filter(0.22 μm,Millipore).Then,it was quickly injected into deionized water(10.0 mL) under a continuous bath ultrasound.After continuous sonication for 10 min,it was filtered through a polyethersulfone(PES) filter(0.22 μm,Millipore).The methanol solvent was removed by nitrogen purge at room temperature.The residue was washed and centrifuged with a 30 kDa centrifuge filter tube(Millipore) at 3500 r/min for 8 min,the procedure was repeated for five times.The desired solution was diluted to 1.0 mL with sterile deionized water for storage.
1.4 Photothermal Conversion Efficiency
An aqueous suspension of SP-PPh3NPs at different concentrations(10,15,20,25,30 μg/mL,0.5 mL) was injected in a centrifuge tube and irradiated with an 808 nm laser at a power density of 1.0 W/cm2for 6 min.Temperature changes were recorded by a near-infrared camera.The photothermal stabi-lity of the nanoparticles(20 μg/mL,0.5 mL) was tested by five on/off cycles.The photothermal conversion efficiency(η) is calculated by the following formula:

(1)
whereh[W/(m2·K)] is the heat transfer coefficient;S(m2) is the surface area of the sample container;Tmax(K) is the highest temperature;Tsurr(K) is the temperature of the surroundings;Q0(W) is the baseline energy input of the sample container and solvent;I(W) is the power of 808 nm laser;Aλis the UV-Vis absorption of the sample at 808 nm.hScould be determined by the following formula:
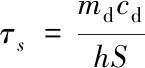
(2)
whereτs(s) is the characteristic thermal constant;md(kg) is the mass of the solution,andcd[J/(kg·K)] is its specific heat capacity.τsis determined by the linear regression of the cooling time of the material and the negative natural logarithm ofθ:
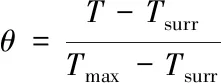
(3)
1.5 Cytotoxicity Assessment
The cytotoxicity of SP-PPh3NPs was determined by the MTT kit.HeLa(cervical adenocarcinoma epithelial cells) was cultured in cell culture medium[V(DMEM)∶,V(FBS)∶,V(penicillin)=100∶,10∶,1] at 37 ℃,5% CO2environment.HeLa was placed in a 96-well plate at 1×104cells per well(100 μL) and incubated in an incubator for 24 h to ensure cell attachment.The medium was removed and gradient concentrations of SP-PPh3NPs(10,20,30,40,50 μg/mL) were added.After incubated for another 24 h,the SP-PPh3NPs solution was removed and MTT(100 μL per well) was added,the resulting mixture was cultured for 4 h.Then,MTT was replaced by DMSO(100 μL per well).After shanked for 10 min,cell viability was assessed by the OD values at 490 nm by a microplate reader.
1.6 Determination of Half-maximal Inhibitory Concentration
SP-PPh3NPs were dissolved in LB(Luria-Bertani) medium as stock solutions,and 100 μL of bacteria(1×106CFU/mL) suspended in LB medium with an equal volume of different concentration samples were inoculated into 96-well plates and mixed(n=3).The bacterial solution alone and the medium containing SP-PPh3NPs were used as positive and negative controls,respectively.The negative and positive controls were placed on the same well plate.The well plates were incubated in an incubator at 37 ℃ for 14—16 h.And the half-maximal inhibitory concentration(IC50) was calculated from the absorbance values at 600 nm obtained by the microplate reader.
1.7 In vitro Antibacterial Activity
Theinvitroantibacterial activity of SP-PPh3NPs againstE.coliandS.aureuswas evaluated by plate counting.This part of the experiment was divided into the control group,the laser group,the SP-PPh3NPs group,and the “SP-PPh3NPs+laser” group,respectively.For the “SP-PPh3NPs+laser” group,E.coliorS.aureus(200 μL,OD600=0.6,respectively) suspension was centrifuged(8000 r/min) for 5 min and the supernatant was removed carefully.Then,a solution of SP-PPh3NPs(200 μL,20 μg/mL) was added and incubated with bacteria for 5 min in a dark environment.The mixed solution was irradiated with a power density of 808 nm laser at 1.0 W/cm2for 6 min.After that,the solution was diluted with PBS and uniformly coated on an agar plate.For the laser group,the same procedure was carried out without the usage of SP-PPh3NPs.The same procedure was performed for the SP-PPh3NPs group without laser irradiation.For the control group,the same procedure was carried out without the usage of SP-PPh3NPs and laser.All groups were incubated in an incubator for 16 h until obvious colonies were formed.All experiments were repeated three times.The number of bacteria was counted by a counter.
1.8 Morphological Observation of Bacteria
After treating bacteria in the same way asinvitroantibacterial,the samples were centrifuged(8000 r/min,5 min) and washed with PBS.The precipitate was fixed with 2.5% glutaraldehyde for 12 h at 4 ℃,then glutaraldehyde was removed.The mixture was washed twice with PBS,dehydrated with different gradients of ethanol(30%,50%,70%,90% and 100%,15 min).7 μL of the suspension was dropped on a silicon wafer,and the morphology of the bacteria was observed by SEM.
