Azacitidine decreases reactive oxygen species production in peripheral white blood cells:A case report
2020-04-08
Hidekazu Hasunuma,Department of Blood Transfusion,Toho University Medical Center Sakura Hospital,Sakura 2858741,Japan
Naomi Shimizu,Department of Hematology,Toho University Medical Center Sakura Hospital,Sakura 2858741,Japan
Hiromitsu Yokota,Clinical Laboratory Program,Education Development Center,Faculty of Science Toho University,Funabashi 2748510,Japan
Ichiro Tatsuno, Center for Diabetes,Metabolism and Endocrinology,Toho University Medical Center Sakura Hospital,Sakura 2858741,Japan
Abstract BACKGROUND In myelodysplastic syndrome (MDS),oxidative stress is closely related to iron overload and DNA damage. A recent study suggested the possibility that increased oxidative stress causes not only iron overload but also disease progression of MDS with DNA damage. We present a case of MDS with decreased reactive oxygen species (ROS) production in peripheral white blood cells (WBCs) and decreased diacron-reactive oxygen metabolites (d-ROMs) in serum after azacitidine therapy.CASE SUMMARY A 74-year-old man presented to the hematological department with the chief complaint of anemia. His vital signs were within normal limits at admission with a heart rate of 80 bpm and blood pressure of 135/60 mmHg. Laboratory tests indicated pancytopenia,a WBC count of 2190 cells/μL,a hemoglobin level of 6.2 g/dL and a platelet count of 7.4 × 104/μL. The patient was diagnosed with MDS with fibrosis after a bone marrow examination. This case showed decreased ROS production in WBCs,d-ROMs in serum and Wilms’ tumor 1 after azacitidine therapy,after which his hematopoiesis recovered.CONCLUSION Azacitidine therapy can improve hematopoiesis and decrease ROS and d-ROM production.
Key Words: Myelodysplastic syndrome; Reactive oxygen species; Diacron-reactive oxygen metabolites; Ferritin; Azacitidine; Case report
INTRODUCTION
DNA damage caused by oxidative stress plays an important role in the initiation and carcinogenic process of mutation. A recent study suggested that oxidative stress leads to increased mutation frequency in a murine model of myelodysplastic syndrome(MDS)[1,2]and that additional mutations lead to MDS progression. Patients with MDS often require blood transfusion and consequently develop iron overload,which causes oxidative stress. Antioxidant therapy and iron chelation are therefore considered suitable for managing patients with MDS[3,4]. A correlation has also been reported between serum ferritin levels and serum reactive oxygen species (ROS) levels in patients with MDS patients[4]. MDS is a heterogeneous group of clonal hematopoietic stem cell disorders that transform into acute myeloid leukemia. DNA hypermethylation is one of the most important pathogenic mechanisms of MDS[1].Azacitidine is the first DNA hypomethylating agent that has demonstrated superiority over conventional therapy in treating MDS in patients unable to undergo allogeneic stem cell transplantation. A relationship between oxidative stress and hypermethylation has also been reported; oxidative stress is likely to be involved in MDS disease extension[1]. We report on a patient with MDS and fibrosis,who presented with decreased ROS in the peripheral white blood cells (WBCs),decreased diacron-reactive oxygen metabolites (d-ROMs) in serum and decreased Wilms’ tumor 1 (WT1). The patient was withdrawn from transfusion dependence after azacitidine therapy. The Ethics Committee of Toho University approved this study,and we obtained the patient’s informed consent.
CASE PRESENTATION
Chief complaints
A 74-year-old man presented with dyspnea on exertion that started two months earlier.
History of present illness
The patient presented anemia and a hemoglobin level of 6.4 g/dL during an ophthalmology consultation for cataract treatment. The patient presented dyspnea on exertion,a symptom that had aggravated over the past two months. The patient visited the hematological department.
History of past illness
The patient had a past medical history of cataract and ossification of a posterior longitudinal ligament.
Personal and family history
The patient had been a smoker for 50 years.
Physical examination
Vital signs were within normal limits at presentation,with a heart rate of 80 bpm,blood pressure of 135/60 mmHg,and a temperature of 36.1 ℃. His height and weight were 171 cm and 75 kg,respectively.
Laboratory examinations
Laboratory tests indicated a WBC count of 2190 cells/μL,a hemoglobin level of 6.2 g/dL,a platelet count of 7.4 × 104/μL,a lactate dehydrogenase level of 283 IU/L,and a ferritin level of 620.41 ng/mL. The WT1 mRNA was 4800/μg RNA of peripheral WBCs,and the serum d-ROM level was 435 Carratelli units. The bone marrow aspiration was dry tap,and the bone marrow biopsy showed MDS with fibrosis.