1.9 Wound Healing Experiment
BALB/c mice(3—4 weeks,female) were purchased from Beijing Huafukang Bioscience Co.Inc.The mice were divided into three groups: control group(PBS),SP-PPh3NPs group and “SP-PPh3NPs+laser” group with 5 mice per group.Mice were anesthetized with isoflurane and a wound with a diameter of 0.8 cm was made on the back of mice.The wound was infected withS.aureus(1×107CFU/mL,120 μL).After 12 h,for the “SP-PPh3NPs+laser” group,a solution of SP-PPh3NPs(50 μL,20 μg/mL) was inoculated into the wound.Five min later,the wound was irradiated with 808 nm laser(1.5 W/cm2) for 4 min.The SP-PPh3NPs group was treated in the same way besides the usage of an 808 nm laser.The control group was infection without irradiation or SP-PPh3NPs.
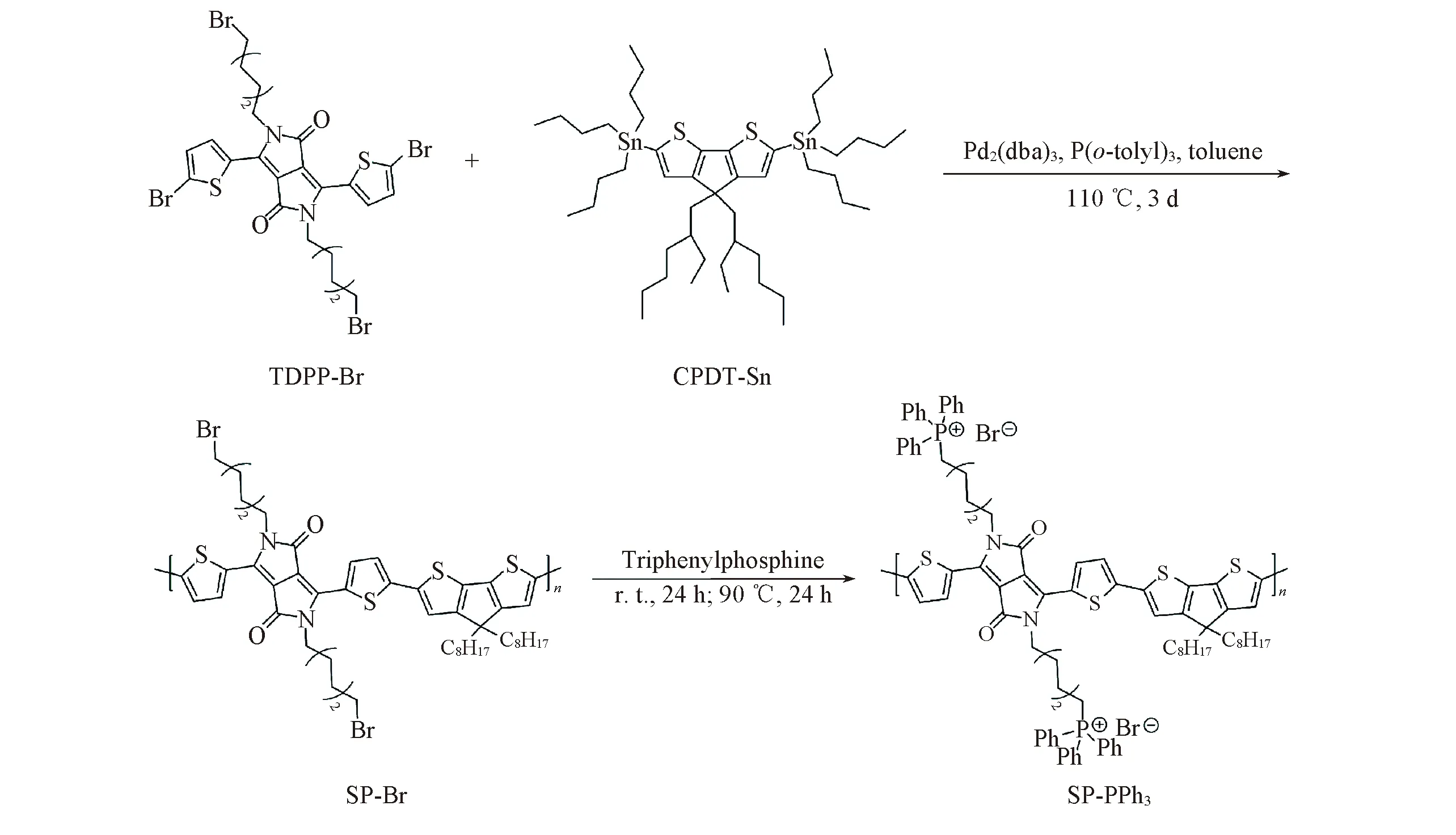
Fig.1 Synthetic route of SP-PPh3
The wound was photographed on the 0,3,6,9 and 12 d.The wounds with the adjacent skin(3,6,9 and 12 d) were collected and stored in 10% formaldehyde solution.After 12 d,organs(heart,liver,spleen,lung,kidney) of mice were harvested.The obtained organ and skin tissue were embedded in paraffin blocks and processed into slices.After hematoxylin and eosin(H&E) staining,the slices of skin and organs were used for histological and liver function analysis.