FINAL DIAGNOSIS
The patient was diagnosed with MDS with fibrosis.
TREATMENT
The patient was administered 75 mg/m2of azacitidine for 7 d once a month,underwent iron chelation therapy and took deferoxamine during the azacitidine therapy.
OUTCOME AND FOLLOW-UP
To date,the patient has undergone 11 cycles of azacitidine therapy. After the fourth cycle,he was released from transfusion. Figure 1 shows the changes in hematopoiesis during the azacitidine therapy. The patient presented with decreased ROS production in the peripheral WBCs,decreased d-ROMs in serum and decreased WT1 after azacitidine therapy. Figure 2 shows the decrease in ROS production in the WBCs after the ninth cycle of azacitidine therapy. The black line shows ROS production in a healthy control,while the colored line shows the patient’s production.
DISCUSSION
Gonçalveset al[5]reported the relationship between ROS and other mitochondrial dysfunction markers in patients with MDS. The levels of ROS stained with DCFH2-DA showed increases in each fraction of WBCs in patients with MDS,as was the case in our experiment. Moreover,patients with MDS and high ROS showed lower overall survival. The authors also analyzed the variants in genes involved in oxidative stress in patients with MDS and acute myeloid leukemia and showed that a number of superoxide dismutase 2 and glutathione peroxidase 1 genotypes increased the susceptibility for developing MDS with increased ROS production[6].
We have previously reported that the other oxidative stress markers (serum d-ROMs) transiently decreased after each azacitidine therapy. Furthermore,these oxidative stress markers strongly correlated with WT1 levels[7]. This case also suggests the mechanisms by which azacitidine prevents MDS disease progression by inhibiting oxidative stress.
Kornickaet al[8]reported that azacitidine showed reduced ROS accumulationin vitro. In the present case,we also evaluated ROS production in WBCs,in addition to serum d-ROM,ferritin and WT1 production during azacitidine therapy. Our patient was released from blood transfusion with a decrease in WT1,serum d-ROMs and ROS production in the WBCs after azacitidine therapy (Figure 1 and 2). As shown in Figure 1,there was no relationship with ferritin and oxidative stress markers. We