2 Results and Discussion
2.1 Synthesis and Characterization
In order to make the absorption of the semiconductor polymer(SP-PPh3) into the near-infrared region(NIR),TDPP-Br and CPDT-Sn were reacted in anhydrous toluene by a Stille coupling reaction to form a donor(D)-acceptor(A)(Fig.1).
Positively charged polymer can adhere to the membrane of bacteria,which is helpful to transmit heat to the cell membrane effectively.Through the nucleophilic substitution reaction of triphenylphosphine with alkyl bromide on SP-Br,the triphenylphosphine group was introduced into the alkyl chain of SP-Br to form the cationic polymer SP-PPh3.We characterized SP-PPh3with Fourier transform infrared spectroscopy(FTIR).In Fig.2(C) and (D),the characteristic peaks of triphenylphosphine(531,501 cm-1) indicated that the triphenylphosphine groups were linked with the alkyl chain.There was no stretching vibration peak at 558 cm-1of C—Br,which indicated that SP-Br was converted to the product SP-PPh3.In order to make it dispersed in an aqueous medium,SP-PPh3NPs were preparedviaa nanoprecipitation approach using the amphiphilic copolymers Pluronic F127.The prepared SP-PPh3NPs were characterized by transmission electron microscope(TEM) and dynamic light scattering(DLS).SP-PPh3NPs had spherical morphology,and the average diameter was about 40 nm[Fig.3(B)].The size of the particles by DLS was slightly larger than the size in TEM.DLS tests the hydrated particle size of particles,which results in a slight difference compared with the electron microscopy.Thezetapotential value of SP-PPh3NPs was +13.8 mV.The UV-vis spectra showed that the maximum absorption of SP-PPh3NPs was about 790 nm[Fig.3(A)],which contributed to a higher absorption rate under 808 nm laser.The high absorption efficiency can contribute a high conversion efficiency and good photothermal performance.
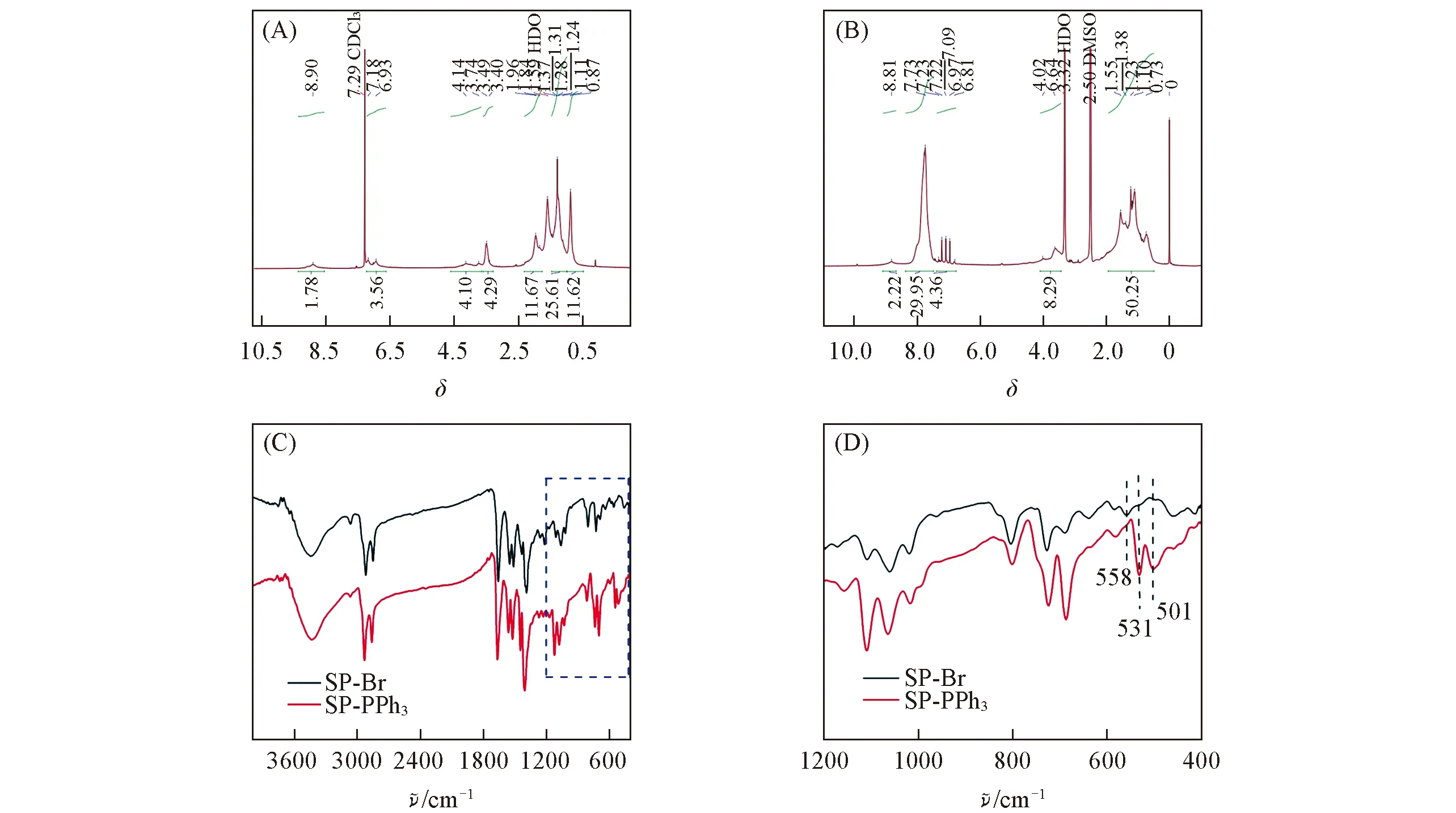
Fig.2 1H NMR(A,B) and FTIR(C,D) spectra of SP-Br(A) and SP-PPh3(B) (D) is the amplification of the marked part in (C).
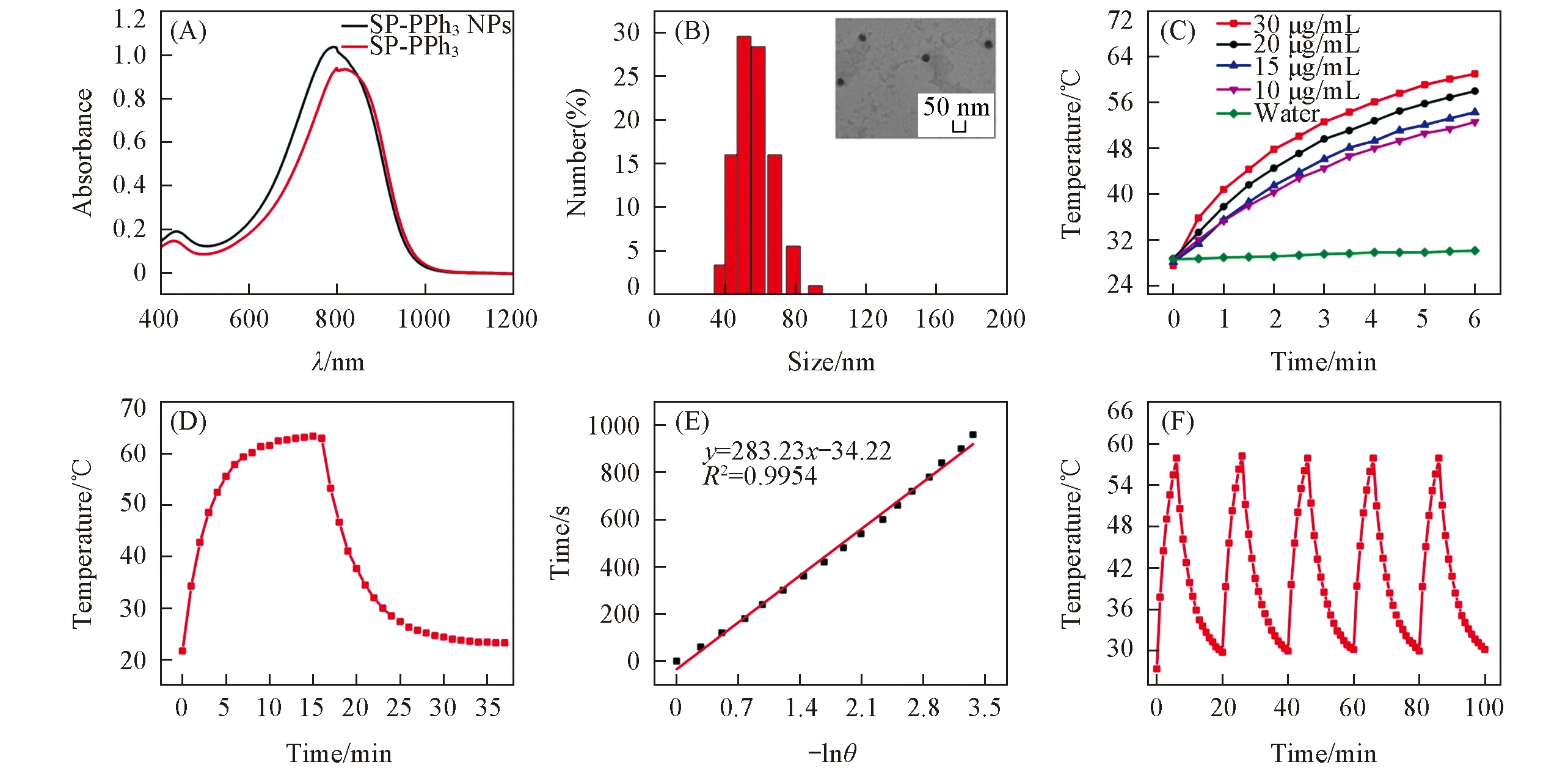
Fig.3 Characterization and photothermal activity of materials(A) UV-Vis absorption spectra of SP-PPh3 and SP-PPh3 NPs; (B) DLS and TEM image(inset) of SP-PPh3 NPs; (C) temperature elevation of SP-PPh3 NPs aqueous solution(10,15,20,30 μg/mL) and water under 808 nm laser(1.0 W/cm2); (D) temperature change of SP-PPh3 NPs aqueous solution(20 μg/mL) under irradiation of 808 nm laser(1.0 W/cm2) and turn off laser follow by natural cooling; (E) time-dependent natural logarithm of the temperature of SP-PPh3 NPs during the natural cooling after 16 min; (F) cyclic heating curve of SP-PPh3 NPs(20 μg/mL) under laser irradiation for five circles.