therefore speculate that oxidative stress is related to the MDS state without iron overload. We also analyzed other patients with MDS undergoing azacitidine therapy.The patients in the responder group showed decreased ROS production in WBCs;however,the nonresponder group showed increased ROS and WT1 production(unpublished data). We therefore consider that ROS production the most suitable factor for evaluating MDS progression.
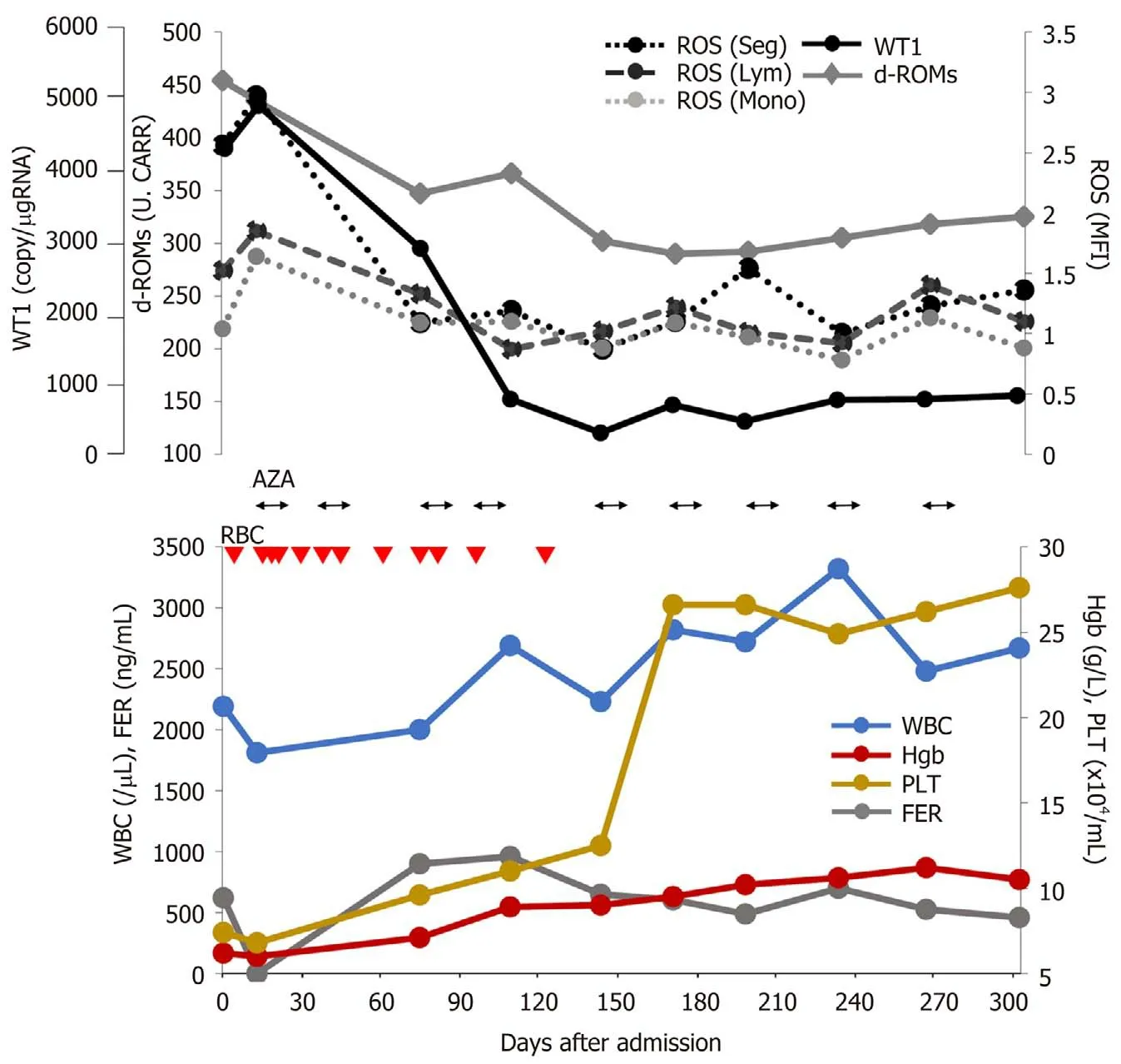
Figure 1 Clinical course with laboratory data during azacitidine therapy. Red triangle (2 units):RBC:Red blood cell transfusions; ROS:Reactive oxygen species; AZA:Azacitidine,WT1:Wilms’ tumor 1; d-ROM:Derivatives of reactive oxygen metabolite; FER:Ferritin; Normal ranges:Serum d-ROM; < 300 Carratelli units; Ferritin:18.6-261 ng/dL.
CONCLUSION
This is the first report indicating that azacitidine decreases ROS production in WBCs and decreases serum d-ROM levels in a patient with MDS. ROS,d-ROM and WT1 levels showed the same behavior during azacitidine therapy,and these parameters appear to have no relationship with ferritin levels. We speculate the novel mechanisms regarding the suppression of oxidative stress by the DNA hypomethylating agent azacitidine. Further study is therefore needed to clarify this point.
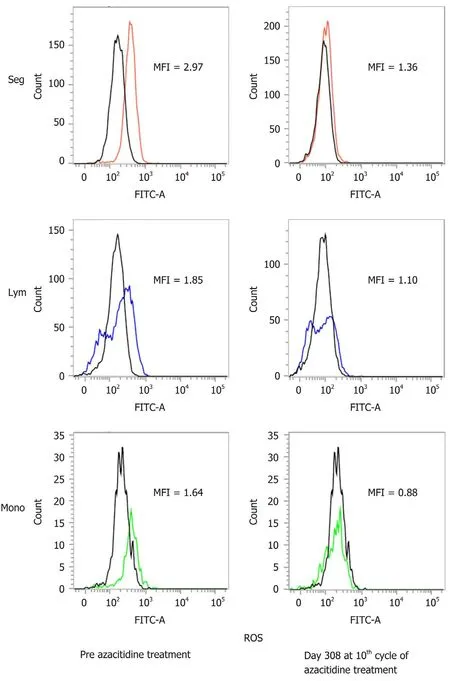
Figure 2 Flow cytometry analyses of reactive oxygen species in blood cells. Each white blood cell was gated and its fluorescence distribution histogram and mean fluorescence intensity (MFI) are shown. MFI shows this patient value when the healthy control is 1. ROS:Reactive oxygen species; Seg:Segment; Ly:Lymphocyte; Mono:Monocyte.
杂志排行
World Journal of Clinical Cases的其它文章
- COVID-19:A review of what radiologists need to know
- Holistic care model of time-sharing management for severe and critical COVID-19 patients
- Bioequivalence of two esomeprazole magnesium enteric-coated formulations in healthy Chinese subjects
- Osteoprotegerin,interleukin and hepatocyte growth factor for prediction of diabetes and hypertension in the third trimester of pregnancy
- High serum lactate dehydrogenase and dyspnea:Positive predictors of adverse outcome in critical COVID-19 patients in Yichang
- Risk factors analysis of prognosis of adult acute severe myocarditis