The photothermal conversion prole of SP-PPh3NPs was investigated.In Fig.3(C),when the SP-PPh3NPs solution(20 μg/mL) was irradiated for 6 min under an 808 nm laser at the power of 1.0 W/cm2,the temperature of the SP-PPh3NPs solution was raised from 28.7 ℃ to 58.0 ℃.The changing temperature(ΔT) of SP-PPh3NPs was 29.3 ℃.At the same time,the temperature of the water solution was increased from 29.6 ℃ to 30.1 ℃ and the ΔTwas 1.5 ℃.These results show that SP-PPh3NPs have a good photothermal effect.The temperature change of SP-PPh3NPs showed an increasing trend with the increase of concentration and laser irradiation time within 6 min.In order to further evaluate the photothermal performance,SP-PPh3NPs(20 μg/mL) were irradiated until the temperature reached equilibrium,and the laser was turned off[Fig.3(D)].According to the natural cooling time(t) and negative natural logarithm(-lnθ) of SP-PPh3NPs in aqueous solution(20 μg/mL),the characteristic thermal constant(τs) was calculated as 283.2[Fig.3(E)].Accordingly,the photothermal conversion efficiencies(η) of SP-PPh3NPs was calculated to be 43.8%.After five heating/cooling cycles,the temperature rise of SP-PPh3NPs was basically unchanged,indicating that SP-PPh3NPs have good photothermal stability[Fig.3(F)].These data support that SP-PPh3NPs is a good photothermal therapeutic agent.

Fig.4 IC50 of SP-PPh3 NPs for E. coli(A) and S. aureus(B)
2.2 In vitro Antibacterial Test
Positively charged materials can adhere to the negatively charged bacterial surfaces due to the electrostatic interaction[29—31].With the interaction between SP-PPh3NPs and bacteria,the local high charge on the surface of bacteria destroys the cell membrane and causes the bacteria to die.To investigate the antimicrobial properties of SP-PPh3NPs,the half-maximal inhibitory concentration(IC50) value of nanoparticle againstE.coliandS.aureuswas tested.The IC50of SP-PPh3NPs forE.coliis 32 μg/mL and forS.aureusis 16 μg/mL,respectively(Fig.4).The value of IC50is higher for Gram-negativeE.colithan that for Gram-positiveS.aureus.This heterogeneity is due to the differences between the two species’ membrane structure.The surface of Gram-negative bacteria has more lipopolysaccharide outer membrane than Gram-positive bacteria,which makes it more difficult for SP-PPh3NPs to inhibit the growth ofE.coli[32].

Fig.5 In vitro antibacterial activity evaluation and SEM images(A) Photographs of bacterial colony formation in the presence of E.coli(A1—A4) and S.aureus(A5—A8).(A1) PBS; (A2) laser; (A3) SP-PPh3 NPs; (A4) SP-PPh3 NPs+laser; (A5) PBS; (A6) laser; (A7) SP-PPh3 NPs; (A8) SP-PPh3 NPs+laser.(B) The relative bacterial viability of the control group(a),the laser group(b),the SP-PPh3 NPs group(c),the SP-PPh3 NPs+laser group(d).The concentration of SP-PPh3 NPs is 20 μg/mL and 808 nm laser power is 1.0 W/cm2.(C) SEM images of E.coli(C1—C3) and S.aureus(C4—C6) contacting with control(C1,C4),20 μg/mL SP-PPh3 NPs(C2,C5),SP-PPh3 NPs+laser(C3,C6).The 808 nm laser power is 1.0 W/cm2.
To achieve an effective antibacterial activity,the temperature of the photothermal agent needs to be above 70 ℃[33—35].However,the excessive temperature may damage surrounding tissue[36].The positive charge surface of SP-PPh3NPs also can inhibit the bacterial activity.The combination of positive charge and photothermal effect can enhance the antibacterial rate with less side effects.We evaluated the synergistic antibacterial activity of the nanoparticles against Gram-negativeE.coliand Gram-positiveS.aureusby plate counting.As shown in Fig.5(A),the number of colonies on the LB agar plates were almost no difference between the blank group[Fig.5(A1) and 5(A5)] and laser group[Fig.5(A2) and 5(A4)].The growth of bacteria was not inhibited by 808 nm laser irradiation at the power of 1.0 W/cm2for 6 min.When SP-PPh3NPs(20 μg/mL) were incubated withE.colifor 5 min in dark,compared with the blank group,the bacterial colony of the SP-PPh3group were decreased significantly[Fig.5(A3) and 5(A7)].On the basis of the positive charge effect,SP-PPh3NPs showed a certain antibacterial effect in 5 min.A significant decrease of bacteria was observed when bacteria were incubated with SP-PPh3NPs(20 μg/mL) for 5 min in dark and then irradiated at 808 nm laser[Fig.5(A4) and 5(A8)].The survival rate ofE.coliandS.aureusdecreased to 0.06% and 1.4%,respectively[Fig.5(B)].The above results show that the synergistic effect of SP-PPh3NPs has a good antibacterial effect.Positive charged SP-PPh3NPs adhere to the bacteria,and the local high-density charge could destroy the integrity of bacterial cell membranes[28].In addition,when SP-PPh3NPs stick to the bacteria,the heat caused by 808nm laser irradiation is directly transmitted to the bacteria.So,the heat caused by SP-PPh3NPs and the laser is directly used to kill the bacteria effectively[37—39].The morphology of SP-PPh3NPs interacted withE.coliandS.aureuswas imaged by SEM[Fig.5(C)].The bacteria in the control group(PBS) maintained their original morphology and the cell surface was smooth and intact.After the interaction of SP-PPh3NPs(20 μg/mL) with bacteria under dark conditions for five min,the bacteria were damaged with the lesions and holes.The shape ofE.coliunder laser irradiation with SP-PPh3NPs was fold and the surface of the bacteria collapses.And forS.aureus,there was exudation of the bacterial cytoplasmic matrix.SP-PPh3NPs present efficacious antibacterial activityinvitrobased on the synergistic effect of positive charge and photothermal treatment.
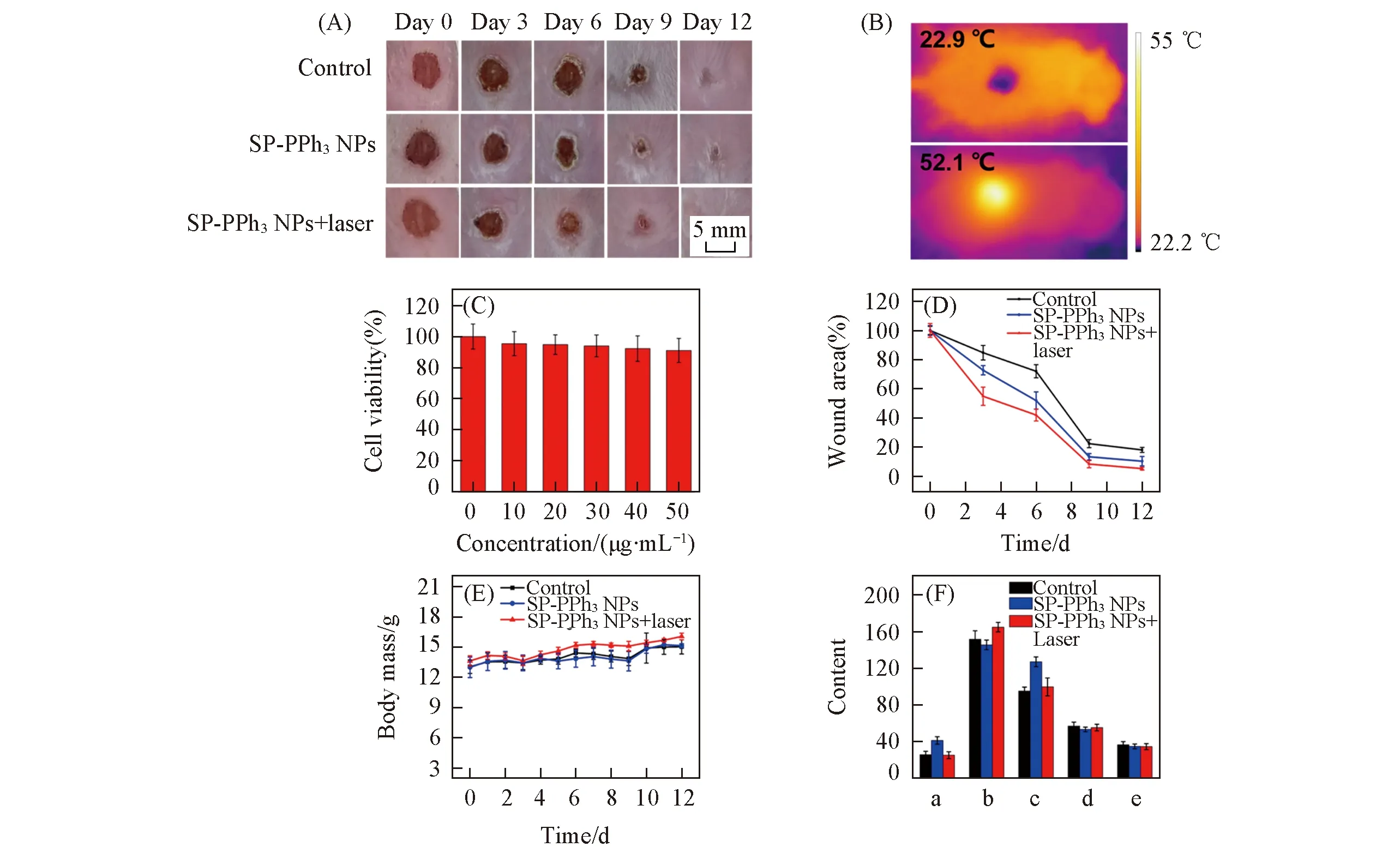
Fig.6 Cytotoxicity and mice model experiments(A) Photographs of wounds in mice treated with PBS(control),SP-PPh3 NPs and SP-PPh3 NPs+laser on days 0,3,6,9,12; (B) IR thermal images of the wound treated without SP-PPh3 NPs and with SP-PPh3 NPs respectively under the laser irradiation(808 nm,1.5 W/cm2); (C) cytotoxicity of SP-PPh3 NPs(10,20,30,40,50 μg/mL); (D) wounds area value on day 0,3,6,9,and 12 during antibacterial therapy; (E) the body mass of mice treated with PBS(control),SP-PPh3 NPs and SP-PPh3 NPs+laser; (F) analysis of liver function in mice after 12 d by blood,including alanine aminotransferase(a,U/L),aspartate aminotransferase(b,U/L),alkaline phosphatase(c,U/L),total protein(d,g/L),albumin(e,g/L).
2.3 In vivo Antibacterial Treatment Evaluation
In order to apply SP-PPh3NPsinvivo,we tested the cytotoxicity of SP-PPh3NPs.Even the concentration is up to 50 μg/mL,SP-PPh3NPs show little cytotoxicity[Fig.6(C)].Encouraged by low cytotoxicity,we applied SP-PPh3NPs for the treatment of bacterial infection woundinvivo.Under anesthesia,the wound(diameter: 0.8 cm) was made on the back of the mouse(BALB/c) and infected withS.aureus(1×107CFU/mL,120 μL).The mice were divided into three groups: control group(PBS),SP-PPh3NPs group,SP-PPh3NPs+laser group.The concentration of SP-PPh3NPs was 20 μg/mL(50 μL).In Fig.6(B),the tempe-rature of the wound treated with the SP-PPh3NPs+laser group increased to approximately 52.1 ℃ in 4 min,which indicated SP-PPh3NPs still kept good photothermal performanceinvivo.To further understand the antibacterial effect,the wound size on days 0,3,6,9,and 12 were recorded[Fig.6(A)].The relative wound area sizes of mice on days 0,3,6,9,and 12 were shown in Fig.6(D).The results showed that the SP-PPh3NPs+laser group had the smallest wound area,and the wound area of the mice treated with SP-PPh3NPs was smaller than that of the control group.SP-PPh3NPs with the laser can promote the wound healing on the skin effectively.
To further evaluate the pathological changes,skin tissues around the wounds of the mice were obtained and stained by H & E staining on days 3,6,9,and 12.As shown in Fig.7(A),all three groups had neutrophils(black arrows) and necrotic lesions(red matrix) were present on the third day,indicating that the mice were infected successfully byS.aureus.At the same time,a large number of fibroblasts(blue arrows) and macrophages(black matrix) appeared in the SP-PPh3NPs+laser group.Fibroblasts and macrophages are responsible for the involvement of tissue damage repair.The control group still had necrotic lesions,while granulation tissue(blue matrix) appeared in the treatment group indicating the healing of the wound after 6 d.On day 9,a large number of squamous epithelial cells(yellow matrix) appeared in the SP-PPh3NPs+laser group,and macrophages gradually disappeared.After 12 d,in the treatment group,the lesions almost disappeared and granulation tissue(green matrix) was filled in the original lesion,which indicated a good treatment effect.In addition,there was no significant change in the daily mass of the mice[Fig.6(E)].Histological analysis of major organs[Fig.7(B)] and liver function tests[Fig.6(F)] showed no significant damage.The above results show that SP-PPh3NPs have a good therapeutic effect on skin wound,and have basically no side effects.
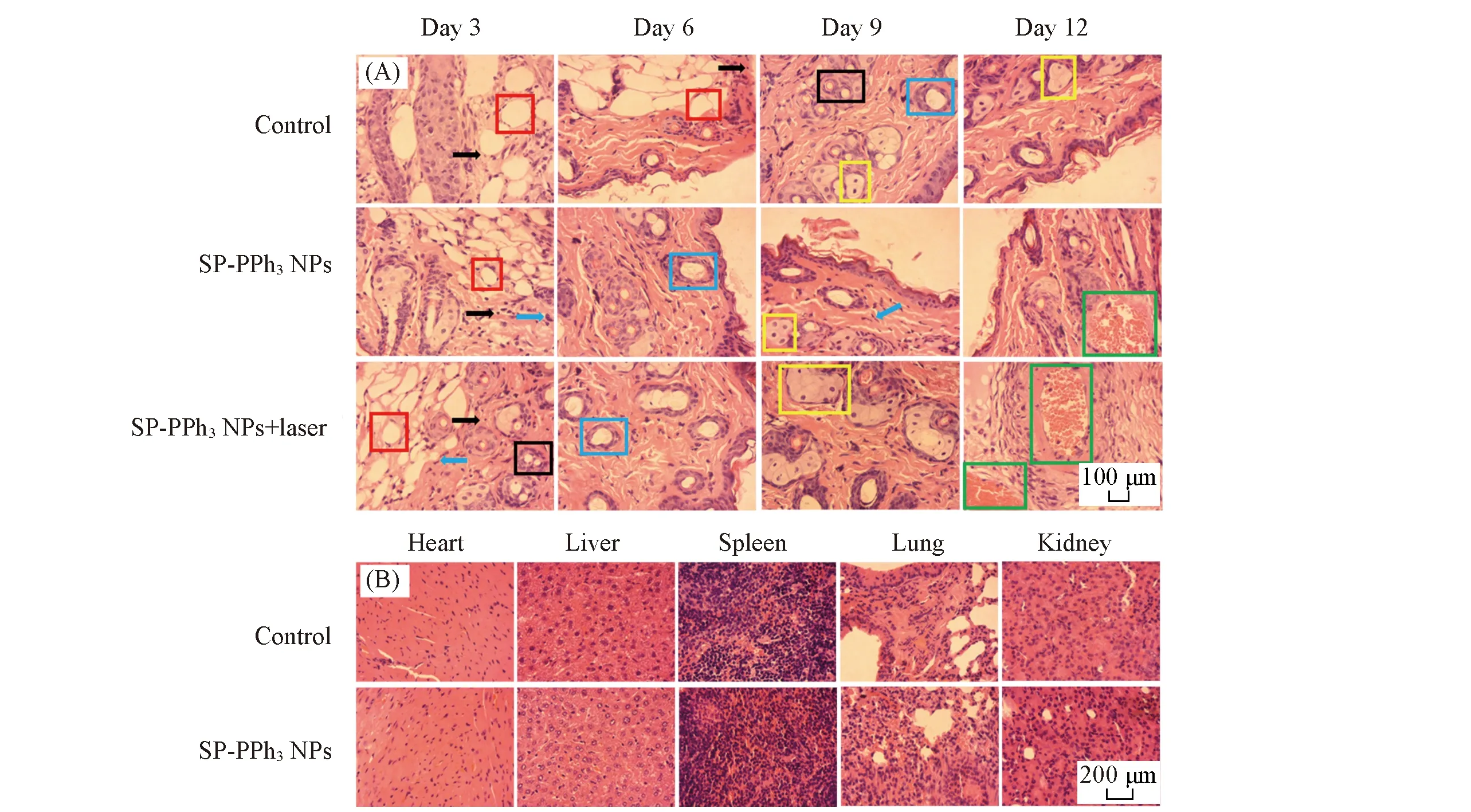
Fig.7 H&E staining analysis of skin and major organs(A) H & E staining slices of wound skin tissue on days 3,6,9,and 12; (B) mouse organ H & E staining sections(heart,liver,spleen,lung and kidney) of the control group and the SP-PPh3 NPs treated group.
3 Conclusions
In summary,we developed a new antibacterial material by using synergistic interaction of positive charge and photothermal therapy.SP-PPh3NPs not only have good photothermal performance but also have an antibacterial ability due to the positive charge.SP-PPh3NPs exhibited potent antibacterial properties against Gram-negativeE.coliand Gram-positiveS.aureus.The IC50values of SP-PPh3NPs forE.coliandS.aureuswere 32 and 16 μg/mL,respectively.In addition,SP-PPh3NPs showed satisfactory therapeutic effects in the mouse model ofS.aureusinfection,and promote wound healing.These results indicate that SP-PPh3NPs are expected to be candidates for the treatment of antibacterial infection.
This paper is supported by the Natural Science Foundation of China(Nos.81961138011,21775010),the Beijing Natural Science Foundation,China(No.7192106) and the Fundamental Research Funds for the Central Universities,China(Nos.XK1901,XK1802-6).